Abstract
To explore the effect of prior thyroid cancer on the survival of primary liver cancer (PLC). Eligible PLC patients were selected from the Surveillance, Epidemiology, and End Results (SEER) database during 2004–2016. Propensity score matching (PSM) was used to create a highly comparable control group that PLC patients without prior thyroid cancer. All PLC patients were divided into three groups based on the survival information: (1) PLC-specific death; (2) death due to other causes; (3) alive. The effect sizes were presented by the corresponding hazard ratio (HR) and 95% confidence intervals (CI). Totally, 142 PLC patients with prior thyroid cancer and 1420 PLC patients without prior thyroid cancer were included. During the follow-up period, 714 (45.71%) PLC patients died of liver cancer while 638 (40.85%) PLC patients were alive. Median survival time for PLC patients was 11.00 months, respectively. PLC patients with prior thyroid cancer have a lower risk of death (HR = 0.64; 95% CI: 0.48–0.86). Subgroup analyses stratified by gender displayed the similar relation in female patients with PLC. Prior thyroid cancer may be a protective factor for liver cancer death in PLC patients, especially in female patients.
Similar content being viewed by others
Introduction
Primary liver cancer (PLC) is the fourth most prevalent leading cause of cancer-related death around the world1,2,3. Despite enormous progress in the modern therapeutic era, 5-year net survival of PLC ranging from 5 to 30%4. Over the past 20 years, liver cancer has been the tumor with the greatest increased mortality5. By 2030, the number of liver cancer cases worldwide could rise to 1 million6. The steadily increased incidence and high mortality rates have aroused people’s attention7.
With the development of cancer therapy and an increase in the rates of early diagnosis, the number of long-term cancer survivors continues to rise8. Better survival expectations increase the possibility of developing a second primary malignancy among survivors of cancers9,10. There is a pressing need to understand the clinical characteristics and survival outcomes of these populations. Several studies have reported that prior cancer history appeared to have an impact on second primary cancer survival11,12,13. Thyroid cancer, as cancer with a high survival rate, its influence on primary cancer survival has also been discussed14,15,16. Thyroid cancer has been found a benefit to the survival of ovarian16 and breast cancer15. The results of the previous study have encouraged the exploration of the effect of thyroid cancer on other cancers. A study by Mantovani et al. demonstrated that hypothyroidism was associated with a severe degree of nonalcoholic fatty liver disease (NAFLD)17. Furthermore, decreased thyroid function was related to a variety of metabolic abnormalities, such as insulin resistance, dyslipidemia, and lipid peroxidation, which all may increase the risk of liver damage18. Abnormal thyroid function may have an adverse effect on liver cancer mortality19. The association between prior thyroid cancer and the survival of PLC need to be explored.
Herein, we used data from the Surveillance, Epidemiology, and End Results (SEER) database to analyze the survival of PLC patients with or without a prior thyroid cancer. We conducted this study to determine whether prior thyroid cancer affects the survival of PLC patients.
Materials and methods
Study design and population
In this retrospective cohort study, we selected patients with PLC (the International Classification of Diseases for Oncology, Third Edition [ICD-O-3] code: C22.0, liver; C22.1, intrahepatic bile duct) from the SEER database during 2004 and 2016 based on November 2018 submission. SEER database provides complete patient data abstracted from 18 geographically diverse populations that represent rural, urban, and regional populations. Excluding criteria were as follows: (1) patients with a history of liver cancer; (2) patients demographic information lost; (3) < 18 years old; (4) patients whose follow-up time was 0 or lost to follow-up. Follow-up time for each patient was calculated from the date of cohort entry to the date of death or the end of follow-up (31 December 2016), and patients were followed up once a month. As all SEER data were accessed with approval from the SEER database, this article does not contain any studies with human participants or animals performed by any of the authors.
Data selection
We gathered variables of age, gender (female or male), race (white; black; others), marital status [married (including common law) or not married], tumor size (less than 5 cm; greater than or equal to 5 cm; unknown), regional nodes positive (yes; no; unknown), primary site (ICD-O-3, code C22.0 or C22.1), radiation therapy (none or unknown; yes), surgery (none or unknown; surgery performed), chemotherapy (none or unknown; yes), histologic grade (grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated; unknown), American Joint Committee on Cancer (AJCC) T status (T1; T2; T3; T4; unknown), AJCC N status (N0; N1; unknown), AJCC M status (M0; M1; unknown), SEER historic stage (localized; regional; distant; unknown), survival months, vital status (alive or dead), SEER cause death classification (alive; death due to liver cancer; death due to other cause).
Variable definitions
The primary site code (C22.0, liver) and the ICD-O-3 histology codes were used to identify cases with hepatocellular Carcinoma: from 8170 to 8175. The primary site code (C22.0, liver) and the ICD-O-3 histology codes of cases with intrahepatic cholangiocarcinoma was 8160. Prior thyroid cancer was defined as being diagnosed with thyroid cancer (the ICD-O-3 primary site code: C73.9, thyroid gland) before patients were diagnosed with liver cancers.
The eligible patients’ survival status was divided into three groups: (1) PLC-specific death; (2) death due to other causes; (3) alive.
Statistical analysis
Propensity Score Matching (PSM) is a commonly used statistical method that establishes a new control group by discarding outlier control subjects so that reduce the unwanted influences of covariates as well as measures the intended variable properly20. We used PSM with a caliper value of 0.1 and a ratio of 1:10 for matching, i.e. each patient with prior thyroid cancer was matched by age and gender with 10 patients without prior thyroid cancer (Supplementary Table 1).
Kolmogorov–Smirnov normality test was used for the measurement data. And measurement data of normal distribution was described as mean ± standard deviation (mean ± SD), while the measurement data of non-normal distribution were presented as median and quartile [M (Q1, Q3)]. And an independent-sample t test and Mann–Whitney U rank-sum test were conducted, respectively. The enumeration data were manifested as cases and the constituent ratio [n (%)] and were compared using the chi-square test or the Fisher’s exact test.
Cox proportional hazard model was used to explore the effect of prior thyroid cancer on the risk of PLC-specific death. And the product-limit (KM) method was used to plot the cumulative incidence function curve. In the competing risk model, the prior thyroid cancer was taken as the main variable, survival month and survival status (i.e. PLC-specific death; other cause-specific death; alive) were used as the outcome variables. Model 1 was undusted model. Model 2 was adjusted for age and gender, and Model 3 was adjusted for all confounding factors, i.e. age, gender, race, marital status, tumor size, regional nodes positive, primary site, histology groupings, radiation therapy, surgery, chemotherapy, histologic grade, AJCC T status, AJCC N status, AJCC M status, SEER historic stage.
SAS software (SAS Institute Inc., Cary, NC, USA; version 9.4) was used for all analyses. All the statistical tests were two-sided, and P < 0.05 was considered to be statistically significant.
Ethics declarations
Our data are from SEER database. Since all subjects in the database were anonymous, informed consent and ethical approval were not required. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Baseline information of participants
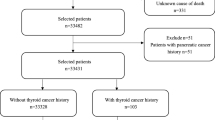
We originally selected 100,063 patients with liver cancer from the SEER database, 793 patients with a history of liver cancer, 850 patients whose ages were younger than 18 years old, 14,711 patients whose survival months were zero, 3522 patients who were lost follow-up, 4144 patients with unknown demographic information were excluded. Finally, a total of 76,043 patients were included in this study, among them, 1420 PLC patients without prior thyroid cancer and 142 PLC patients with prior thyroid cancer after PSM matching processing. The characteristics of the eligible patients before PSM matching processing are shown in Supplementary Table 2. From January 1, 2004, to December 31, 2016, the loss rate of follow-up in this study was 4%. The patient’s selection strategy is present in Fig. 1.
The average age of patients was 65.45 ± 10.83 years old, and the majority of patients were males (1052, 67.35%), white race (1029, 65.88%), and married (827, 52.94%). Comparing PLC without prior thyroid cancer, PLC patients who had prior thyroid cancer had longer survival months (P < 0.001). And PLC patients with prior thyroid cancer had higher proportion of females (P < 0.001), white race (P = 0.039), dead (P = 0.020), and death due to other causes (P < 0.001) than those who did not have thyroid cancer before. The main characteristics of the eligible patients are illustrated in Table 1.
Confounding factors
Variables including age, tumor size, regional nodes positive, primary site, histology groupings, surgery, chemotherapy, histologic grade, AJCC T status, AJCC N status, AJCC M status, and SEER historic stage all showed significant association with the risk of PLC-specific death were included in competing risk model (Table 2). Gender was included in the model as a variable that was often adjusted. Considering that marital status may be related to outcome variables, it was also included in the model in our study.
The effect of prior thyroid cancer on the survival of PLC patients
The cumulative incidence of PLC-specific death in PLC patients with and without prior thyroid cancer are shown in Fig. 2. The result showed that PLC patients with prior thyroid cancer had a lower risk of death from liver cancer.
Supplementary Table 3 showed that PLC patients who had a thyroid cancer history had a decreased PLC-specific death [hazard ratio (HR) = 0.618; 95% confidence interval (CI): 0.477–0.801, P < 0.001]. Compared with PLC patients who did not have prior thyroid cancer, the risk of death from liver cancer was reduced by 0.37 folds in patients with prior thyroid cancer [hazard ratio (HR) = 0.63; 95% confidence interval (CI): 0.50–0.81, P < 0.001]. After adjusting the age and gender, the risk of death from liver cancer was decreased by 0.39 folds in patients who had prior thyroid cancer (HR = 0.61; 95% CI: 0.48–0.79, P < 0.001). When all the confounders were adjusted, among patients with prior thyroid cancer, the risk of death from liver cancer was significantly lower than those without (HR = 0.64; 95% CI: 0.48–0.86, P = 0.003). Thus, prior thyroid cancer may be a protective factor for liver cancer death in patients with PLC (Table 3).
In the sensitivity analysis, the result before PSM treatment (Supplement Table 2) was similar to that after treatment (Table 3), i.e. prior thyroid cancer was related to the decreased risk of PLC-specific death in patients with PLC.
Association of prior thyroid cancer with the survival of PLC patients based on gender grouping
Among gender grouping, female patients with prior thyroid cancer had a lower risk of PLC death (HR = 0.49; 95% CI: 0.34–0.71, P < 0.001). In Model 2, this difference remained significant in the subgroups mentioned above. In Model 3, the potential protective effect of prior thyroid cancer was only found in female patients with PLC (HR = 0.53; 95% CI: 0.32–0.90, P = 0.018) (Table 4). The cumulative incidence of PLC-specific death between PLC patients with and without prior thyroid cancer in females is depicted in Fig. 3a. The cumulative incidence of PLC-specific death between PLC patients with and without prior thyroid cancer in males is displayed in Fig. 3b.
Discussion
An increase in cancer survivors has recently been observed due to advances in diagnosis and treatment strategies. With longer survival, patients are more likely to be subsequently diagnosed with second primary malignancy. It is important to determine the clinical significance and survival outcome of previous malignancies, as these factors may influence clinical trial design, oncology practice, and treatment decisions. Hypothyroidism has recently been identified as a predisposing factor for the development of liver cancer21. However, the role of thyroid cancer in PLC remains unclear. The present study evaluated the relationship between thyroid cancer and liver cancer mortality in a seer database consisting of middle-aged 1562 subjects. The result of this study indicated that among PLC patients, especially in female patients, prior thyroid cancer reduces the risk of PLC-specific death.
Our study observed that prior thyroid cancer may be a protective factor for liver cancer death. Thyroid cancer patients would suffer from hypothyroidism which is a common condition of thyroid hormone (TH) deficiency22 after undergoing a series of treatments to damage the diseased thyroid gland, such as surgery and the use of radioiodine23. While hypothyroidism is associated with poorer overall and recurrence-free survival of hepatocellular carcinoma patients receiving liver transplantation24. Pinter et al. observed that thyroid-stimulating hormone (TSH) and free tetraiodothyronine (FT4) were associated with prognostic factors of hepatocellular carcinoma3. A study by Kowalik et al. suggests that re-activation of the thyroid hormone triiodothyronine (T3)/thyroid hormone receptor β (TRβ) axis induces differentiation of neoplastic cells towards a more benign phenotype and that T3 or its analogues, particularly agonists of the TRβ, can represent useful tools in liver cancer therapy. In addition, T3 was shown to increase mitochondrial function and respiration in hepatocellular carcinoma cells25. The protective effect of prior thyroid cancer on PLC survival may be explained by the effects of TH on PLC. Changes in dietary patterns after the first primary cancer may also lead to better survival with the second primary cancer. Cancer patients tend to have good eating habits after being diagnosed, such as reducing alcohol consumption26. Previous studies speculated that a history of alcohol consumption may be a potential prognostic factor for liver cancer patients27,28.
In addition, the intensified medical surveillance after a first primary cancer leads to earlier detection of second primary cancers29. Early detection can effectively improve the prognosis of cancer patients and reduce the mortality rate1. A study by Huang et al. supported that increased surveillance after a first primary cancer leads to better outcomes for second primary cancers30. The first primary cancer may guide the frequency of second primary malignancy surveillance, which may lead to early diagnosis and better survival outcomes.
In this study, prior thyroid cancer may be a protective factor for PLC death in female patients rather than male patients. Thyroid cancer is reported to be female predominant while male patients have more aggressive behaviors and worse prognosis compared with female31. Liver metastases from thyroid cancer were more frequently diagnosed in male patients32. Some studies showed that estrogens may play a role in favoring the malignant progression of thyroid tissue to cancer33,34. We speculate that the better prognosis in female patients may be the reflection of the higher aggressive biology and mortality of thyroid cancer in male.
We conducted a retrospective cohort study based on the SEER database during 2004–2016, and found that prior thyroid cancer may decrease the risk of death for PLC. This finding suggests that prior cancers may influence the risk of subsequent cancers’ specific death, and researchers and clinicians should pay more attention to the possible impact of disease history on the results of their research or diagnosis. Our research has the following advantages. First of all, large-scale information from the SEER database is the highlights of our study. Moreover, the SEER database has high data accuracy. Furthermore, to our knowledge, it is the first attempt to explore the effect of prior thyroid cancer on the survival of PLC. However, the following limitations should be taken into consideration. Some information (such as the use of TH replacement drugs) was not investigated in the SEER database, so there may be some potential confounding factors that had not been considered. To reduce the impact of these limitations, clinical trials with a larger sample size should be conducted to verify our results.
Conclusion
Prior thyroid cancer may reduce the risk of PLC-specific death among PLC patients, especially female patients. It means that prior thyroid cancers may have an important effect on the development of PLC, and researchers and clinicians should pay more attention to the potential impact of prior thyroid cancer on their research or diagnosis related to PLC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mei, K. et al. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: Cohort A report in a multicenter phase Ib/II trial. J. Immunother. Cancer. https://doi.org/10.1136/jitc-2020-002191 (2021).
Barsouk, A., Thandra, K. C., Saginala, K., Rawla, P. & Barsouk, A. Chemical risk factors of primary liver cancer: An update. Hepatic Med. 12, 179–188. https://doi.org/10.2147/hmer.S278070 (2020).
Pinter, M. et al. The impact of thyroid hormones on patients with hepatocellular carcinoma. PLoS One 12, e0181878. https://doi.org/10.1371/journal.pone.0181878 (2017).
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London, England) 391, 1023–1075. https://doi.org/10.1016/s0140-6736(17)33326-3 (2018).
Llovet, J. M., Villanueva, A., Lachenmayer, A. & Finn, R. S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 12, 408–424. https://doi.org/10.1038/nrclinonc.2015.103 (2015).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Satriano, L., Lewinska, M., Rodrigues, P. M., Banales, J. M. & Andersen, J. B. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat. Rev. Gastroenterol. Hepatol. 16, 748–766. https://doi.org/10.1038/s41575-019-0217-8 (2019).
Joung, J. Y. et al. Second primary cancer risk among kidney cancer patients in Korea: A population-based cohort study. Cancer Res. Treat. 50, 293–301. https://doi.org/10.4143/crt.2016.543 (2018).
Zhuang, L., Yan, X. & Meng, Z. Second primary malignancy in patients with cholangiocarcinoma: A population-based study. Cancer Manag. Res. 11, 1969–1983. https://doi.org/10.2147/cmar.S187614 (2019).
Zhang, G., Wu, B., Wang, X. & Li, J. Second primary malignancy in patients with esophageal adenocarcinoma and squamous cell carcinoma. Medicine 98, e17083. https://doi.org/10.1097/md.0000000000017083 (2019).
Liu, J. et al. Impact of prior cancer history on the overall survival of younger patients with lung cancer. ESMO Open 5, e000608. https://doi.org/10.1136/esmoopen-2019-000608 (2020).
Al-Husseini, M. J. et al. Impact of prior malignancies on outcome of colorectal cancer; Revisiting clinical trial eligibility criteria. BMC Cancer 19, 863. https://doi.org/10.1186/s12885-019-6074-6 (2019).
Wu, Y. et al. Effect of prior cancer on survival outcomes for patients with advanced prostate cancer. BMC Urol. 21, 26. https://doi.org/10.1186/s12894-021-00792-w (2021).
Fligor, S. C., Lubitz, C. C. & James, B. C. ASO author reflections: Does timely surgery matter in papillary thyroid cancer?. Ann. Surg. Oncol. 28, 3567. https://doi.org/10.1245/s10434-021-09799-x (2021).
Cheng, W., Shen, X. & Xing, M. Decreased breast cancer-specific mortality risk in patients with a history of thyroid cancer. PLoS One 14, e0221093. https://doi.org/10.1371/journal.pone.0221093 (2019).
Yang, Q. et al. Thyroid cancer benefits the prognosis of ovarian cancer: A SEER-based study. Adv. Ther. 36, 1211–1220. https://doi.org/10.1007/s12325-019-00918-5 (2019).
Mantovani, A. et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Thyroid 28, 1270–1284. https://doi.org/10.1089/thy.2018.0257 (2018).
Ferrari, S. M., Fallahi, P., Antonelli, A. & Benvenga, S. Environmental issues in thyroid diseases. Front. Endocrinol. 8, 50. https://doi.org/10.3389/fendo.2017.00050 (2017).
Sohn, W. et al. Abnormal and euthyroid ranges of thyroid hormones in serum and liver cancer mortality: A cohort study. Cancer Epidemiol. Biomark. Prev. 29, 2002–2009. https://doi.org/10.1158/1055-9965.Epi-20-0283 (2020).
Kane, L. T. et al. Propensity score matching: A statistical method. Clin. Spine Surg. 33, 120–122. https://doi.org/10.1097/bsd.0000000000000932 (2020).
Bian, X. et al. The effects of a prior malignancy on the survival of patients with ovarian cancer: A population-based study. J. Cancer 11, 6178–6187. https://doi.org/10.7150/jca.46584 (2020).
Chaker, L., Bianco, A. C., Jonklaas, J. & Peeters, R. P. Hypothyroidism. Lancet (London, England) 390, 1550–1562. https://doi.org/10.1016/s0140-6736(17)30703-1 (2017).
Schlumberger, M. & Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 17, 176–188. https://doi.org/10.1038/s41574-020-00448-z (2021).
Zhang, N. et al. Hypothyroidism is associated with worse outcomes of hepatocellular carcinoma patients after liver transplantation. Cancer Med. 7, 5870–5878. https://doi.org/10.1002/cam4.1797 (2018).
Chi, H. C. et al. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 36, 5274–5284. https://doi.org/10.1038/onc.2017.136 (2017).
Malalur, P. et al. Cancer survivorship in hematologic malignancies: Lifestyle changes after diagnosis. Cancer Med. 10, 1066–1073. https://doi.org/10.1002/cam4.3698 (2021).
Ilić, M., Radoman, K., Konević, S. & Ilić, I. Liver cancer mortality and food consumption in Serbia, 1991–2010: An ecological study. Cent. Eur. J. Public Health 24, 103–108. https://doi.org/10.21101/cejph.a4168 (2016).
Schwartz, L. et al. Alcohol consumption, one-carbon metabolites, liver cancer and liver disease mortality. PLoS One 8, e78156. https://doi.org/10.1371/journal.pone.0078156 (2013).
Preyer, O. et al. The relative risk of second primary cancers in Austria’s western states: A retrospective cohort study. BMC Cancer 17, 699. https://doi.org/10.1186/s12885-017-3683-9 (2017).
Huang, H. Y. et al. Clinic image surveillance reduces mortality in patients with primary hepato-gastrointestinal cancer who develop second primary lung cancer: A STROBE-compliant retrospective study. Medicine 99, e23440. https://doi.org/10.1097/md.0000000000023440 (2020).
Hwang, S. H., Kim, E. K., Moon, H. J., Yoon, J. H. & Kwak, J. Y. Risk of thyroid cancer in euthyroid asymptomatic patients with thyroid nodules with an emphasis on family history of thyroid cancer. Korean J. Radiol. 17, 255–263. https://doi.org/10.3348/kjr.2016.17.2.255 (2016).
de Ridder, J. et al. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 7, 55368–55376. https://doi.org/10.18632/oncotarget.10552 (2016).
Zane, M. et al. Estrogen and thyroid cancer is a stem affair: A preliminary study. Biomed. Pharmacother. 85, 399–411. https://doi.org/10.1016/j.biopha.2016.11.043 (2017).
Shi, L. Y. et al. Clinic-pathologic features and prognostic analysis of thyroid cancer in the older adult: A SEER based study. J. Cancer 9, 2744–2750. https://doi.org/10.7150/jca.24625 (2018).
Author information
Authors and Affiliations
Contributions
H.L. designed the study and wrote the manuscript. H.X. and Y.T. collected and analyzed the data. Z.Y. critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, H., Heng, X., Tian, Y. et al. Effect of prior thyroid cancer on survival of primary liver cancer: a study based on the SEER database. Sci Rep 12, 13887 (2022). https://doi.org/10.1038/s41598-022-17729-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17729-4
This article is cited by
-
Value of thyroid cancer history in the prognosis of pancreatic cancer: a SEER population-based study
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.