Abstract
Culicoides biting midges (Diptera: Ceratopogonidae) are the major vectors of bluetongue, Schmallenberg, and African horse sickness viruses. This study was conducted to survey Culicoides species in different parts of Ethiopia and to develop habitat suitability for the major Culicoides species in Ethiopia. Culicoides traps were set in different parts of the country from December 2018 to April 2021 using UV light Onderstepoort traps and the collected Culicoides were sorted to species level. To develop the species distribution model for the two predominant Culicoides species, namely Culicoides imicola and C. kingi, an ensemble modeling technique was used with the Biomod2 package of R software. KAPPA True skill statistics (TSS) and ROC curve were used to evaluate the accuracy of species distribution models. In the ensemble modeling, models which score TSS values greater than 0.8 were considered. Negative binomialregression models were used to evaluate the relationship between C. imicola and C. kingi catch and various environmental and climatic factors. During the study period, a total of 9148 Culicoides were collected from 66 trapping sites. Of the total 9148, 8576 of them belongs to seven species and the remaining 572 Culicoides were unidentified. The predominant species was C. imicola (52.8%), followed by C. kingi (23.6%). The abundance of these two species was highly influenced by the agro-ecological zone of the capture sites and the proximity of the capture sites to livestock farms. Climatic variables such as mean annual minimum and maximum temperature and mean annual rainfall were found to influence the catch of C. imicola at the different study sites. The ensemble model performed very well for both species with KAPPA (0.9), TSS (0.98), and ROC (0.999) for C. imicola and KAPPA (0.889), TSS (0.999), and ROC (0.999) for C. kingi. Culicoides imicola has a larger suitability range compared to C. kingi. The Great Rift Valley in Ethiopia, the southern and eastern parts of the country, and the areas along the Blue Nile and Lake Tana basins in northern Ethiopia were particularly suitable for C. imicola. High suitability for C. kingi was found in central Ethiopia and the Southern Nations, Nationalities and Peoples Region (SNNPR). The habitat suitability model developed here could help researchers better understand where the above vector-borne diseases are likely to occur and target surveillance to high-risk areas.
Similar content being viewed by others
Introduction
African horse sickness (AHS) and bluetongue (BT), as well as Schmallenberg virus (SBV), are among the best-known animal diseases transmitted by adult female Culicoides biting midges1,2,3. AHS is a non-contagious viral disease of equids1,3 while BT, and SBV affect ruminants1,4. AHS disease is endemic in many parts of Africa, especially in the central and eastern parts of the continent, where it periodically makes short excursions beyond these areas5,6. BT has also historically been endemic in many countries located between 40° north and 35° south latitude7. However, since 1998, an unprecedented spread of BT has been observed in the Mediterranean basin8. SBV is a recent arboviral disease known to cause abortions, stillbirths, and congenital malformations in cattle, sheep, and less commonly, goats9. SBV was first detected in Germany in 2011 and since then the disease has spread to almost all European countries4. The disease has also been reported outside Europe from Ethiopia10.
Ethiopia experiences serious and repeated outbreaks of AHS every year. From 2007 to 2010, about 737 outbreaks were reported in different parts of the country11. However, the status of BT and SBV is not well understood, which may be due to misdiagnosis of the diseases with other common ruminant diseases such as foot and mouth disease (FMD), peste des petits ruminants (PPR), lumpy skin disease, and sheep and goat pox, which cause similar clinical symptoms12. However, there are some serological reports of BT13,14. For SBV, although there is no molecular evidence or virus isolation, a high apparent seroprevalence of 56.6% has been reported10.
Culicoides biting midges (Diptera: Ceratopogonidae) is a genus of the smallest blood-sucking flies, which measures up to 3 mm in size. The genus has a worldwide distribution, except in Antarctica and New Zealand, and it has more than 1400 known species15,16. Culicoides are known to transmit a wide range of pathogens. More than 50 arboviruses belonging to the Bunyaviridae, Reoviridae, and Rhabdoviridae families have been isolated from various Culicoides species17.
Culicoides breed in a variety of habitats and tend to stay near their hosts, including in and around farms, decaying vegetation, manure, pond edges, and moist soils. Female Culicoides often seek blood as a protein source for the development of their eggs. Therefore, they often bite their hosts such as amphibians, birds, and mammals, including humans and domestic animals to feed on their blood18.
Several studies have investigated the occurrence and species composition of Culicoides species throughout the world19,20,21,22,23. Some have predicted the potential current and future geographic distribution of Culicoides midges using different climatic and environmental variables24,25,26,27,28,29. To date, several Culicoides species have been detected in Ethiopia, including C. milnei, C. zuluensis, C. imicola, C. neavei, C. fulvithorax, and C. isioloensis30 and C. fuscicaudae31.
For effective risk management, it is essential to know the species composition and abundance of Culicoides populations in an area32. This study is, therefore, aimed to survey Culicoides species in different parts of Ethiopia and to develop habitat suitability for the prevalent species.
Materials and methods
Culicoides collection sites
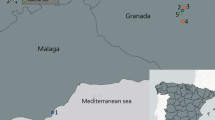
The current study was conducted in different parts of Ethiopia belonging to two regions. Hawassa town, Gamo Gofa, Konso and Wolayita in the South Nation Nationalities and People Region (SNNPR) and Jimma, Oromia Special Zone, East Shewa and Borena in the Oromia Region (Fig. 1). The areas were selected based on previous reports of Culicoides-borne diseases, namely AHS, bluetongue and SBV. The study is in accordance with relevant guidelines and regulations.
Map of Culicoides collection sites. The map was created using QGIS software v 3.22.2 (https://qgis.org/).
Culicoides collection and identification
Culicoides trapping was conducted from December 2018 to April 2021, consisting of two rounds. Sampling of the first round was conducted from December 2018 to April 2019 in Hawassa town, Gamo Gofa, Konso, Wolayita, East Shewa and Borena zones, while the second round was conducted from November 2020 to April 2021 in East Shewa, Jimma and Oromia Special zones. Culicoides were collected using UV light/suction traps developed by Onderstepoort Veterinary Institute (OVI, South Africa) and powered by a 12 V car battery. Trap locations were selected to ensure that they were locations conducive to the reproduction of Culicoides species. These include areas near water bodies, wetlands, livestock farms, and/or equine stables (Table 1). The location of each trap site was obtained with Global Positioning System (GPS). Traps were set from dusk (6:00 p.m.) to dawn (6:00 a.m.) and were placed both outdoors and indoors. Traps were hung from tree branches or building eaves at a height of 1.5–2 m above the ground. Insects were collected overnight in the cups of the traps and retrieved the next morning. All catches were transported to a local laboratory and placed in a − 4 °C refrigerator for 15 min to tranquillize insects. Culicoides species were first separated from other insects under a stereomicroscope, then transferred to an insect collection tube containing 70% ethanol, and finally transported to the entomology laboratory at the National Animal Health Diagnostic and Investigation Centre (NAHDIC) for species identification.
Species identification and enumeration were performed by observation of morphological features of Culicoides under a stereomicroscope. Identification was made according to previously published identification keys31,33,34. Most Culicoides midges have a wing pigmentation pattern and a distribution of wing macrotrichia consisting of grey and white spots; these patterns are unique to each species and can be easily observed under a dissecting microscope. The specimens were mounted on a slide and under the light microscope, morphological features such as shape, size, the number of female spermathecae, and the distance between eyes were observed33. Then we observed the ratio of antennae XI/X (length of segment XI divided by the length of segment X) and the shape and size of the 3rd palpal segment. Finally, we compared all the observed features with the images in the IIKC database (Interactive Identification Key for Culicoides)33.
Spatial distribution modeling (SDM)
The geographic distribution of the two most common Culicoides spp. was predicted using an ensemble modeling technique35. Species distribution models consists of three main aspects: species occurrence data (dependent variable), layers of environmental variables (independent variables), and a modeling algorithm.
Climate and environmental variables that characterize favorable habitats for Culicoides were selected based on a literature review of presence and abundance models25,36,37,38. Minimum, mean, and maximum temperature, precipitation, solar radiation (kJ m−2 day−1), wind speed (ms−1), water vapor pressure (kPa) and altitude were downloaded from the WorldClim database version 2 (http://worldclim.org/).
Land cover data was downloaded from the European Space Agency’s GlobCover Portal (http://due.esrin.esa.int/page_globcover.php). Livestock distribution data was downloaded from the website of FAO livestock systems (http://www.fao.org/livestock-systems/). Soil type was downloaded from (https://www.iiasa.ac.at/web/home/research/researchprograms/water/HWSD.html). All data layers were projected in the same projection system with a spatial resolution of 2.5 arc minutes using QGIS 3.4.1.
Data analysis
Ensemble modeling technique using biomod235 package of R software (http://cran.r-project.org/web/packages/biomod2/index.html) was used to develop the SDM. The package uses ten different methods: general linear models (GLM), general boosted models (GBM, also called boosted regression trees), general additive models (GAM), classification tree analysis (CTA), artificial neural networks (ANN), surface range envelope (SRE), flexible discriminant analysis (FDA), multiple adaptive regression splines (MARS), random forests (RF), and maximum entropy (MAXENT)35. Because the above ten modeling techniques require both absence and presence data to determine the suitability range of species, pseudo-absence data were generated using Surface Range Envelope (SRE). The data was split into two parts, 80% was used to train the model and 20% was used to test model performance. Models were evaluated using the results of threefold cross-validation in 30 models (10 techniques × 3 replicates).
The performance of models were evaluated using the true-skill statistic (TSS), the area under the receiver-operating characteristic curve (ROC) curve, and the Cohen's kappa statistic (Kappa). TSS is a threshold-dependent evaluation (sensitivity + 1, specificity − 1), with values closer to one indicating model accuracy39. For ensemble modeling, only models which score TSS values greater than 0.8 were considered. AUC scores range from 0 to 1, with models with scores above 0.5 providing better predictions than random draws40.
Since there is overdispersion in our multivariable Poisson model, a negative binomial regression models were used to assess the relationship between C. imicola and C. kingi catch with various environmental and climatic factors including agro-ecological zonation, habitat, livestock density, soil type, mean annual minimum and maximum temperatures, annual precipitation, solar radiation, wind speed, land cover and water vapour pressure. Univariable negative binomial regression models were first developed and only variables which are significant on univariable analysis were used to develop the multivariable negative binomial regression models. Multicollinearity among explanatory variables was checked using variance inflation factor (VIF) analysis, with the “vifstep” command in the “usdm” package of R. Variables which have VIF values less than or equal to 10 were considered in the analysis41. Mean annual maximum temperature was removed from C. imicola model due to collinearity and mean annual minimum temperature, altitude and solar radiation were removed from C. kingi model due to multicollinearity.
Ethics approval and consent to participate
Ethical approval was obtained from ethics committee of the College of Veterinary Medicine and Agriculture of Addis Ababa University. Written informed consents were obtained from all households who participated in the study.
Result
Entomological survey
During the study period, a total of 9148 Culicoides were collected from 66 trapping sites. Of the total 9148, 8576 of them belongs to seven species and the remaining 572 Culicoides were unidentified. Of the seven species identified, C. imicola was the most abundant species with 4830 (52.8%), followed by C. kingi with 2160 (23.6%), C. milnei, C. schultezi and C. Zuluensis, which accounted for 9%, 6.8%, and 1.4% of the catch, respectively (Table 2). Most Culicoides were caught in Jimma zone (28.5%), followed by Hawassa town (19.4%) and Oromia special zone (17.4%).
Most Culicoides were collected in the vicinity of the animal pen 6926 (75.7%), 1000 Culicoides were collected near rivers, which constitute 11% of the catch (Table 3).
Factors associated with Culicoides imicola and Culicoides kingi occurrence
The impact of different environmental and climatic variables on the abundance of C. imicola and C. kingi was evaluated using multivariable negative binomial regression (Tables 4, 5). Agro-ecological zonation, habitat, soil type, mean annual minimum temperature, altitude, and water vapour pressure were found to have a significant effect on the number of C. imicola catches (Table 4). Culicoides imicola catches were higher near animal pen and in nitisols and vertisols soil types. When mean annual minimum temperature and altitude increases, C. imicola catches decrease (Table 4).
Agro-ecological zonation of trapping sites, habitat, soil type and mean annual maximum temperature are significantly related to the occurrence of C. kingi. The catches of C. kingi were higher in humid areas, and in leptosols, nitisols and vertisols soil types. Mean annual maximum temperature is found to have a antagonistic effect with the C. kingi catches, as mean annual maximum temperature increases C. kingi catches decreases (Table 5).
Species distribution modeling
We chose to model the distribution of the two most common Culicoides species, C. imicola and C. kingi. The ensemble model performed very well for both species with (KAPPA (0.9), TSS (0.98), and ROC (0.999) for C. imicola and (KAPPA (0.889), TSS (0.999), and ROC (0.999) for C. kingi.
Culicoides imicola has a wider suitability range compared to C. kingi. The Great Rift Valley in Ethiopia and southern and eastern Ethiopia have a high suitability range for C. imicola. Suitability of C. imicola is also high in northern Ethiopia, particularly along the Blue Nile and Lake Tana catchments. Central Ethiopia has a patchy suitability range. The model predicts Gambela, Benshangul Gumuz, western parts of Oromia, Tigray, Afar, and Somali region as moderately suitable (Fig. 2).
Probability of C. imicola occurrence in Ethiopia. Highly suitable areas are represented with red color while unsuitable areas are represented using blue colour. The map was created using QGIS software v 3.22.2 (https://qgis.org/).
A high suitability range for C. kingi was observed in central Ethiopia and in SNNPR. The ensemble model predicted the other parts of the country as moderately suitable, with the exception of the Afar and Somali regions, for which the model predicted lower suitability (Fig. 3).
Probability of C. kingi occurrence in Ethiopia. Highly suitable areas are represented with red color while unsuitable areas are represented using blue color. The map was created using QGIS software v 3.22.2 (https://qgis.org/).
According to the results of this ensemble modeling, the distribution of C. imicola depends mainly on soil type (15.7%), altitude (12.5%), livestock distribution (12.5%), solar radiation (12.1%), and mean annual minimum temperature (11.8%). For C. kingi, they are wind speed (43.4%), soil type (10.8%), altitude/elevation (10.6%), and vapor pressure (8.8%) (Table 6).
Discussion
AHS, BT bluetongue and SBV are economically important Culicoides-borne viral diseases affecting equids and ruminants. The importance of these arboviral diseases derives from their very wide geographic distribution, potential for rapid spread, and large economic impact1,2. A considerable number of studies reported widespread occurrence of these diseases in Ethiopia. Demissie42 reported AHS from Gamo Gofa, Wolaita and Hadiya zones of SNNPR. Zeleke et al.43 reported the occurrence of AHS in southern (Awassa, Hossana, Wondogenet, and Hagereselam), western (Jimma, Bedelle, Nekemte, Horroguduru, and Chaliya), and central (Bishoftu, Meki, Zeway, Filtimo, and Bekejo) Ethiopia. Ayelet et al.44 reported AHS from Ada’a, Bahir Dar, Mecha, Dangla, Jimma and Sodo and Aklilu et al.11 reported AHS from central Ethiopia. Gizaw et al.13 reported the presence of group-specific antibodies against bluetongue virus from Adami Tulu, Amibara, Areka, Arsi Negelle, Bene Tsemay, Doyo Gena, G/Mekeda, Fafan, and Jinka. Abera et al.14 reported the presence of bluetongue antibodies from Jimma, Bonga and Bedelle and Gulima45 reported the presence of bluetongue antibodies from Amhara regional state in northern Ethiopia. There is only one study on the sero-prevalence of SBV in Ethiopia. The author reported a very high apparent seroprevalence of 56.6%10.
These diseases are transmitted by females of several species of midges belonging to the large genus Culicoides (Diptera: Ceratopogonidae) (which includes more than 1300 described species worldwide46,47. In this study, morphological identification confirmed the presence of seven species in Ethiopia. In the current study, various Culicoides species were collected, of which C. imicola was the largest number 4830 (52.8%). Similar to these results, entomological surveys carried out in many sub-Saharan African countries show that C. imicola is the dominant species22,23,48,49,50. A previous study by Mulatu and Hailu30, reported the presence of C. imicola, C. milnei, C. neavei, C. zuluensis, C. fulvithorax and C. isioloensis in western parts of Ethiopia and Khamala and Kettle31 reported the presence of C. fuscicaudae. This study identified three species of Culicoides that had not been previously described in Ethiopia, including C. kingi, C. schultzei and C. pycnostictus.
Multivariable negative binomial regression models were used to model the impact of various environmental and climatic factors on C. imicola and C. kingi catch. For both species, significantly higher catch was obtained in the subhumid agro-ecological zone. This finding suggests that Culicoides species require breeding habitat with high relative humidity51. In the current study, higher numbers of Culicoides were caught in traps placed near animal pens. This result is consistent with Riddin et al.52 that reported high Culicoides catches near horse barns. The abundance of Culicoides near animal pens is mainly due to the presence of suitable breeding sites represented by moist soil sites, leaking animal watering troughs, and pond edges contaminated with feces53.
Soil type appears to be very important in determining the distribution and abundance of Culicoides23,54,55. According to the studies, the largest numbers of Culicoides were found in areas with a high, moisture-retentive clay soil, whilst the lowest numbers were encountered in rapidly draining sandy soils. In this study, C. imicola is mainly collected from nitisols and vertisols soil type, these soils types are clay-rich and characterized by their high moisture retention capacity56,57. C. kingi was mainly collected from diverse soil types including leptosols, nitisols and vertisols soil types. Leptosols are mountain soils known by their high waterlogging capacity58. These soil types are believe to create suitable breeding sites for different Culicoides species.
The present study shows that climatic variables to be an important determinants of Culicoides catches, temperature being the most important determinant for both species. Studies demonstrated that, Culicoides activity generally declines or even ceases at low temperatures, and high temperatures. Temperature ≥ 40 °C could be lethal38. Foxi et al.53 also reported relatively poor tolerance of Culicoides to lower temperatures. Eventhough it is not evident in our multivariable negative binomial regression models, previous study by Gusmão et al.21 suggests that persistent or heavy rain can create conditions for biting midges to proliferate but it can also be a barrier to the activity of adult (winged) biting midges and prevent them from flying. Rain can prevent adults from leaving their shelters and could affect catch.
Various climatic and environmental factors were used to model the species distribution of C. imicola and C. kingi. Soil type, altitude/elevation, livestock distribution, solar radiation, and mean minimum annual temperature were the most important variables for the C. imicola model. Wind speed, soil type, altitude/elevation, and vapor pressure were the variables that contributed most to the model for C. kingi. Although there is no previous information on the climatic requirements of C. kingi, numerous studies have examined the role of various climatic and environmental factors on the distribution and abundance of C. imicola. Global ensemble modeling of C. imicola by Leta et al.29 reported temperature covariates contributing 64% to their model. This is supported by Veronesi et al.59 which showed that temperature can affect fecundity, hatching, and survival of C. imicola. When reared at a higher temperature (28 °C), C. imicola exhibited higher variability in fecundity and lower hatch rates, and the mean emergence rate from pupae was highest at 20 °C. The distribution of C. imicola is probably directly limited by its relatively low tolerance to lower temperatures60. As temperatures rise, adults hatch and populations gradually increase to reach a peak in abundance in spring or summer, depending on the site, which is a function of spring temperatures and summer drought. Because temperature shortens larval development time and the time between two blood meals, thus increasing laying frequency, which has a positive effect on population dynamics (and their growth), we expected that temperature would have a significant effect on abundance24.
Our study also showed that solar radiation and livestock distribution are influential variables in the spread of C. imicola. According to Conte et al.61, intense solar radiation on C. imicola larval habitat combined with high nighttime temperatures accelerates larval development, resulting in multiple generations/season. The importance of livestock as a source of blood meals for C. imicola is well established62. Culicoides imicola is a bloodsucking insect that tends to feed on blood and breed near livestock and humans. The frequency of contact between Culicoides and vertebrate hosts is closely related to the multiplication of the pathogen and the risk of transmission62,63.
In this study, C. imicola was found to have a larger suitable range compared to C. kingi. Globally, C. imicola is widespread in tropical and subtropical regions of Africa, the southern part of Europe, and some parts of Asia64. The model describes that all regions have a small to large range of suitable areas, with Oromia and SNNPR regions having a larger range of suitable areas. High suitability for C. imicola was also demonstrated in the Amhara region, particularly adjacent to the Blue Nile basin and Lake Tana, in southern Afar, and in areas of the Somali region adjacent to Oromia. The results of the current model overlap in many ways with the previously published model of Leta et al.29. The current model emphasizes at national level by elaborating the distribution of C. imicola and C. kingi suitability in different regions of the country.
In conclusion, the entomological study shows the occurrence of C. imicola and C. kingi in different parts of Ethiopia, with C. imicola predominating. The widespread occurrence of these species indicates a higher risk of SBV, BT and AHS in different parts of Ethiopia. The models could help to understand the risk of introduction and spread of SBV, BT, and AHS.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author up on request.
Change history
02 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-18764-x
References
MacLachlan, N. J. & Guthrie, A. J. Re-emergence of bluetongue, African horse sickness, and other Orbivirus diseases. Vet. Res. https://doi.org/10.1051/vetres/2010007 (2010).
Koenraadt, C. J. M. et al. Bluetongue, Schmallenberg—What is next? Culicoides-borne viral diseases in the 21st Century. BMC Res. Notes 10, 77 (2014).
Dennis, S. J., Meyers, A. E., Hitzeroth, I. I. & Rybicki, E. P. African horse sickness: A review of current understanding and vaccine development in the. Viruses 11, 844 (2019).
Collins, Á. B., Doherty, M. L., Barrett, D. J. & Mee, J. F. Schmallenberg virus: A systematic international literature review (2011–2019) from an Irish perspective. Ir. Vet. J. 72, 1–22 (2019).
Tkuwet, G. & Firesbhat, A. A review on African horse sickness. Eur. J. Appl. Sci. 7, 213–219 (2015).
Mellor, P. S. & Hamblin, C. African horse sickness. Vet. Res. 35, 445–466 (2004).
Coetzee, P., Stokstad, M., Venter, E. H., Myrmel, M. & Van Vuuren, M. Bluetongue: A historical and epidemiological perspective with the emphasis on South Africa. Virol. J. 9, 198 (2012).
Cagienard, A., Griot, C., Mellor, P. S., Denison, E. & Stärk, K. D. Bluetongue vector species of Culicoides in Switzerland. Med. Vet. Entomol. 20, 239–247 (2006).
Oluwayelu, D., Adebiyi, A. & Tomori, O. Endemic and emerging arboviral diseases of livestock in Nigeria: A review. Parasit. Vectors 11, 1–12 (2018).
Sibhat, B., Ayelet, G., Gebremedhin, E. Z., Skjerve, E. & Asmare, K. Seroprevalence of Schmallenberg virus in dairy cattle in Ethiopia. Acta Trop. 178, 61–67 (2018).
Aklilu, N. et al. African horse sickness outbreaks caused by multiple virus types in Ethiopia. Transbound. Emerg. Dis. 61, 185–192 (2014).
Rojas, J. M., Rodríguez-Martín, D., Martín, V. & Sevilla, N. Diagnosing bluetongue virus in domestic ruminants: Current perspectives. Vet. Med. Res. Rep. 10, 17 (2019).
Gizaw, D., Sibhat, D., Ayalew, B. & Sehal, M. Sero-prevalence study of bluetongue infection in sheep and goats in selected areas of Ethiopia. Ethiop. Vet. J. 20, 105 (2016).
Abera, T. et al. Bluetongue disease in small ruminants in south western Ethiopia: Cross-sectional sero-epidemiological study. BMC Res. Notes 11, 112 (2018).
Mellor, P. S., Boorman, J. & Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 45, 307–340 (2000).
Carpenter, S., Groschup, M. H., Garros, C., Felippe-Bauer, M. L. & Purse, B. V. Culicoides biting midges, arboviruses and public health in Europe. Antivir. Res. 100, 102–113 (2013).
Sick, F., Beer, M., Kampen, H. & Wernike, K. Culicoides biting midges—Underestimated vectors for arboviruses of public health and veterinary importance. Viruses 11, 376 (2019).
Blanda, V. et al. Geo-statistical analysis of Culicoides spp. distribution and abundance in Sicily, Italy. Parasit. Vectors 11, 78 (2018).
Vasić, A. et al. Species diversity, host preference and arbovirus detection of Culicoides (Diptera: Ceratopogonidae) in south-eastern Serbia. Parasit. Vectors 12, 1–9 (2019).
Martin, E. et al. Culicoides species community composition and infection status with parasites in an urban environment of east central Texas, USA. Parasit. Vectors 12, 1–10 (2019).
Gusmão, G. M. C., Brito, G. A., Moraes, L. S., Bandeira, M. D. C. A. & Rebêlo, J. M. M. Temporal variation in species abundance and richness of Culicoides (Diptera: Ceratopogonidae) in a tropical equatorial area. J. Med. Entomol. https://doi.org/10.1093/jme/tjz015 (2019).
Sghaier, S. et al. New species of the genus Culicoides (Diptera Ceratopogonidae) for Tunisia, with detection of Bluetongue viruses in vectors. Vet. Ital. 53, 357–366 (2017).
Gordon, S. J. G. et al. The occurrence of Culicoides species, the vectors of arboviruses, at selected trap sites in Zimbabwe. Onderstepoort J. Vet. Res. 82, e1–e8 (2015).
Villard, P. et al. Modeling Culicoides abundance in mainland France: Implications for surveillance. Parasit. Vectors 12, 1–10 (2019).
Diarra, M. et al. Spatial distribution modelling of Culicoides (Diptera: Ceratopogonidae) biting midges, potential vectors of African horse sickness and bluetongue viruses in Senegal. Parasit. Vectors 11, 341 (2018).
Calvete, C. et al. Spatial distribution of Culicoides imicola, the main vector of bluetongue virus, Spain. Vet. Rec. 158, 130–131 (2006).
Purse, B. V. et al. Modelling the distributions of Culicoides bluetongue virus vectors in Sicily in relation to satellite-derived climate variables. Med. Vet. Entomol. 18, 90–101 (2004).
Purse, B. V. et al. Spatial and temporal distribution of bluetongue and its Culicoides vectors in Bulgaria. Med. Vet. Entomol. 20, 335–344 (2006).
Leta, S. et al. Modeling the global distribution of Culicoides imicola: An ensemble approach. Sci. Rep. 9, 1–9 (2019).
Mulatu, T. & Hailu, A. The occurrence and identification of Culicoides species in the Western Ethiopia. Acad. J. Entomol. 12, 40–43 (2019).
Khamala, C. P. M. & Kettle, D. S. The Culicoides Latreille (Diptera: Ceratopogonidae) of East Africa. Trans. R. Entomol. Soc. Lond. 123, 1–95 (1971).
Venter, G. J. Specie di Culicoides (Diptera: Ceratopogonidae) vettori del virus della Bluetongue in Sud Africa. Vet. Ital. 51, 325–333 (2015).
Mathieu, B. et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasit. Vectors 5, 137 (2012).
Glick, J. I. Culicoides biting midges (Diptera: Ceratopogonidae) of Kenya. J. Med. Entomol. 27, 85–195 (1990).
Thuiller, W., Lafourcade, B., Engler, R. & Araújo, M. B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography (Cop.) 32, 369–373 (2009).
Baylis, M., Bouayoune, H., Touti, J. & El Hasnaoui, H. Use of climatic data and satellite imagery to model the abundance of Culicoides imicola, the vector of African horse sickness virus, in Morocco. Med. Vet. Entomol. 12, 255–266 (1998).
Diarra, M. et al. Modelling the abundances of two major culicoides (Diptera: Ceratopogonidae) species in the Niayes area of Senegal. PLoS One 10, e0131021 (2015).
Ramilo, D. W., Nunes, T., Madeira, S., Boinas, F. & da Fonseca, I. P. Geographical distribution of Culicoides (DIPTERA: CERATOPOGONIDAE) in mainland Portugal: Presence/absence modelling of vector and potential vector species. PLoS One 12, e0180606 (2017).
Ben Rais Lasram, F. et al. The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Chang. Biol. 16, 3233–3245 (2010).
Tiffin, P. & Ross-Ibarra, J. Goal-oriented evaluation of species distribution models accuracy and precision: True Skill Statistic profile and uncertainty maps. PeerJ PrePints https://doi.org/10.7287/peerj.preprints.488v1 (2014).
Graham, M. H. Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815 (2003).
Demissie, G. H. Seroepidemiological study of African horse sickness in southern Ethiopia. Open Sci. Repos. Vet. Med. 10, e70081919 (2013).
Zeleke, A., Sori, T., Powel, K., Gebre-Ab, F. & Endebu, B. Isolation and identification of circulating serotypes of African horse sickness virus in Ethiopia. J. Appl. Res. Vet. Med. 3, 40–43 (2005).
Ayelet, G. et al. Outbreak investigation and molecular characterization of African horse sickness virus circulating in selected areas of Ethiopia. Acta Trop. 127, 91–96 (2013).
Gulima, D. Seroepidemiological study of bluetongue in indigenous sheep in selected districts of Amhara National Regional State, north western Ethiopia. Ethiop. Vet. J. 13, 1–15 (2009).
Borkent, A. & Dominiak, P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa 4787, 1–377 (2020).
Borkent, A. & Wirth, W. W. World species of biting midges (Diptera: Ceratopogonidae). Bull. Am. Museum Nat. Hist. 233, 5–195 (1997).
Guichard, S. et al. Worldwide niche and future potential distribution of Culicoides imicola, a major vector of bluetongue and African horse sickness viruses. PLoS One 9, e112491 (2014).
Becker, E. E. E., Venter, G. J., Labuschagne, K., Greyling, T. & van Hamburg, H. Occurrence of Culicoides species Diptera: Ceratopogonidae) in the Khomas region of Namibia during the winter months. Vet. Ital. 48, 45–54 (2012).
Capela, R. et al. Spatial distribution of Culicoides species in Portugal in relation to the transmission of African horse sickness and bluetongue viruses. Med. Vet. Entomol. 17, 165–177 (2003).
Calvete, C. et al. Modelling the distributions and spatial coincidence of bluetongue vectors Culicoides imicola and the Culicoides obsoletus group throughout the Iberian peninsula. Med. Vet. Entomol. 22, 124–134 (2008).
Riddin, M. A., Venter, G. J., Labuschagne, K. & Villet, M. H. Culicoides species as potential vectors of African horse sickness virus in the southern regions of South Africa. Med. Vet. Entomol. 33, 498–511 (2019).
Foxi, C. et al. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasit. Vectors 9, 440 (2016).
Musuka, G. N., Mellor, P. S., Meiswinkel, R., Baylis, M. & Kelly, P. J. Prevalence of Culicoides imicola and other species (Diptera: Ceratopogonidae) ateight sites in Zimbabwe: To the editor. J. S. Afr. Vet. Assoc. 72, 62–63 (2001).
Meiswinkel, R. The 1996 outbreak of African horse sickness in South Africa—the entomological perspective. Arch. Virol. Suppl. 14, 69–83 (1998).
Jean Pierre, T. et al. Characteristics, classification and genesis of vertisols under seasonally contrasted climate in the Lake Chad Basin, Central Africa. J. Afr. Earth Sci. 150, 176–193 (2019).
Elias, E. Characteristics of Nitisol profiles as affected by land use type and slope class in some Ethiopian highlands. Environ. Syst. Res. 6, 1–15 (2017).
Nachtergaele, F. The classification of leptosols in the world reference base for soil resources.
Veronesi, E., Venter, G. J., Labuschagne, K., Mellor, P. S. & Carpenter, S. Life-history parameters of Culicoides (Avaritia) imicola Kieffer in the laboratory at different rearing temperatures. Vet. Parasitol. 163, 370–373 (2009).
Verhoef, F. A. A., Venter, G. J. & Weldon, C. W. Thermal limits of two biting midges, Culicoides imicola Kieffer and C. bolitinos Meiswinkel (Diptera: Ceratopogonidae). Parasites Vectors 7, 384 (2014).
Conte, A., Goffredo, M., Ippoliti, C. & Meiswinkel, R. Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus Complex in Italy. Vet. Parasitol. 150, 333–344 (2007).
Martinez-de la Puente, J., Navarro, J., Ferraguti, M., Soriguer, R. & Figuerola, J. First molecular identification of the vertebrate hosts of Culicoides imicola in Europe and a review of its blood-feeding patterns worldwide: Implications for the transmission of bluetongue disease and African horse sickness. Med. Vet. Entomol. 31, 333–339 (2017).
Purse, B. V. et al. Impacts of climate, host and landscape factors on Culicoides species in Scotland. Med. Vet. Entomol. 26, 168–177 (2012).
Leta, S. et al. Updating the global occurrence of Culicoides imicola, a vector for emerging viral diseases. Sci. Data 6, 1–8 (2019).
Acknowledgements
We extend our gratitude to who let us sample from their homestead.
Funding
This study was funded by the Addis Ababa University. The funding organization did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
E.F.: Conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing. G.T.: Formal analysis, investigation, writing—original draft. H.D.: Data curation, investigation. D.M.: Data curation, investigation. T.T.: Data curation, investigation. D.T.: Data curation, investigation. H.N.: Conceptualization, formal analysis, funding acquisition, methodology, supervision, writing—original draft, writing—review and editing. T.M.: Data curation, investigation, methodology, supervision, writing—original draft, writing—review and editing. M.B.J.: Conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing. S.L.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors, where it was unclear that three identification keys were used for the identification of the species. Materials and methods were corrected and two new references were included and listed in the paper as References 31 and 34.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fetene, E., Teka, G., Dejene, H. et al. Modeling the spatial distribution of Culicoides species (Diptera: Ceratopogonidae) as vectors of animal diseases in Ethiopia. Sci Rep 12, 12904 (2022). https://doi.org/10.1038/s41598-022-16911-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16911-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.