Abstract
For management of Budd-Chiari syndrome (BCS), a step-wise therapeutic approach starting with medical treatment, followed by endovascular recanalization, transjugular intrahepatic portosystemic shunt, and finally liver transplantation has been adopted. We retrospectively analyzed 51 patients with symptomatic short segment (≤ 30 mm) hepatic vein (HV)-type BCS who underwent percutaneous transluminal balloon angioplasty (PTBA) with/without stenting to determine the feasibility, clinical effectiveness, and long-term outcomes. The intervention was technically successful in 94.1% of cases (48/51)—32 patients underwent PTBA and 16 patients underwent HV stenting. Procedure-related complications occurred in 14 patients (29.1%). The clinical success rate at 4 weeks was 91.7% (44/48). Nine patients underwent reintervention, six patients due to restenosis/occlusion and three patients with clinical failure. The mean primary patency duration was 64.6 ± 19.9 months (CI, 58.5–70.8; range, 1.2–81.7 months). The cumulative 1-, 2-, and 5-year primary patency rates were 85.4, 74.5, and 58.3%, respectively. The cumulative 1-, 2-, and 5-year secondary patency rates were 93.8, 87.2, and 75%, respectively. The cumulative 1-, 2-, and 5-year survival rates were 97.9, 91.5, and 50%, respectively. Percutaneous transluminal angioplasty with and without stenting is effective and achieves excellent long-term patency and survival rates in patients with symptomatic HV-type BCS. With its lower incidence of re-occlusion and higher clinical success rate, HV angioplasty combined with stenting should be the preferred option especially in patients with segmental HV-type BCS.
Similar content being viewed by others
Introduction
Budd–Chiari syndrome (BCS) is a rare disorder defined as partial or complete obstruction of the hepatic venous outflow tract anywhere along its course from the small hepatic veins (HVs) to the suprahepatic portion of the inferior vena cava (IVC) in the absence of cardiac or pericardial disease or hepatic veno-occlusive disease/sinusoidal obstruction syndrome1,2,3.
Primary BCS is diagnosed when venous outflow is obstructed from within the vein (venous thrombosis or membranous obstruction) whereas secondary BCS is caused by compression or invasion from the outside (by tumor, abscess, cyst, etc.)4,5.
Based on the site of hepatic venous outflow compromise—HVs, IVC, or IVC and HVs—HV-type, IVC-type, and combined-type BCS can be distinguished6,7.
A stepwise therapeutic approach proceeding according to the response to therapy has been adopted starting with medical treatment (anticoagulants and diuretics), followed by percutaneous recanalization (thrombolysis, percutaneous transluminal balloon angioplasty (PTBA) with or without stenting), transjugular intrahepatic portosystemic shunt (TIPS), and finally liver transplantation (LTx) in patients who have not responded to any of the previous treatment steps1,6,8,9,10.
Recanalization of the IVC is the most commonly performed treatment in IVC-type BCS and most patients with combined-type BCS, of whom approximately 86–89% have a compensatory patent accessory HV (AHV)6,11.
In patients with HV-type BCS, endovascular recanalization (PTBA with/without stenting) is considered the first-line treatment specifically in patients with short segment stenosis, followed by TIPS5,7,12,13. TIPS is considered the first-line treatment in patients with diffuse thrombosis of HVs, where percutaneous angioplasty is not technically feasible and has low long‐term patency rates5,14.
The aim of the present study is to evaluate the feasibility, clinical effectiveness, and long-term outcomes of PTBA with and without stenting for endovascular treatment of HV-type BCS.
Results
Patient demographics
The study included 51 patients (31 female, mean age, 27.2 ± 9.1 years; range, 14–52 years). No risk factor was identified in 11 patients (21.6%) while factor V Leiden mutation (FVLM) and protein C deficiency were the most frequent risk factors, identified in 9 (17.6%) and 8 patients (15.7%), respectively. Thirty-four patients (66.7%) had occlusion of all 3 main HVs while 17 patients (33.3%) had occlusion of two HVs. Obstruction was segmental in 37 patients (72.5%; mean length of obstructed segment, 24.68 ± 4.86 mm; range, 14–30 mm). Ascites was the most common symptom (47 patients, 92.2%). A Rotterdam score of 1.22 ± 0.62 (class II), Clichy score of 5.36 ± 0.88, and revised Clichy score of 4.49 ± 1.30 suggested a moderate prognosis. Patient demographics are presented in Table 1.
Technical success
Endovascular recanalization was technically successful in 48 patients (94.1%). Thirty-two patients underwent PTBA alone (62.7%) and 16 patients (31.4%) underwent PTBA with HV stent insertion. Angioplasty was not successful in 3 patients (5.9%) with occlusion of all 3 Main HVs. These patients were treated with TIPS insertion.
Recanalization of one main HV was sufficient to restore hepatic venous drainage in most of the patients (43/48), while recanalization of 2 HVs was necessary in 5 patients (10.4%). The right HV was most frequently selected as the target vein in 25 patients (52.1%, Fig. 1). Mean free HV pressure (FHVP) significantly decreased from 43.13 ± 6.64 before recanalization to 15.35 ± 2.20 after treatment (P value < 0.001). Descriptive analysis of interventional details is listed in Table 1.
Angiography images of a 15-year-old female patients with FVLM: factor V Leiden mutation (FVLM) with obstruction of the right hepatic vein for 6 months. Transhepatic venography revealed short segment obstruction involving right HV ostium with multiple dilated vascular collaterals (a). Balloon dilatation was performed (b), venography revealed residual stenosis and persistence of collaterals (c). Repeat dilatation was performed (d) and control venography revealed good recanalization of the HV with disappearance of vascular collaterals and without residual stenosis (e). Patient presented with recurrent symptoms 10 months following HV angioplasty, and color Doppler ultrasonography revealed thrombosis of the previously treated right HV. Angiography revealed progressive thrombosis of the treated HV compared to the primary intervention with vascular collaterals (f). The obstruction was bypassed using a transhepatic approach (g) followed by stent insertion (h). Final venography following stent dilatation revealed free flow of contrast agent across the stent with disappearance of intrahepatic collaterals (j).
Procedure-related complications
Procedure-related complications were found in 14 patients (29.1%). Intraperitoneal bleeding and subcapsular hematoma occurred in 2 patients managed with a combined transhepatic-transjugular approach. Subsegmental pulmonary embolism was observed during balloon angioplasty in 3 patients who suffered a drop in O2 saturation. The diagnosis was confirmed by postinterventional pulmonary computed tomography angiography (CTA). No stent migration was noted after HV stenting. All complications were successfully managed by conservative treatment. All complications were minor (classes A and B according to North American Society of Interventional Radiology (SIR) classification)15.
Clinical success
The clinical success rate was 93.7% (45/48) at two weeks and 91.7% (44/48) at four weeks. In patients with clinical success, BCS-related symptoms were significantly relieved within one month of the intervention (complete success rate of 72.9% (35/44 patients) vs. 37.5% (18/45 patients) two weeks following angioplasty). There was no clinical improvement in 3 patients at two-week follow-up despite target HV patency in follow-up Doppler US and venography. Clinical failure in these patients was attributed to deterioration of liver function since they had a long history of BCS complicated by cirrhosis. Those patients were managed by TIPS shunt insertion. In addition, improvement in dilated vascular collaterals (abdominal wall collaterals or varices) was observed earlier than relief of other presenting symptom such as abdominal distention and ascites, which diminished gradually over a longer period of time.
Mean serum total bilirubin improved from 2.05 ± 0.45 mg/dl (range 1.12–2.93) before treatment to 1.2 ± 0.31 mg/dl (0.72–2.10) two weeks after treatment (P = 0.01). Similarly, serum total bilirubin, albumin, aspartate aminotransferase, and alanine aminotransaminase levels significantly improved one month after the intervention (P value < 0.001).
Patency
The mean follow-up duration was 40.1 ± 16.8 months (95% CI 35.2–45 months, range 8.8–81.7 months). Eight patients were lost to follow-up. Re-obstruction was in the form of re-thrombosis of the target HV in 4 patients with segmental HV thrombosis and restenosis/occlusion in 2 patients with membranous obstruction. The mean primary patency duration was 64.6 ± 19.9 months (95% CI, 58.5–70.8 months; range, 1.2–81.67 months). The cumulative 1-, 2-, 3-, and 5-year primary patency rates were 85.4, 74.5, 65.5, and 58.3%, respectively (Table 2, Fig. 2).
Reintervention
Nine patients underwent reintervention—six patients due to restenosis/occlusion of the target HV and 3 patients with clinical failure manifesting as refractory ascites/vascular collaterals despite patent recanalized target HV. Among the 9 patients, 5 underwent HV stent insertion (3 patients who were primarily managed with PTBA and 2 patients primarily managed with HV stent, P = 0.44) while 4 patients underwent TIPS insertion (one patient with segmental HV re-thrombosis following PTBA and 3 patients in whom clinical failure was encountered despite a patent recanalized target vein).
Among the 9 patients, 5 underwent a short-term reintervention after a primary patency duration of 1.2–2.43 months (4 patients underwent TIPS insertion and one patient who was primarily managed with PTBA underwent HV stent insertion).
The mean secondary patency duration was 64.2 ± 2.7 months (95% CI, 58.9–69.6 months; range, 12.1–81.7 months). The cumulative 1-, 2-, 3-, and 5-year secondary patency rates were 93.8, 87.2, 86.2 and 75%, respectively (Table 2, Fig. 2).
In ROC analysis of different prognostic indices for prediction of target vein patency following intervention, the Clichy score and Rotterdam score demonstrated the largest AUC of 0.798 for prediction of patency (CI of 0.000–0.464 and 0.255–0.379 for Clichy and Rotterdam scores, respectively) (Table 3).
In univariate regression analysis, no significant factors influencing HV patency following angioplasty or patient survival either in the whole study population or in subgroup analysis of patients with segmental obstruction were identified (Fig. 3, Supplementary Tables 1, 2, 3 and 4).
Survival
The mean survival time was 50.62 ± 5.33 months (95% CI 40.17–61.08). The cumulative 1-, 2-, 3-, and 5-year survival rates were 97.9, 91.5, 80.6, and 50%, respectively (Fig. 2). Two patients died 13.4 and 8.8 months after treatment. Causes of death were liver failure with variceal bleeding (n = 1) and fulminant liver failure related to hepatitis B (n = 1).
Comparison of PTBA alone and PTBA with HV stent
There was no significant difference in the re-obstruction rate between patients who were managed with PTBA alone (n = 4/32, 12.5%) and those who underwent additional HV stent insertion (n = 2/16, 12.5%, P = 0.472). The occluded segment in the HV stent group was significantly longer than in the PTBA group (27.3 mm vs. 22.7 mm, P = 0.002). The complete clinical success rate 2 weeks following the intervention was higher in the HV stent group (7/16 patients, 43.8%) than in the PTBA group (11/32, 34.3%, P = 0.42). No significant difference in complications was noted. Refractory ascites and refractory vascular collaterals were only observed in the PTBA group (2 and 3 patients, P = 0.55 and 0.54, respectively). Two patients experienced PE in the PTBA group vs. one in the HV stent group (P = 1.000).
In subgroup analysis of patients in whom angioplasty was not technically successful (n = 3, managed by TIPS) and patients in whom PTBA with or without stenting was complicated by either re-occlusion (n = 6) or clinical failure (n = 3), we observed that (a) the duration of BCS-related symptoms before the intervention was significantly longer (9.8 ± 2.1 vs. 5.4 ± 2.5 months, P = 0.000) and that (b) FHVP before the intervention was significantly higher (47.1 ± 6.7 vs. 42.2 ± 6.4 cmH2O, P = 0.04). Ten patients had segmental HV obstruction while only two patients had membranous obstruction. The length of the occluded segment was longer in these patients (26.1 ± 4.4 mm vs. 24.2 ± 5 mm, P = 0.284). Furthermore, eleven patients had occlusion of all three Main HVs, and one patient had occlusion of two HVs. In the nine patients who underwent reintervention, 7 patients were primarily managed with PTBA while only two patients had HV stent placement.
Discussion
For management of BCS, a step-wise treatment strategy starting with medical treatment, followed by endovascular revascularization and TIPS shunt insertion and finally LTx has proved to be effective and achieves good long-term survival7,16.
The primary aim of HV recanalization is to relieve liver congestion, improve liver functions, and alleviate patients’ symptoms11,13,17. Recently, HV recanalization is being increasingly recognized and recommended in the EASL and APASL guidelines for BCS as the most preferred invasive radiological intervention in patients with BCS, especially in patients with short segment HV thrombosis or ostial stenosis since it restores hepatic circulation closest to physiology1,18.
The effectiveness of medical treatment is controversial. Earlier studies reported medical treatment alone to be ineffective and associated with poor long-term outcome17,19,20. On the other hand, Kulkarni et al.8 and Zeitoun et al.21 observed no significant survival benefit of percutaneous recanalization or surgical shunting compared to patients managed by medical treatment alone. In patients with persistent symptoms, endovascular intervention (PTBA with or without stenting) is performed, which is most effective in patients with short segment HV or IVC obstruction6,12,13,17,22,23, whereas TIPS is reserved for symptomatic patients in whom endovascular management has failed or is not technically feasible such as patients with diffuse HV thrombosis or combined-type BCS11,24,25,26,27. Indications for LTx include end-stage chronic liver disease due to progressive deterioration of liver function despite medical and/or interventional management (10–20% of BCS patients), fulminant liver failure as well as selected patients with BCS complicated by hepatocellular carcinoma (HCC) and still eligible for LTx5,28,29. The present study investigated technical success, clinical effectiveness, and long-term outcomes of endovascular treatment in HV-type BCS.
The etiology of BCS is known to be variable with thrombosis being more common in western countries and membranous obstruction in the Asian population30,31. The results of our study are consistent with previous findings obtained in patients with BCS in Egypt with FVLM, protein C deficiency, and methylene tetrahydrofolate reductase (MTHFR) mutation as the most common prothrombotic risk factors in Egyptian BCS patients32,33,34.
The good technical and clinical success achieved in the present study further corroborates the strategy recommended by several previous study groups, namely that recanalization of one HV with the shortest obstruction should be sufficient to drain the entire liver parenchyma and relieve hepatic venous flow compromise because of the well-established intrahepatic collateral circulation in patients with BCS12,13,17,22,35,36.
The primary patency rates and patency duration in our patients are comparable to several previous studies investigating endovascular treatment in HV-type BCS6,12,13,17,22,36,37,38,39. In addition, the high cumulative secondary patency rates suggest that endovascular HV recanalization is repeatable when restenosis occurs17. These good long‐term patency and survival rates further corroborate the stepwise approach in the management of BCS. In a meta-analysis by Zhang et al. including over 2000 BSC patients, percutaneous HV recanalization was found to have a technical success rate of 93.1% (CI 91.8–94.3) and 1-year and 5-year survival rates of 95.9% (CI 93.4–98.3) and 88.6% (CI 82.4–94.8), respectively40.
The primary and secondary 5-year patency rates in the present study were significantly lower than in the study of Ding et al. (90 and 98.6%, respectively)17. This could be explained by the larger diameter of balloons (12–20 mm vs. 14 mm in our study) as well as the significantly larger proportion of patients with membranous obstruction (91 patients vs. 2 patients with segmental obstruction) in their study.
In the present study, HV recanalization was primarily performed using PTBA alone with HV stenting being reserved for cases with residual stenosis/restenosis or recurrent/refractory symptoms following PTBA. Although statistically not significant, the reintervention rates due to re-occlusion/re-stenosis or clinical failure were higher in patients who underwent PTBA alone than those who were managed by HV stent placement, especially for segmental obstruction. This observation suggests that PTBA combined with stent insertion might be superior to PTBA alone in endovascular recanalization of HV-type BCS, increasing the clinical success rate and lowering the frequency of re-occlusion. The nonsignificant difference might be explained by the relatively small number of patients in our study. Similarly, Eapen et al. suggested that re-occlusion was more common in patients undergoing PTBA alone than in patients with additional stent placement; however, the difference was not statistically significant41. In the study by Han et al., PTBA alone without stenting was a predictor of re-occlusion, which was in turn a predictor of survival in univariate and multivariate regression analysis. Consequently, they recommend PTBA and stenting to lower the incidence of reintervention and improve survival6. Conversely, Cheng et al. recommend to reserve HV stenting for patients with residual stenosis > 25% and a pressure gradient across the stenosis/occlusion > 3 mmHg, while, in general, PTBA should be the preferred option since stent implantation is permanent and might increase the risk of complications and render reintervention in case of occlusion more difficult10. We exclusively used uncovered stents for HV recanalization. Cheng et al. suggest that uncovered stents are superior since covered stents might hinder development of collateral circulation, which in turn could affect the clinical response following angioplasty10.
The majority of patients, either with technical failure or those who underwent reintervention, had segmental HV obstruction, and the length of the occluded segment was nonsignificantly longer in these patients. However, segmental HV obstruction was not an independent risk factor for re-occlusion in our analysis, contrary to previous studies by Cui et al.13 and Chen et al.7 This could be explained by the fact that we only included patients with short segment stenosis/occlusion < 30 mm.
Neither age nor sex had an effect on patency following recanalization or patient survival in our univariate analysis, which is consistent with previous studies by Sakr et al.33 and Qi et al.42. None of the prognostic indices we analyzed turned out to be a significant predictor of primary patency or survival in our analysis, and this might be explained by the low number of patients as well as high survival and patency rates. Our results are in agreement with previous studies by Chen et al.7 and Cui et al.13 but disagree with several other studies. In the study by Sakr et al., none of the prognostic indices was a significant predictor of one-year patency while the revised Clichy score was an independent predictor of one-year survival with a cut-off of 3.7533. In the study by Rautou et al., all prognostic indices, except the BCS-TIPS score, were significant predictors of transplant-free and invasive therapy-free survival43. In the study by Han et al., the Child–Pugh score, Clichy score, and BCS-TIPS score were significant predictors of survival in univariate analysis6. In the study by Tripathi et al., the Child–Pugh score, Model of End Stage Liver Disease (MELD) score, revised Clichy score, and Rotterdam score were significant predictors of survival in univariate analysis in addition to age22. However, the use of prognostic indices has so far been limited to the stratification of patients in clinical studies. Several reports suggest that prognostic indices are of limited use in individual patient management and should not be relied on for decision making about the type or timing of interventions22,33,42,43.
Recanalization was not complicated by hepatic encephalopathy (HE) in the present study. The incidence of HE following venoplasty is minimal because the normal physiologic pathway of hepatic blood flow is restored compared to TIPS, where portal flow is diverted, which is associated with a 17% risk of HE and deterioration of liver function by decreasing hepatopedal portal flow in segmental branches17,39,44. Tripathi et al. investigated venoplasty in 63 patients with BCS and compared their results with a previously reported series of 59 patients treated by TIPSS. Angioplasty yielded similar patency and survival rates while it is less invasive and has significantly fewer procedure-related complications (9.5% vs. 27.1%) and HE (0% vs. 18%) in comparison to TIPS22.
The good patency and survival rates in the present study suggest that HV angioplasty should be preferred over medical treatment even in patients with early HV-type BCS (i.e., without symptoms of portal hypertension) to prevent deterioration of liver function and development of portal hypertension-related symptoms. Kulkarni et al.8 reported that, despite a nonsignificant difference in terms of patient survival, patients treated by percutaneous recanalization had significantly lower rates of recurrent symptoms and hospital admissions compared with medical treatment, which is in agreement with our results. Furthermore, Shin et al. report success rates of 33–54% and 0–7% with medical management alone in patients with early HV-type and combined-type BCS, respectively45. These rates are relatively low compared to endovascular interventions.
Anticoagulant therapy was initiated during the intervention and continued afterwards as suggested by Zhang et al. They strongly recommended anticoagulant therapy following stent insertion in patient with segmental HV and/or IVC obstruction since patients without anticoagulant therapy following venoplasty had a higher incidence of stent occlusion46.
Limitations
This study has some limitations. First, the retrospective study design could be a source of selection bias. Second, a major limitation in the present study was measurement of the FHVP as an indicator of technical success following the intervention without measuring the wedged hepatic venous pressure (WHVP) and consequently hepatic vein pressure gradient (HVPG) which would have been a more accurate indicator of technical success. Currently, measurement of HVPG is considered a “gold standard” to evaluate and diagnose portal hypertension and for risk stratification in patients with liver cirrhosis since it reflects the degree of architectural disruption of the liver and provides prognostic information about the degree of liver cirrhosis47,48. Third, the sample size was small, especially the subset of patients who underwent HV stenting. Forth, the number of patients managed with PTBA versus those with stent insertion was not the same, which might have rendered the nonrandomized, noncontrolled comparison between the two interventions less accurate. Further prospective randomized controlled studies are recommended to investigate the potential advantage of HV stenting over PTBA alone.
Conclusion
Endovascular hepatic vein recanalization using angioplasty with or without stenting is effective in patients with HV-type BCS and achieves excellent long-term patency and survival rates. With its lower incidence of re-occlusion and higher clinical success rate, HV angioplasty combined with stenting should be the preferred option especially in patients with segmental HV-type BCS.
Patients and methods
Patient population and study design
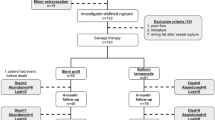
We retrospectively identified 51 patients with symptomatic short segment HV-type BCS (≤ 3 cm) who underwent endovascular treatment with PTBA with or without stenting at our institution between October 2013 and January 2019 (Fig. 4) by examination of the picture archiving and communication system (PACS) and patients’ electronic medical records. The Study was performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki) and approved by the institutional review board (Ethics committee—National Liver Institute). Informed consent was waived by the ethics committee ((Ethics committee—National Liver Institute).
Exclusion criteria were: long segment HV occlusion (> 3 cm), IVC-type or combined-type BCS, diffuse thrombosis of the 3 main HVs, asymptomatic BCS due to well-established intrahepatic vascular collateral, BCS secondary to malignant tumor, clinical success of medical treatment (anticoagulation and diuretics), portal vein thrombosis, and previous management with TIPS, surgical shunt, or LTx.
Diagnosis, definition, and evaluation of symptomatic HV-type BCS
The diagnosis of BCS was established in accordance with the guidelines of the European Association for the Study of the Liver (EASL)1. HV-type BCS was primarily diagnosed using abdominal Doppler ultrasonography (US) and CTA or magnetic resonance angiography (MRA) when obstruction exclusively involved the HVs4,49. Symptomatic BCS was diagnosed when any of the following clinical manifestations was present: ascites, abdominal distention, abdominal pain, hepatomegaly, jaundice, variceal bleeding, dilated vascular collaterals of the thoraco-abdominal wall, or hepatic encephalopathy6.
Laboratory parameters and severity indices for BCS
Liver function tests (LFTs) (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], serum total bilirubin, and serum albumin), serum creatinine, international normalized ratio (INR), and prothrombin time (PT), performed within three days before the intervention, were selected for the present analysis.
The Child–Pugh score50, MELD score51, and specific prognostic indices developed for BCS, including the original Clichy score21, revised Clichy score52, and Rotterdam score53, were calculated using the findings obtained at the time of HV recanalization. Prior to the intervention, patients with high-grade (grades III and IV) esophageal varices (n = 8) underwent prophylactic endoscopic management.
Interventional procedures
All interventional procedures were performed under local anesthesia and conscious. Coagulation disorder was corrected aiming at INR < 2.0.
Target vein selection
The main criterion for selection of the target HV was the length of the occlusion. From the three main HVs, the one with the shortest obstruction was selected. Other selection criteria were good caliber (≥ 7 mm), straight course, and echo-free lumen of the vein. If all three HVs were eligible according to these criteria, our preference was the right HV (easier to manipulate), followed by a common ostium of left and middle HVs, assuming that nearly 50% of the liver parenchyma can be drained by either selection. A compensatory (diameter ≥ 5 mm) but obstructed AHV would have been chosen as the target vein if the occluded segment of it was shorter than that of any of the three main HVs. However, we did not encounter any patients in whom the AHV could have been selected. Computed tomography angiography or MRA was used for primary measurement of length of obstruction and selection of the target vein. Final selection was based on HV angiography during the intervention.
Approaches for percutaneous HV recanalization
A transjugular approach was the primary route for PTBA. All patients underwent US-guided internal jugular vein (IJV) puncture (right IJV access in 48 patients, left IJV access in 3 patients due to right IJV thrombosis). Inferior vena cava angiography was performed using a 5-Fr straight catheter with multiple side holes (Cook Medical, Bloomington, USA) to ensure patency and reveal morphology of the IVC and orifices of the HVs. A Rösch-Uchida transjugular liver access set device (Cook Medical, Bloomington, USA) was used to traverse the occluded HV segment.
A combined transjugular-transhepatic approach was used in patient with failed HV access through the jugular vein (n = 18). In this approach, percutaneous transhepatic US-guided access of the target HV was accomplished using a Neff percutaneous access set (Cook Medical) or 21-gauge Chiba needle (Cook Medical) followed by HV angiography through a 6-Fr sheath (Terumo, Tokyo, Japan). Paracentesis was performed in patients with ascites (n = 14) before the percutaneous transhepatic approach. The occlusion/stenosis was negotiated using different sets of guidewires (0.035″ angled tip and straight hydrophilic standard and stiff guidewires (Radiofocus M; Terumo) or Glidewire (Boston Scientific, Natick, Mass, USA) and catheters including a 5-Fr Cobra 2 angiographic catheter (Radiofocus, Terumo; Cordis, Warren, New Jersey, USA) and 4-Fr Headhunter catheter (Cook Medical). If the obstructed segment could not be traversed with this technique, the stiff back end of the guidewire was advanced very slowly under continuous fluoroscopic guidance and road-mapping, limiting each movement to maximum of 3 mm.
After successful passage of the occlusion/stenosis, the guidewire was snared in the IVC or right atrium using a gooseneck snare (Amplatz snare, Medtronic, Minneapolis, MN, USA) and withdrawn through the jugular sheath. Hepatic vein angiography was performed to demonstrate morphology of the HVs, length and location of the stenosis/occlusion, and presence of intrahepatic collaterals. Angioplasty with/without stenting was performed through the jugular approach as it facilitated introduction of larger-caliber balloons without increasing the risk of hepatic capsular injury compared with use of the transhepatic access.
Percutaneous balloon angioplasty (PTBA)
Balloon angioplasty was performed using balloon catheters (Cook; Cordis; or Bard, Murray Hill, NJ, USA) of various sizes (8–14 mm diameter, 40–60 mm length). Balloons were dilated over a stiff hydrophilic (Terumo) or extra-stiff guidewire (0.035′′ Super Stiff Amplatz guidewire, J-Tip, Boston Scientific, Marlborough, MA). The balloon was manually dilated (2–11 times), with each dilatation procedure lasting approximately 1 min until the waist disappeared. Repeat HV venography was performed 15 min after dilatation (15 min recoil test) to look for significant residual stenosis, recoiling, or persistence of intrahepatic collaterals.
Self-expandable stent placement
Metal stents of 10–14 mm diameter and 40–60 mm length (Wallstent; Boston Scientific) were inserted when initial balloon dilatation was insufficient as evidenced by any of the following criteria in HV venography after 15 min: (a) significant residual stenosis of > 30% or recoiling following angioplasty; (b) pressure gradient > 15 cmH2O (1 cmH2O = 0.098 kPa) between HV segments proximal and distal to the occlusion/stenosis; and (c) persistence of intrahepatic vascular collaterals.
The balloon or the stent extended at least 10 mm beyond the lesion ends. The diameter of the stent or balloon needed to be 2 mm larger than the diameter of the target HV. Free HV pressure was measured by a piezometer tube before and after recanalization. At the end of the procedure, the transhepatic track was embolized using gel foam strips pushed through the introducer sheath.
Postprocedure management and follow-up
Heparin infusion was started during the intervention (50 IU/kg), overlapping with postprocedure oral anticoagulants until the target INR was reached (2–3 according to EASL guidelines1). Thereafter, patients were maintained on long-term oral anticoagulation. Anticoagulant was continued for at least 6 months following PTBA in patients with membranous obstruction and for life in patients with segmental obstruction46. INR was monitored weekly until the target level was achieved, then once monthly. Management of the underlying hypercoagulable state was done in consultation with hematologists. In addition, symptomatic therapy (low-salt diet, diuretic therapy and/or beta-blockers) was adjusted as needed8.
Follow-up data were obtained from the medical records, whenever possible at prespecified intervals (14 days, 1, 3, 6, and 12 months after treatment and then annually or whenever symptoms recurred). Data retrieved included clinical assessment for recurrence of symptoms, laboratory investigations (bilirubin, INR), and imaging (color Doppler US, CT, or MRI). Follow-up ended at the specified timepoint (March 2020) or the timepoint of being lost to follow-up, if the patient underwent TIPS, surgical shunt, or LTx, or by patient death.
Study endpoints and definitions
The primary endpoints were technical success, clinical success, primary patency duration, and survival. The secondary endpoints were complications and evaluation of factors that could predict long-term patency following recanalization. Technical success was defined as successful recanalization of the target HV with disappearance of intrahepatic collaterals. In patients with stent insertion, technical success also included correct stent positioning, adequate stent expansion (residual stenosis < 30%), and absence of immediate stent migration.
Clinical success was defined as an improvement of BCS-related symptoms (such as ascites, hepatomegaly, hepatic encephalopathy, as well as resolution of portal hypertensive bleeding) and liver chemistries (evidenced by normalization of serum AST/ALT and total bilirubin level,i.e. < 1.5 mg/dL) after technically successful HV recanalization. Complete success was defined as complete elimination of symptoms (diuretics are no longer required). Patients who achieved all except one or two of these parameters including reduction in the dose of diuretics are considered to be partial responders. Clinical failure was considered if there was (a) no improvement, new onset, or recurrence of clinical symptoms, (b) no reduction in the required dose of diuretics, or (c) development or progressive deterioration of liver dysfunction6,18,36,54.
Primary patency was defined as the period between the initial angioplasty and re-appearance of outflow obstruction (i.e., recurrence of symptoms which was confirmed by re-occlusion on imaging) that necessitated re-intervention. In patients without re-occlusion, it was the interval until end of follow-up, last follow-up, management by TIPS, surgical shunt, or liver transplant, or patient death. Re-occlusion was suspected on color Doppler US if there was no or retrograde flow, or if there was significant narrowing (> 30%) of the treated segment with formation of intrahepatic collateral vessels and recurrence of BCS-related symptoms. Definite diagnosis of re-occlusion was confirmed by angiography. Secondary patency was defined as the total interval between initial HV angioplasty with the contribution of subsequent recanalization procedures (apart from TIPS) until the last follow-up, end of predefined follow-up period, surgical interventions (surgical shunt or LTx), or death.
Complications were classified according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee15,54. Overall survival was defined as the time from start of treatment to last follow-up, end of follow-up, or patient death.
Statistical analysis
Continuous variables are presented as mean or median and were compared using the independent sample t-test. Categorical variables were compared using the x2 test or Fisher exact test. Survival and patency durations were calculated using Kaplan–Meier curves and compared using the log-rank test. Independent predictors of primary patency were calculated using Cox regression analysis. Receiver operator characteristics (ROC) and regression analysis were performed to investigate predictors of hepatic vein patency following angioplasty. A P-value < 0.05 was considered statistically significant. All statistical calculations were performed by using Stata/MP version 16.0 (StataCorp, College Station, Texas, USA).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
EASL Clinical Practice Guidelines. Vascular diseases of the liver. J. Hepatol. 64, 179–202 (2016).
Valla, D. C. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol. Int. 12, 168–180. https://doi.org/10.1007/s12072-017-9810-5 (2018).
Cura, M., Haskal, Z. & Lopera, J. Diagnostic and interventional radiology for Budd-Chiari syndrome. Radiographics 29, 669–681. https://doi.org/10.1148/rg.293085056 (2009).
DeLeve, L. D., Valla, D. C. & Garcia-Tsao, G. Vascular disorders of the liver. Hepatology 49, 1729–1764. https://doi.org/10.1002/hep.22772 (2009).
Khan, F. et al. Review article: a multidisciplinary approach to the diagnosis and management of Budd-Chiari syndrome. Aliment. Pharmacol. Ther. 49, 840–863. https://doi.org/10.1111/apt.15149 (2019).
Han, G. et al. Percutaneous recanalization for budd-chiari syndrome: an 11-year retrospective study on patency and survival in 177 Chinese patients from a single center. Radiology 266, 657–667. https://doi.org/10.1148/radiol.12120856 (2013).
Chen, Z. K., Fan, J., Cao, C. & Li, Y. Endovascular treatment for hepatic vein-type Budd-Chiari syndrome: effectiveness and long-term outcome. Radiol. Med. 123, 799–807. https://doi.org/10.1007/s11547-018-0907-2 (2018).
Kulkarni, C. B. et al. Budd-Chiari syndrome managed with percutaneous recanalization: Long-term outcome and comparison with medical therapy. Int. J. Gastrointest. Interv. 8, 74–81. https://doi.org/10.18528/ijgii180001 (2019).
Darwish Murad, S. et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann. Intern. Med. 151, 167–175. https://doi.org/10.7326/0003-4819-151-3-200908040-00004 (2009).
Cheng, D.-L. et al. Interventional treatment strategy for primary Budd-Chiari syndrome with both inferior vena cava and hepatic vein involvement: patients from two centers in China. Cardiovasc. Intervent. Radiol. 42, 1311–1321. https://doi.org/10.1007/s00270-019-02267-w (2019).
Fu, Y. F. et al. Percutaneous recanalization for combined-type Budd-Chiari syndrome: strategy and long-term outcome. Abdom. Imaging 40, 3240–3247. https://doi.org/10.1007/s00261-015-0496-7 (2015).
Sang, H. F. & Li, X. Q. Endovascular treatment of Budd-Chiari syndrome with hepatic vein obstruction in China. J. Laparoendosc. Adv. Surgery Tech. A 24, 846–851. https://doi.org/10.1089/lap.2014.0095 (2014).
Cui, Y.-F., Fu, Y.-F., Li, D.-C. & Xu, H. Percutaneous recanalization for hepatic vein-type Budd-Chiari syndrome: long-term patency and survival. Hepatol. Int. 10, 363–369. https://doi.org/10.1007/s12072-015-9676-3 (2016).
Wang, Q. et al. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: a randomised controlled trial. Lancet Gastroenterol. Hepatol. 4, 686–697. https://doi.org/10.1016/S2468-1253(19)30177-3 (2019).
Sacks, D., McClenny, T. E., Cardella, J. F. & Lewis, C. A. Society of Interventional radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 14, S199-202. https://doi.org/10.1097/01.rvi.0000094584.83406.3e (2003).
Seijo, S. et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology 57, 1962–1968. https://doi.org/10.1002/hep.26306 (2013).
Ding, P. X. et al. Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd-Chiari syndrome. J. Gastroenterol. Hepatol. 31, 222–228. https://doi.org/10.1111/jgh.13025 (2016).
Shukla, A. et al. Budd-Chiari syndrome: consensus guidance of the Asian Pacific association for the study of the liver (APASL). Hepatol. Int. 15, 531–567. https://doi.org/10.1007/s12072-021-10189-4 (2021).
Ringe, B. et al. Which is the best surgery for Budd-Chiari syndrome: venous decompression or liver transplantation? a single-center experience with 50 patients. Hepatology 21, 1337–1344 (1995).
Kohli, V. et al. Management of hepatic venous outflow obstruction. Lancet 342, 718–722. https://doi.org/10.1016/0140-6736(93)91712-u (1993).
Zeitoun, G. et al. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology 30, 84–89. https://doi.org/10.1002/hep.510300125 (1999).
Tripathi, D. et al. Long-term outcomes following percutaneous hepatic vein recanalization for Budd-Chiari syndrome. Liver Int. 37, 111–120. https://doi.org/10.1111/liv.13180 (2017).
Fu, Y. F. et al. Combined thrombus aspiration and recanalization in treating Budd-Chiari syndrome with inferior vena cava thrombosis. Radiol. Med. 120, 1094–1099. https://doi.org/10.1007/s11547-015-0554-9 (2015).
Tripathi, D. et al. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome. Aliment. Pharmacol. Ther. 39, 864–872. https://doi.org/10.1111/apt.12668 (2014).
Qi, X. et al. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome: techniques, indications and results on 51 Chinese patients from a single centre. Liver Int. 34, 1164–1175. https://doi.org/10.1111/liv.12355 (2014).
Hayek, G. et al. Long-term outcome and analysis of dysfunction of transjugular intrahepatic portosystemic shunt placement in chronic primary budd-chiari syndrome. Radiology 283, 280–292. https://doi.org/10.1148/radiol.2016152641 (2017).
Fitsiori, K. et al. Transjugular intrahepatic portosystemic shunt for the treatment of Budd-Chiari syndrome patients: results from a single center. Cardiovasc. Interv. Radiol. 37, 691–697. https://doi.org/10.1007/s00270-013-0697-9 (2014).
Plessier, A. & Valla, D. C. Budd-Chiari syndrome. Semin. Liver Dis. 28, 259–269. https://doi.org/10.1055/s-0028-1085094 (2008).
Orloff, M. J., Daily, P. O., Orloff, S. L., Girard, B. & Orloff, M. S. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann. Surg. 232, 340–352. https://doi.org/10.1097/00000658-200009000-00006 (2000).
Qi, X. et al. Review article: the aetiology of primary Budd-Chiari syndrome - differences between the West and China. Aliment. Pharmacol. Ther. 44, 1152–1167. https://doi.org/10.1111/apt.13815 (2016).
Valla, D.-C. Hepatic venous outflow tract obstruction etiopathogenesis: Asia versus the West. J. Gastroenterol. Hepatol. 19, S204–S211. https://doi.org/10.1111/j.1440-1746.2004.03642.x (2004).
Sakr, M. et al. Epidemiological aspects of Budd-Chiari in Egyptian patients: a single-center study. World J. Gastroenterol. 17, 4704–4710. https://doi.org/10.3748/wjg.v17.i42.4704 (2011).
Sakr, M. et al. Validation of prognostic indices in Egyptian Budd-Chiari syndrome patients: a single-center study. World J. Gastroenterol. 23, 629–637. https://doi.org/10.3748/wjg.v23.i4.629 (2017).
El Sebay, H. M. et al. Association of factor V Leiden, Janus kinase 2, prothrombin, and MTHFR mutations with primary Budd-Chiari syndrome in Egyptian patients. J. Gastroenterol. Hepatol. 31, 235–240. https://doi.org/10.1111/jgh.13066 (2016).
Mammen, T. et al. Intrahepatic collateral recanalization in symptomatic Budd-Chiari syndrome: a single-center experience. J. Vasc. Interv. Radiol. 21, 1119–1124. https://doi.org/10.1016/j.jvir.2010.03.008 (2010).
Rathod, K. et al. Endovascular treatment of Budd-Chiari syndrome: single center experience. J. Gastroenterol. Hepatol. 32, 237–243. https://doi.org/10.1111/jgh.13456 (2017).
Fan, X. et al. Good clinical outcomes in Budd-Chiari syndrome with hepatic vein occlusion. Dig. Dis. Sci. 61, 3054–3060. https://doi.org/10.1007/s10620-016-4208-0 (2016).
Shalimar, et al. Hepatic venous outflow tract obstruction: treatment outcomes and development of a new prognostic score. Aliment. Pharmacol. Ther. 43, 1154–1167. https://doi.org/10.1111/apt.13604 (2016).
Li, T. et al. Feasibility and midterm outcomes of percutaneous transhepatic balloon angioplasty for symptomatic Budd-Chiari syndrome secondary to hepatic venous obstruction. J. Vasc. Surg. 50, 1079–1084. https://doi.org/10.1016/j.jvs.2009.06.049 (2009).
Zhang, F., Wang, C. & Li, Y. The outcomes of interventional treatment for Budd-Chiari syndrome: systematic review and meta-analysis. Abdom. Imaging 40, 601–608. https://doi.org/10.1007/s00261-014-0240-8 (2015).
Eapen, C. E. et al. Favourable medium term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut 55, 878–884. https://doi.org/10.1136/gut.2005.071423 (2006).
Qi, X., Ren, W., Wang, Y., Guo, X. & Fan, D. Survival and prognostic indicators of Budd-Chiari syndrome: a systematic review of 79 studies. Expert Rev. Gastroenterol. Hepatol. 9, 865–875. https://doi.org/10.1586/17474124.2015.1024224 (2015).
Rautou, P. E. et al. Prognostic indices for Budd-Chiari syndrome: valid for clinical studies but insufficient for individual management. Am. J. Gastroenterol. 104, 1140–1146. https://doi.org/10.1038/ajg.2009.63 (2009).
Fisher, N. C. et al. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut 44, 568–574. https://doi.org/10.1136/gut.44.4.568 (1999).
Shin, N. et al. Redefining Budd-Chiari syndrome: a systematic review. World J. Hepatol. 8, 691–702. https://doi.org/10.4254/wjh.v8.i16.691 (2016).
Zhang, C. Q. et al. Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World J. Gastroenterol. 9, 2587–2591. https://doi.org/10.3748/wjg.v9.i11.2587 (2003).
Bosch, J. & García-Pagán, J. C. Calculating hepatic venous pressure gradient: feel free to stay free. J. Vasc. Interv. Radiol. 27, 1138–1139. https://doi.org/10.1016/j.jvir.2016.03.048 (2016).
Bochnakova, T. Hepatic venous pressure gradient. Clin. Liver Dis. (Hoboken) 17, 144–148. https://doi.org/10.1002/cld.1031 (2021).
Valla, D. C. Primary Budd-Chiari syndrome. J. Hepatol. 50, 195–203. https://doi.org/10.1016/j.jhep.2008.10.007 (2009).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649. https://doi.org/10.1002/bjs.1800600817 (1973).
Kamath, P. S. et al. A model to predict survival in patients with end-stage liver disease. Hepatology 33, 464–470. https://doi.org/10.1053/jhep.2001.22172 (2001).
Langlet, P. et al. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J. Hepatol. 39, 496–501. https://doi.org/10.1016/s0168-8278(03)00323-4 (2003).
Darwish Murad, S. et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology 39, 500–508. https://doi.org/10.1002/hep.20064 (2004).
Leoni, C. J., Potter, J. E., Rosen, M. P., Brophy, D. P. & Lang, E. V. Classifying complications of interventional procedures: a survey of practicing radiologists. J. Vasc. Interv. Radiol. 12, 55–59. https://doi.org/10.1016/s1051-0443(07)61403-1 (2001).
Acknowledgements
The authors thank Bettina Herwig for language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.E. did the literature search, collected the data, helped in interpretation of data, study design, was responsible for study coordination, and drafted the manuscript. M.A., T.D. helped with study design und interpretation of the data, revised the manuscript critically for important intellectual content. D.G. helped with designed the study, performed the statistics and data analysis, revised the manuscript critically for important intellectual content. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkilany, A., Alwarraky, M., Denecke, T. et al. Percutaneous transluminal angioplasty for symptomatic hepatic vein-type Budd-Chiari syndrome: feasibility and long-term outcomes. Sci Rep 12, 14095 (2022). https://doi.org/10.1038/s41598-022-16818-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16818-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.