Abstract
Vigorous spontaneous breathing has emerged as a promotor of lung damage in acute lung injury, an entity known as “patient self-inflicted lung injury”. Mechanical ventilation may prevent this second injury by decreasing intrathoracic pressure swings and improving regional air distribution. Therefore, we aimed to determine the effects of spontaneous breathing during the early stage of acute respiratory failure on lung injury and determine whether early and late controlled mechanical ventilation may avoid or revert these harmful effects. A model of partial surfactant depletion and lung collapse was induced in eighteen intubated pigs of 32 ±4 kg. Then, animals were randomized to (1) SB‐group: spontaneous breathing with very low levels of pressure support for the whole experiment (eight hours), (2) Early MV-group: controlled mechanical ventilation for eight hours, or (3) Late MV-group: first half of the experiment on spontaneous breathing (four hours) and the second half on controlled mechanical ventilation (four hours). Respiratory, hemodynamic, and electric impedance tomography data were collected. After the protocol, animals were euthanized, and lungs were extracted for histologic tissue analysis and cytokines quantification. SB-group presented larger esophageal pressure swings, progressive hypoxemia, lung injury, and more dorsal and inhomogeneous ventilation compared to the early MV-group. In the late MV-group switch to controlled mechanical ventilation improved the lung inhomogeneity and esophageal pressure swings but failed to prevent hypoxemia and lung injury. In a lung collapse model, spontaneous breathing is associated to large esophageal pressure swings and lung inhomogeneity, resulting in progressive hypoxemia and lung injury. Mechanical ventilation prevents these mechanisms of patient self-inflicted lung injury if applied early, before spontaneous breathing occurs, but not when applied late.
Similar content being viewed by others
Introduction
Patients with severe lung injury usually have a high respiratory drive, resulting in an intense inspiratory effort1. The patient’s effort has emerged as a potential driver of damage to the lungs, coining the concept of “patient self-inflicted lung injury” (P-SILI)2,3. Nevertheless, this phenomenon is based only on clinical observations and its pathophysiological plausibility. The proposed mechanisms involved in P-SILI may be similar to those classically described for ventilator-induced lung injury (VILI), including uneven ventilation distribution associated with local and global lung strain exceeding the thresholds for lung damage2,4,5.
Harmful effects of spontaneous effort have been demonstrated in experimental models of uninjured and injured lungs under mechanical ventilation (MV), but mechanisms are still poorly understood6,7. Spontaneously breathing patients with acute hypoxemic respiratory failure (AHRF), who failed a trial of non-invasive ventilation, presented higher minute ventilation and markedly higher levels of intrathoracic pressure swings than patients that did not fail. The therapeutic implications of this concept are of utmost importance, as starting invasive MV in patients with AHRF could become a protective strategy by stopping the detrimental effects associated to high respiratory drive8.
We aimed to determine the effects of spontaneous breathing (SB) on lung injury and inflammation, as well as the associated mechanisms, during the initial phase of acute respiratory failure. In addition, we wanted to determine whether early or late controlled protective MV can modify these effects. We hypothesize that SB may further injure the lungs due to the presence of vigorous inspiratory efforts, generating a heterogeneous distribution of ventilation and increasing the risk of P-SILI. Early and late controlled MV can prevent or reverse these deleterious effects by decreasing intrathoracic pressure oscillations and improving regional air distribution.
Methods
Animal preparation
We studied 18 piglets (Sus scrofa domestica) of 2–3 months old, weighing 32 ± 4 kg. The animals were anesthetized with an intramuscular injection of xylazine (2 mg/kg) and ketamine (20 mg/kg), followed by a continuous intravenous infusion of ketamine (30 mg/kg/h), fentanyl (0.5–1.0 μg/kg/h), midazolam (0.1 mg/kg/h), and rocuronium (0.3 mg/kg/h). Ringer’s lactate 30 ml/kg/h was infused IV during the first hour. Then it was decreased to 10 ml/kg/h until the end of the experiment.
The animals were placed in supine position and, after tracheal intubation, were mechanically ventilated (Carescape R860, GE Healthcare, USA) in a volume-controlled ventilation (VCV) mode with the following settings: tidal volume (VT) of 8 ml/kg, respiratory rate (RR) 30 bpm, positive end-expiratory pressure (PEEP) 5 cmH2O, inspiratory to expiratory ratio (I:E) 1:2, and oxygen inspired fraction (FIO2) of 1. The animals were continuously monitored with electrocardiogram and pulse oximetry. Invasive systemic arterial pressure was monitored using the PICCO system (PV2015L20, Pulsion, Munich, Germany), placed in a femoral artery. A pulmonary artery catheter was inserted through the femoral vein under ultrasound viewing. A bladder catheter was surgically inserted to measure urine output per hour. NICO-monitor (Philips, Wallingford, CT, USA) was connected for volumetric capnography measurements.
Experiment protocol
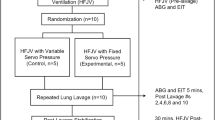
After the initial preparation, the pigs were stabilized for 30 min, and baseline measurements were recorded. We adapted a previously established model of alveolar instability based on partial surfactant depletion and lung collapse9,10. Briefly, deeply anesthetized animals on controlled MV received 30 ml/kg of warmed isotonic saline through the endotracheal tube. After 10 s, the saline solution was passively drained by gravity. Finally, endotracheal suction until SpO2 was less than 80% was performed. After an initial stabilization of 10 min, and before starting the 8-h-study period, animals were allocated into three groups by a six-sized block randomization using an online randomization tool (https://www.randomizer.org)11 (Fig. 1).
-
SB: Animals were ventilated with pressure support ventilation (PSV) set, at the beginning, to achieve Vt of 6–8 ml/kg, PEEP between 0 and 5 cmH2O (to maintain SpO2 > 92%), and FIO2 of 1.
-
Early controlled MV: Animals were ventilated with VCV using VT of 6 to 8 ml/kg, RR of 30 breaths per minute (bpm), PEEP of 5 cmH2O, I:E ratio 1:2, and FIO2 of 1.
-
Late controlled MV: Animals were ventilated with PSV with the same settings as the SB group for 4 h. Then they were switched to VCV (same settings as Early controlled MV group) for the next 4 h.
Study design and timeline. Preparation corresponds to anesthesia and invasive monitoring, which took around 2 h, baseline was measured at the end of this period. Alveolar collapse model corresponds to partial surfactant depletion and lung collapse model. After induction of the model, animals were randomized for spontaneous breathing, early controlled MV and late controlled MV by a period of 8 h. Arrows: data record points.
During controlled mechanical ventilation, pigs were anesthetized, keeping the initial infusion of ketamine, fentanyl, midazolam, and rocuronium. To switch to SB, we stopped midazolam and rocuronium infusion, keeping ketamine (30 mg/kg/h) and fentanyl (0.5–1.0 μg/kg/h) until the onset of ventilatory efforts sufficient to maintain VT close to 6 ml/kg. The respiratory effort was monitored clinically and looking at the inspiratory deflection of esophageal pressure. Animals were euthanized after completing the 8-h-study period.
Measurements
Data were collected at (1) Baseline: before inducing the alveolar collapse model; (2) H0: after stabilization of alveolar collapse model (3) H4: 4 h after H0; (4) H8: 8 h after H0 at the end of the protocol (Fig. 1).
-
1.
Respiratory mechanics and gas exchange
Respiratory mechanics were assessed with a NICO monitor, using airway pressure and flow waveforms. For SB-group we considered Ppeak as the highest airway pressure during the respiratory cycle. Esophageal balloon-tipped catheters (NeuroVent Research Inc, Toronto, Canada) were placed, and pressures were monitored and registered through a double-channel pneumotachometer (Data Acquisition System, Hans Rudolf, Inc., USA). Gas exchange analysis was performed using a bedside blood analyzer (i-STAT®-1 immunoready).
-
2.
Electrical impedance tomography (EIT)
A 16 electrode-belt was placed in the mid-thoracic region, and continuous lung impedance was assessed by EIT (Pulmovista 500, Dräger Medical Systems, USA). Offline analysis of EIT data was performed, and the following parameters were calculated:
-
Lung inhomogeneity was determined by the Global Inhomogeneity index (GI), which quantifies the homogeneity of the tidal volume distribution through quantitative lung pixel impedance dispersion12.
-
Regional ventilation distribution was assessed by Impedance Ratio (IR), a parameter for determining the dependent and non-dependent regions' air distribution13. An IR > 1 represents a ventral distribution predominance, while an IR < 1 represents mainly a dorsal distribution.
-
Tidal Variation of Impedance (TVI), representing impedance change generated by inspired gas during a respiratory cycle14.
-
End Expiratory Lung Impedance (EELI) corresponds to the impedance value at the end of expiration13,14. Its changes have been correlated with changes in end-expiratory lung volume15.
-
Regional Ventilation Delay (RVD), representing the temporal delay of lung regional ventilation, it is associated to ventilatory heterogeneity and lung tidal recruitment16.
-
-
3.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed with a fiberoptic bronchoscope (Olympus BF 3C40; Olympus Optical Co. Hamburg, Germany). Briefly, we instilled 20 ml of 0.9% saline solution at the ventral and dorsal regions of the left lung. Subsequently, we aspirated the fluid, and the recovered fluid was centrifuged, immediately frozen with liquid nitrogen, and stored at − 80ºC for posterior analysis17. This procedure was performed at H4 and H8.
-
4.
Lung tissue analysis
After euthanasia, during the opening of the chest wall, PEEP 5 cmH2O was set. Then, the inferior cava vein was sectioned, the trachea was clamped at end-expiratory lung volume, and the heart–lung piece was excised. Lung tissue samples were collected from dependent, non-dependent, and intermediate lung regions. Tissues were immersed in 10% buffered formalin, processed, and stained with hematoxylin–eosin for histological analysis17,18. Other samples from the same lung regions were immediately frozen in liquid nitrogen, kept at − 80 °C, and finally processed for biochemical and molecular biology analysis18.
-
5.
Cytokine’s quantification
By the Enzyme-Linked ImmunoSorbent Assay (ELISA) method, the concentrations of interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) as a pathological mechanotransduction-induced protein were measured in BAL fluid (BALF), and lung samples (tissue homogenates)18. The resulting concentrations in BALF were indexed by urea and in tissue by proteins17.
-
6.
Histological score
To assess lung damage, fixed and stained lung tissue samples were analyzed with light microscopy. A validated semiquantitative score was used to evaluate four parameters of lung injury: alveolar hemorrhage, alveolar and septal inflammation, and septal disruption; each of these categories received a score ranging from 0 to 4, where 0 corresponds to no pathologic alteration, 1 corresponds to mild, 2 corresponds to moderate, 3 corresponds to severe and 4 very severe pathologic alteration19. The average was reported of ten random areas for each section, at 200 × magnification18.
-
7.
Statistical analysis
We calculated that a sample size of six animals per group (18 total) was needed to detect a difference of 3.5 ± 2 on the global histological score between groups, with a p < 0.05 and a power of 0.8. The Shapiro–Wilk test was used to test data for normality, and we expressed values as means—standard deviation (SD) or median—interquartile range, accordingly. Intragroup analysis was done using the Friedman test or one-way ANOVA, as appropriate, to study the effect of time over any variable (e.g., Late MV group). For comparisons over time between groups (Early MV and SB groups), regarding each physiological variable, we used a generalized linear mixed model (GLMM), adding a random effect by the subject (pig). Tukey pairwise multiple comparison test was used for posthoc comparisons for the effect of time within the group and between groups. The statistical analyses were conducted by RStudio 2022 (Integrated Development Environment, Boston, MA, USA) and by GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). Statistical tests were carried out with the significance level set at p ≤ 0.05.
Ethics approval and consent to participate
The study was approved by the Animal Ethics Committee in Pontificia Universidad Católica de Chile (ID 170315007) and Universidad Andrés Bello (ID 021/2018). The protocol was designed following the National Institute of Health’s guidelines (NIH) and reported in accordance with ARRIVE guidelines.
Results
No significant differences were found between groups in body weight, hemodynamics, lung mechanics, and gas exchange at baseline (Table 1). The alveolar collapse model was well tolerated and induced an immediate effect on oxygenation and lung mechanics in all the animals.
Effects of spontaneous ventilation
SB versus Early MV groups
-
a.
Hemodynamics
No differences were found in hemodynamics over time. Cardiac output showed interaction, increasing from H0 to H4, and then decreasing at H8 in the Early MV group. (Table 2).
-
b.
Respiratory mechanics and gas exchange
We observed interaction with time and a group effect in RR and PEEP. Changes in PEEP were expected due to the different settings defined by protocol. RR showed a progressive increase from H0 to H8 in the SB group and remained higher than the Early MV group at H4 and H8. (Table 3).
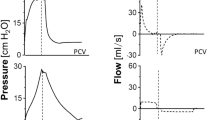
Esophageal pressure swings showed progressive increment between H0 to H4, and H0 and H8 in SB group. Differences between groups were evident at H4 and H8. interaction between groups and time were positive (Figs. 2, 3).
PaO2/FIO2 ratio also showed interaction (p = 0.003), but without time or group effect separately, if not as a whole. In the SB-group, we observed a progressive decrease in PaO2/FIO2 ratio over time (p = 0.02), in contrast to the Early MV group, which increased PaO2/FIO2 ratio above 200 mmHg and then remained stable over time. At the end of the experiment, the Early MV group appeared to have higher oxygenation, a difference which was on the border of statistical significance (138 [107–200] vs. 283 [153–396], p = 0.053), (Fig. 4).
-
c.
Electrical impedance tomography
We observed a progressive dorsal ventilation distribution in the SB-group throughout the study period, as reflected by lower values in IR (Table 4, Fig. 5 and Figure Suppl 1). The early VM group showed no changes over time in the IR. Additionally, there was a progressive increase in temporal heterogeneity of ventilation in the SB-group, as expressed by the increase in RVD along time (Fig. 6).
-
d.
Cytokines in bronchoalveolar lavage fluid, plasma, and lung tissue
When analyzing the concentration of pro-inflammatory cytokines in regional BALF, and lung tissue, in both groups, no significant differences were observed. (Figure Suppl 2 and 3).
-
e.
Histological analysis
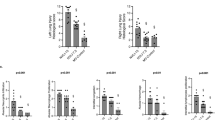
We observed a higher global histological score in the SB-group than in the Early MV (p = 0.002) (Fig. 6). Regionally, the main difference between these groups was found in the ventral region, while no differences were found in the intermediate and dorsal areas (Fig. 7 and Suppl 4).
Table 2 Hemodynamic parameters. Table 3 Ventilatory parameters. Figure 2 Figure 3 Figure 4 Table 4 EIT parameters. Figure 5 Representative images of an animal from the SB-group (right) and another from the Early MV group (left). The pulmonary ventilation distribution assessed by electric impedance tomography showed that SB presented dorsal predominant ventilation, as is shown by the impedance ratio = 0.8; in contrast, Early MV ventilation was predominantly ventral, as is shown by the impedance ratio = 1.3. IR impedance ratio.
Figure 6
Effects of switching to protective controlled MV after a spontaneous breathing period
Late MV group
-
(a)
Hemodynamics
No differences were found in hemodynamics over time.
-
(b)
Respiratory mechanics and gas exchange
At the end of the SB period (H4), the presence of large esophageal pressure swings was observed (Fig. 2). Also, a significant decrease in PaO2/FIO2 was found at H4 which persisted after switching to protective MV (H8) (Fig. 4). No significant differences were observed in the other parameters examined.
-
(c)
Electrical impedance tomography
The Late MV group exhibited an increase in regional inhomogeneity (GI) during the spontaneous breathing period (1.09 [1.1–1.4] at H4 vs. 1.02 [0.9–1.03] at H0, p = 0.02); these changes were reversed during the protective MV period (1.03 [1–1.13] at H8, p = 0.02 vs H4) (Fig. 8). In addition, we observed a significant decrease in tidal ventilation (TVI at H0: 2558 [1909–3245] vs. H8: 1137 [876–1768], p = 0.01) and in temporal heterogeneity (RVD at H0: 6.6 [4.7–9.2] to H8: 2.9 [2.4–4.4], p = 0.04) (Table 4).
-
(d)
Cytokines in BALF, plasma, and lung tissue
No significant findings were observed when analyzing the concentration of pro-inflammatory cytokines in regional BALF or plasma. For lung tissue analysis, we did not find global or regional differences between group. (Figure Suppl 2 and 3).
-
(e)
Histological analysis
We observed that the Late MV group had a higher global histological score than the Early MV group, but it was comparable to that of the SB group. (Fig. 7 and Suppl 4).
Discussion
The main findings of the present experimental study are, in the early phase of ARDS, animals breathing spontaneously had large esophageal pressure swings, predominantly dorsal ventilation, and an uneven temporal and spatial distribution of ventilation. This was associated with a more profound hypoxemia and higher lung injury after 8 h, compared to animals mechanically ventilated and without spontaneous breathing. Late connection to protective MV after 4 h of SB favored a more homogeneous ventilation; however, it failed to reverse hypoxemia or to prevent histological damage.
This study compares the effects of early and late connection to MV to prevent P-SILI on the initial phase of acute respiratory failure. Our group previously showed that SB (using very low tidal volume) presented higher dorsal ventilation than near-apneic controlled ventilation, but we did not observe differences in lung histological damage19. Our current experimental approach was slightly modified from previously published experiences9,10. We induced a model of lung instability and alveolar collapse, trying to avoid excessive inflammation from the model per se, which could mask the inflammation secondary to the ventilatory strategies studied. In addition, we included the Late MV group to extrapolate the condition of a patient with AHRF in whom intubation and connection to controlled MV are delayed. Thus, we tried to determine if controlled MV could reverse the potentially harmful effects of a previous period of spontaneous breathing with increased efforts. Contrary to some previous studies which examined SB as a trigger of lung injury during assisted MV7,20, we have examined the impact of SB with virtually no ventilatory support. Very low levels of support were used to prevent hypoventilation (average 1–2 cmH2O).
The putative underlying mechanisms for P-SILI are similar to those for VILI, and the majority of the evidence available for this concept derives from studies with rather low breathing efforts in the context of assisted MV7,20. However, during the COVID-19 pandemic, the frequent and controversial use of non-invasive MV, high flow nasal oxygenation and awake prone positioning to avert intubation, increased the interest in the risk of P-SILI during the SB period, particularly in those patients who were finally connected to invasive MV5,21. The first evidence supporting this hypothesis was reported by Mascheroni et al. several years ago. They showed that healthy sheep developed lung injury after inducing central hyperventilation. The animals presented progressive lung edema and hypoxemia during the protocol, which lasted 8.4 h on average22, a similar time frame as our study. However, the rest of the evidence that is usually cited to support this hypothesis is not derived from studies with spontaneous breathing and therefore its extrapolation to the context of the non-intubated patient with AHRF is highly debatable.
In this research, we observed that the Early MV group presented a progressive improvement in the PaO2/FIO2 ratio after the induction of the alveolar collapse model. In contrast, the SB group showed progressive deterioration in oxygenation over time (Fig. 4). This difference could be attributed to the use of a different PEEP setting between both groups23. However, we think the difference should be attributed to the whole strategy used (i.e., sedation, neuromuscular blocking, mechanical ventilation settings, among other factors). Although this deterioration in oxygenation may have reflected the progression of lung injury due to P-SILI, we can´t rule out that the lower PEEP used in the SB may have contributed to this result.
Tidal volume was similar in spontaneous and controlled ventilation; however, the higher and progressive intensity of intrathoracic pressure swings values observed in the SB group may suggest an increase in lung elastance or FRC diminishing, which could have mediated an increment in the volumetric strain of the remaining aerated lung tissue22,24. Regrettably, we did not measure lung elastance during SB to confirm these assumptions. In a similar model, Vimláti et al.9,10, demonstrated that this could be done using neuromuscular blockers intermittently. However, we expected that in this model the temporary muscular blocking could induce changes in the air distribution and lung collapse and then progressive reopening with variable temporal dynamics, which could induce a bias in the model. We tried to keep the model closer than we could to the clinical scenario.
We did not observe differences between groups over time regarding EELI. However, the analysis of the evolution of EELI in the SB group showed a progressive decrease throughout the study period. We can hypothesize that the SB-group needed higher inspiratory pressures to keep lung ventilation and to reopen collapsed lung units (tidal recruitment)25,26,27; this mechanism may be coupled to cyclic overdistension as well28.
Vascular stress secondary to high pulmonary blood flow29 and oscillations in the right ventricle stroke volume have been proposed to explain lung injury development during spontaneous breathing in different scenarios as during the practice of high-performance sports, and after upper airway obstruction in healthy humans30. Katira et al., showed that MV with intermittent high positive pressures induced marked oscillations in pulmonary blood flow. They suggested that it may be associated with microvascular injury and increased capillary leak due to capillary stress failure31,32,33,34. Blood flow oscillations could be intensified during respiratory failure and spontaneous breathing since the cyclic inspiratory negative pressures increase venous return, but without the resistance to vascular flow induced by PEEP (i.e., during CMV). We did not assess right ventricle dynamics during the protocol; however, we think this issue should be addressed in future research35.
EIT data showed that, unlike SB, during controlled MV, ventilation was distributed mainly to the non-dependent regions of the lungs. This can be explained due to the presence of alveolar collapse in the dependent areas, secondary to diaphragmatic paralysis (neuromuscular blockade), and low levels of PEEP used. Pellegrini and cols., showed in a porcine model of ARDS that the fraction of aerated lung was significantly smaller (baby lung) in controlled ventilation than during SB because, in the latter, the expiratory tonic activity of the diaphragm seems to preserve lung volume and protect against lung collapse36. Similarly, Yoshida and cols., evaluated the aeration changes with EIT and computed tomography (CT) during spontaneous efforts in mechanically ventilated patients with ARDS. They showed that the presence of SB efforts was associated with the movement of air from non-dependent to dependent zones (pendelluft phenomenon), causing tidal recruitment of dependent regions by concomitant collapse during expiration6. Therefore, beneficial, and harmful scenarios have been proposed depending on the intensity of the effort. The beneficial situation would be when mild spontaneous efforts keep the alveoli of the dependent pulmonary regions recruited27. On the contrary, a harmful situation would occur when vigorous regional negative pressures are associated with the pendelluft mechanism, the opening and closing of unstable lung units (atelectrauma), and more extensive heterogeneity in the distribution of ventilation, increasing the risk of VILI due to excessive lung strain7,37. We did not estimate pendelluft, however, we found important changes in the Regional Ventilation Delay index, suggesting the presence of repetitive recruitment-derecruitment phenomenon in the dorsal lung regions during spontaneous ventilation, denoting regional and temporal heterogeneity in lung ventilation16. Similarly, GI estimates the impedance pixel heterogeneity during tidal ventilation, which is correlated with the degree of pixel ventilation, comparing each individual pixel with the mean of all pixels from the lung field12. Both, RVD and GI point to greater heterogeneity in ventilation distribution during SB. In clinical research, heterogeneity is recognized as a marker of severity and mortality during ARDS38.
Recently, Hurtado and cols. found that spontaneous breathing in lung injury promotes regional strain and strain heterogeneity progression (P-SILI) in a murine model of alveolar instability. In contrast, protective MV prevented regional strain and heterogeneity progression in injured lungs. They conclude that the strain is associated with the diaphragm's vigorous contraction, resulting in an imperfect elastic anisotropic inflation (i.e., heterogeneity) and amplifying the regional lung injury39.
Regarding microstructural and inflammatory consequences of the ventilatory strategies studied, we found higher histological damage scores in SB and Late MV groups than early controlled MV. Surprisingly, we did not find greater histological damage in the dorsal regions in SB. Recently, in an ARDS animal model, Morais and cols. showed that assisted/controlled ventilation plus spontaneous efforts were associated with a higher histological score in the dependent lung regions20. Previously, Yoshida and cols. showed similar results40. However, these models were developed using a two-hit lung injury model with repeated lung lavages plus a variable period of injurious MV. A significant degree of inflammation could have been present since the beginning of the protocol due to the model itself41. Another remarkable difference is that we used PSV similar to clinical practice (very low levels), while in previous experiments, authors used assisted/controlled ventilation (PCV or APRV), allowing spontaneous efforts6,7,20.
Despite the observed differences in ventilatory distribution pattern, we did not find differences in the concentration of inflammatory mediators between SB and Early MV groups. Several reasons could explain this observation, such as a rather short time frame to detect changes in protein tissue concentrations, carry-over effect in the Late MV group, blood and lymphatic flow that could have opposite behavior than the ventilatory pattern, intrabronchial dissemination of inflammatory mediators, among others42,43.
Our study has several limitations. First, we used a model of lung collapse and surfactant depletion. This model alters lung mechanics and gas exchange but does not induce a formal lung injury and is not comparable to human ARDS. In pilot experiments we evaluated more severe models leading to lung injury but animals did not tolerate spontaneous ventilation. Second, the number of animals/group was rather low; some important parameters studied such as EIT indexes and cytokine levels presented high variability so the study may have been underpowered to find differences in some variables. Third, we observed by chance a lower severity of the alveolar collapse model at the beginning of the protocol in the Late MV group compared with the SB and Early MV groups. However, we think this factor did not influence our analysis, as most comparisons were performed between the SB and the Early MV group, while the Late MV group was analyzed mainly over time compared to itself. Fourth, In the Late MV group, some of the results observed at H8 may be explained by a carry-over effect. In this regard, some of the effects of the initial 4-h of spontaneous breathing could have influenced the results of H8. For example, inflammation or edema clearance/production are dynamic phenomena. Therefore, even though SB stopped, the dynamics of the process (i.e., inflammation or edema production) could continue, influencing the results of the next stage (H8). Fifth, we did not measure lung elastance during spontaneous breathing strategy, measuring of lung mechanics would have required to induce transient neuromuscular blocking, and we expected that it could induce changes in the air distribution and lung collapse, which could affect the interpretation of our conclusions.
Conclusions
In conclusion, in this porcine model of acute lung collapse, we showed that marked inspiratory swing pressures associated to vigorous spontaneous breathing efforts induce heterogeneity in the distribution of ventilation, favoring the progression of lung injury. This observation supports the theoretical background that has been proposed to explain the P-SILI concept. The change to controlled MV, despite attenuating lung dysfunction, did not prevent lung tissue injury. Other mechanisms such as the presence of tidal recruitment and increased vascular and alveolar stress are yet to be studied.
Data availability
Authors agree to make the data available. Correspondence and requests for materials should be addressed to JR (jaimeretamal@gmail.com).
Abbreviations
- SB:
-
Spontaneous breathing
- MV:
-
Mechanical ventilation
- P-SILI:
-
Patient self-inflicted lung injury
- VILI:
-
Ventilator-induced lung injury
- AHRF:
-
Acute hypoxemic respiratory failure
- VCV:
-
Volume-controlled ventilation
- VT:
-
Tidal volume
- RR:
-
Respiratory rate
- Bpm:
-
Breaths per minute
- PEEP:
-
Positive end-expiratory pressure
- I:E:
-
Inspiratory to expiratory ratio
- FIO2 :
-
Oxygen inspired fraction
- SpO2 :
-
Pulse oximetry
- PSV:
-
Pressure support ventilation
- PEEP:
-
Positive end-expiratory pressure
- GI:
-
Global Inhomogeneity index
- IR:
-
Impedance ratio
- TVI:
-
Tidal variation of impedance
- EELI:
-
End expiratory lung impedance
- RVD:
-
Regional ventilation delay
- BAL:
-
Bronchoalveolar lavage
- ELISA:
-
Enzyme-linked immunosorbent assay
- IL-8:
-
Interleukin-8
- TNF-α:
-
Tumor necrosis factor-alpha
- TGF-β:
-
Transforming growth factor-beta
- BALF:
-
BAL fluid
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- PaO2/FIO2 :
-
Ratio of arterial oxygen partial pressure to fractional inspired oxygen
- COVID-19:
-
Coronavirus disease 2019
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- CO:
-
Cardiac output
- EVLW:
-
Extravascular lung water
- ITBV:
-
Intrathoracic blood volume
- GEDV:
-
Global end diastolic volume
- CRS:
-
Respiratory system compliance
- Ppeak:
-
Peak pressure
- Pplateau:
-
Plateau pressure
- Pmean:
-
Mean pressure
- DP:
-
Driving pressure
- Crs:
-
Respiratory system compliance
- Vmin:
-
Minute volume
- Pes:
-
Esophageal pressure
- AU:
-
Arbitrary units
References
Vaporidi, K. et al. Respiratory drive in critically Ill patients pathophysiology and clinical implications. Am. J. Respir. Crit. Care Med. 201, 20–32 (2020).
Brochard, L., Slutsky, A. & Pesenti, A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 195, 438–442 (2017).
Cruces, P. et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care 24, 1–10 (2020).
Yoshida, T., Grieco, D. L., Brochard, L. & Fujino, Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr. Opin. Crit. Care 26, 59–65 (2020).
Grieco, D. L., Menga, L. S., Eleuteri, D. & Antonelli, M. Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 85, 1014–1023 (2019).
Yoshida, T. et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am. J. Respir. Crit. Care Med. 188, 1420–1427 (2013).
Yoshida, T., Uchiyama, A., Matsuura, N., Mashimo, T. & Fujino, Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: High transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit. Care Med. 40, 1578–1585 (2012).
Carteaux, G. et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: Role of tidal volume. Crit. Care Med. 44, 282–290 (2016).
Vimláti, L., Larsson, A., Hedenstierna, G. & Lichtwarck-Aschoff, M. Haemodynamic stability and pulmonary shunt during spontaneous breathing and mechanical ventilation in porcine lung collapse. Acta Anaesthesiol. Scand. 56, 748–754 (2012).
Vimláti, L., Larsson, A., Hedenstierna, G. & Lichtwarck-Aschoff, M. Pulmonary shunt is independent of decrease in cardiac output during unsupported spontaneous breathing in the pig. Anesthesiology 118, 914–923 (2013).
Haitsma, J. J. et al. Ventilator-induced coagulopathy in experimental Streptococcus pneumoniae pneumonia. Eur. Respir. J. 32, 1599–1606 (2008).
Zhao, Z., Steinmann, D. & Guttmann, J. Global and local inhomogeneity indices of lung ventilation based on electrical impedance tomography. IFMBE Proc. 22, 256–259 (2009).
Bachmann, M. et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit. Care 22, 1–11 (2018).
Frerichs, I. et al. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J. Appl. Physiol. 93, 660–666 (2002).
Hinz, J. et al. End-expiratory lung impedance change enables bedside monitoring of end-expiratory lung volume change. Intensive Care Med. 29, 37–43 (2003).
Muders, T. et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury*. Crit. Care Med. 40, 903–911 (2012).
Petersen, A. G. et al. Treatment with senicapoc in a porcine model of acute respiratory distress syndrome. Intensive Care Med. Exp. 9, 2–16 (2021).
Retamal, J. et al. High respiratory rate is associated with early reduction of lung edema clearance in an experimental model of ARDS. Acta Anaesthesiol. Scand. 60, 79–92 (2016).
Araos, J. et al. Near-apneic ventilation decreases lung injury and fibroproliferation in an near-apneic ventilation decreases lung injury and fibroproliferation in an acute respiratory distress syndrome model with extracorporeal membrane oxygenation. Am. J. Respir. Crit. Care Med. 199, 603–612 (2018).
Morais, C. C. A. et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am. J. Respir. Crit. Care Med. 197, 1285–1296 (2018).
Schmidt, M. et al. Benefits and risks of noninvasive oxygenation strategy in COVID-19: A multicenter, prospective cohort study (COVID-ICU) in 137 hospitals. Crit. Care 25, 1–13 (2021).
Mascheroni, D. et al. Acute respiratory failure following pharmacologically induced hyperventilation: An experimental animal study. Intensive Care Med. 15, 8–14 (1988).
Spieth, P. M. et al. Open lung approach vs acute respiratory distress syndrome network ventilation in experimental acute lung injury. Br. J. Anaesth. 107, 388–397 (2011).
Mauri, T. et al. Effects of sigh on regional lung strain and ventilation heterogeneity in acute respiratory failure patients undergoing assisted mechanical ventilation*. Crit. Care Med. 43, 1823–1831 (2015).
Pham, T., Telias, I. & Beitler, J. R. Esophageal manometry. Respir. Care 65, 772–792 (2020).
Wrigge, H. et al. Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology 99, 376–384 (2003).
Wrigge, H. et al. Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: A randomized controlled computed tomography trial. Crit. Care 9, 780–789 (2005).
Retamal, J. et al. Preliminary study of ventilation with 4 ml/kg tidal volume in acute respiratory distress syndrome: Feasibility and effects on cyclic recruitment—derecruitment and hyperinflation. Crit. Care 17, 16–23 (2013).
Broccard, A. F. et al. Consequences of vascular flow on lung injury induced by mechanical ventilation. Am. J. Respir. Crit. Care Med. 157, 1935–1942 (1998).
West, J. B. Vulnerability of pulmonary capillaries during severe exercise. Br. J. Sports Med. 40, 24–30 (2006).
Katira, B. H. et al. Adverse heart–lung interactions in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 196, 1411–1421 (2017).
Katira, B. H., Kuebler, W. M. & Kavanagh, B. P. Inspiratory preload obliteration may injure lungs via cyclical ‘on–off’ vascular flow. Intensive Care Med. 44, 1521–1523 (2018).
West, J. B., Tsukimoto, K., Mathieu-Costello, O. & Prediletto, R. Stress failure in pulmonary capillaries. J. Appl. Physiol. 70, 1731–1742 (1991).
West, J. B. & Mathieu-Costello, O. Stress failure of pulmonary capillaries: Role in lung and heart disease. Lancet 340, 762–767 (1992).
Vieillard-Baron, A. & Dreyfuss, D. Ventilator-induced lung injury: Follow the right direction! Another piece of the puzzle in the ventilator-induced lung injury epic. Am. J. Respir. Crit. Care Med. 196, 1366–1368 (2017).
Pellegrini, M. et al. The diaphragm acts as a brake during expiration to prevent lung collapse. Am. J. Respir. Crit. Care Med. 195, 1608–1616 (2017).
Yoshida, T., Uchiyama, A., Matsuura, N., Mashimo, T. & Fujino, Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit. Care Med. 41, 536–545 (2013).
Cressoni, M. et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 189, 149–158 (2014).
Hurtado, D. E. et al. Progression of regional lung strain and heterogeneity in lung injury: Assessing the evolution under spontaneous breathing and mechanical ventilation. Ann. Intensive Care 10, 107–118 (2020).
Yoshida, T. et al. Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. Am. J. Respir. Crit. Care Med. 196, 590–601 (2017).
Borges, J. B. et al. Lung inflammation persists after 27 hours of protective Acute Respiratory Distress Syndrome Network Strategy and is concentrated in the nondependent lung. Crit. Care Med. 43, 123–132 (2015).
Hedenstierna, G. & Lattuada, M. Lymphatics and lymph in acute lung injury. Curr. Opin. Crit. Care 14, 31–36 (2008).
Graf, J. et al. Semi-quantitative tracking of intra-airway fluids by computed tomography. Clin. Physiol. Funct. Imaging 29, 406–413 (2009).
Funding
This work was supported by Project Grant (FONDECYT 1171810) from Fondo Nacional de Desarrollo Científico y Tecnológico, Chile 2017.
Author information
Authors and Affiliations
Contributions
J.R., A.B., G.B. designed the study; M.C.B., P.C., F.D., V.O., M.G., J.V., N.H.V. conducted the experiments, performed data acquisition and analysis; L.F.D., R.B., Y.J., D.C., R.C. performed data acquisition and analysis; MR performed statistical analysis; J.R., P.C., F.D., A.B., M.C.B. developed the manuscript; all authors read, revised, and approved the final manuscript; J.R. was overall supervisor. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bachmann, M.C., Cruces, P., Díaz, F. et al. Spontaneous breathing promotes lung injury in an experimental model of alveolar collapse. Sci Rep 12, 12648 (2022). https://doi.org/10.1038/s41598-022-16446-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16446-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.