Abstract
We present a novel surface coating to resolve the stability of α-AlH3. Inspired by the strong chemical adhesion of mussels, the polymerization of dopamine was first introduced to coat α-AlH3 through simple situ polymerization. The α-AlH3 was used as a substrate. In-depth characterizations confirmed the formation of polydopamine (PDA) on the α-AlH3 surface. The coated α-AlH3 sample was characterized by X-ray diffraction X-ray photoelectron spectrometry and Scanning Electron Microscope. The results show that a strong PDA film is formed on the surface of α-AlH3, and PDA@α-AlH3 retains its primary morphology. The crystal form of α-AlH3 does not change after coating with PDA. The XPS analysis results show that N1 s appears on the material after coating with PDA, indicating that polydopamine is formed on the surface of α-AlH3. The moisture absorption tests show that the moisture absorption rate of α-AlH3 is greatly reduced after being coated with PDA. The excellent intact ability of PDA prevents α-AlH3 from reacting with water in air. The thermal stability of α-AlH3 before and after coating was analyzed by DSC. This work demonstrates the successful applications of dopamine chemistry to α-AlH3, thereby providing a potential method for metastable materials.
Similar content being viewed by others
Introduction
AlH3 is a solid-state hydrogen storage, hydrogen provider and reducing agent. Combined with Ammonium dinitramide (ADN), it becomes a high energy material often used for aerospace and missile industry1,2,3,4. Theoretical studies have shown that the specific impulse value of AlH3 is higher in solid, liquid and solid–liquid propellants than Al5,6,7. AlH3 is a special compound. It has at least seven different crystalline structures depending on the synthesis conditions: α α, β γ δ ε and ζ. Among them, α-AlH3 is the best-studied crystalline8,9,10,11,12. However, α-AlH3 has a small enthalpy of formation and is in a metastable state. Hence, the problem related to stability remains unresolved13,14,15,16.
To date, various researchers in the worldwide have been tackling this problem in improving the stability of AlH3, including surface passivation, surface coating and doping with other substrates17,18,19,20,21,22,23,24. In spite of this, the appropriate coating material and the novel coating technique for coating α-AlH3 should be explored.
Dopamine is a biological neurotransmitter that widely exists in living organisms25. The use of dopamine solution through the oxidation-polymerization of monomers has provided a facile and versatile method for modifying the surfaces of solid materials, which has led to the development of bioinspired PDA for the successful modification of various substrates, including metals, metals with native oxide surfaces, ceramics, semiconductors, carbon materials, and synthetic polymers. PDA-mediated chemistry could provide a general method for the fabrication of numerous multifunctional substrates with specific properties26,27,28,29,30. Dopamine chemistry is a straightforward and versatile coating strategy that may open a door for the surface processing of α-AlH3. However, few works have reported the use of PDA coating on α-AlH3.
Herein, we report a general and facile approach to the coating of α-AlH3, which is bioinspired through the in situ polymerization of dopamine. To the best of our knowledge, this is the first report about the application of dopamine chemistry to α-AlH3. First, dopamine was added to the phosphate buffer saline (PBS). The as-prepared α-AlH3 then underwent dopamine polymerization through immersion in a freshly prepared dopamine solution, with stirring at 200 rpm for 4 h 30. A simple scatter of α-AlH3 in an aqueous dopamine solution can lead to the spontaneous deposition of PDA film. The stability of α-AlH3 is significantly improved through the PDA coating. The present work potentially provides a new method for the modification of metastable.
Results and discussion
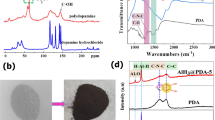
The PDA@α-AlH3 composite and α-AlH3 were subjected to XRD analysis, and the results are shown in Fig. 1. Figure 1 shows that the characteristic peaks of the PDA was amorphous without the characteristic diffraction peak of PDA. composite material appear at 2θ = 27.84°, 38.58°, 40.72°, 46.1°, 49.96°, 57.26°, 63.26°, 66.26°, 68.14°, 72.48°, 73.84°, 82.52°, 86.16°, corresponding to the (012), (104), (006), (202), (024), (116), (122), (018), (214), (300), (208) and (119) planes of α-AlH3 (JCPDF 23-0761), respectively. The position of the diffraction peak of the PDA@α-AlH3 complex is basically the same as the characteristic diffraction peak of α-AlH3. It shows that the position of the characteristic diffraction peak remains unchanged before and after the modification, indicating that α-AlH3 has good crystallization performance and PDA was amorphous without the characteristic diffraction peak of PDA. Observed changes in the relative peak intensities are attributed to the decrease of particle crystallinity andthe increasedscattering power caused by the coated PDA18.
The surface compositions of PDA@α-AlH3 and α-AlH3 were analyzed by photoelectron spectroscopy (XPS), and the results are shown in Fig. 2 and Table 1. Figure 2 shows that the element types on the surface of α-AlH3 before and after coating changed. The surface elements of α-AlH3 before coating only contain three elements: C, Al, and O. After coating, the surface elements of PDA@α-AlH3 contained not only three elements: C, Al, and O but also the characteristic N element peak of the polydopamine film. These observations indicate that the surface of α-AlH3 was successfully coated with a polydopamine film. However, the intensity of the peaks has changed. The intensity of the C1 s peak is significantly increased, indicating that the intensity of the O1 s peak and Al2p corresponding to the carbon element in the PDA coated on the surface of α-AlH3 is significantly reduced. At the same time, it can be seen more specifically from Table 1 that the content of C1 s increased from 18.17% to 52.37%, and the corresponding contents of O and Al were reduced. From Table 1, the content of O1 s is reduced from 38.58% to 22.83%, the content of Al2p is reduced from 43.25% to 21.27%, and the content of N1 s is characteristic of a polydopamine film at 3.53%, indicating that the surface of α-AlH3 is coated with PDA.
To further study the influence of PDA on the micromorphology of α-AlH3, the morphology analysis results of PDA@α-AlH3 and α-AlH3 by SEM are shown in Fig. 3. Figure 3 shows the SEM images and atomic distribution as determined by the EDS mapping images of two samples obtained from different locations. Figure 3a is the SEM and EDS-mapping image of α-AlH3. It has a cubic morphology of polycrystalline irregularity, with a particle size of approximately 10 μm, a relatively smooth surface, and relatively sharp edges and corners. There is a superpositional phenomenon between the particles. Figure 3b is the SEM and EDS mapping image of PDA@α-AlH3. It is a cube with less regularity, with a particle size of approximately 10 μm. The surface is relatively uneven and is attached to polydopamine particles, indicating that PDA is evenly coated on the α-AlH3 surface. It is clear that the elements of α-AlH3 determined by EDS mapping are different in terms of their composition and distribution. EDS mapping shows that the Al shown in green is full of coverage Beause Al is the major element present in α-AlH3. The O distribution is shown in red patches and dots. The Cl distribution is shown in yellow patches and dots. This shows that some Cl minerals included in α-AlH3 have been unremoved by cleaning.
After coating, the elements of PDA@α-AlH3 contained not only Al and O but also the characteristic N and C elements of polydopamine. The C distribution is shown in blue patches and dots. The N distribution is shown in purple patches and dots. The composition of samples determined by EDS is given in Table 1. As seen from Table 2, the C atomic percentage was increased by 24.55%, and when the N atomic percentage was increased by 2.44%, α-AlH3 was coated by PDA, showing a significant enrichment of the C and N contents. At the same time, the Al and the O atomic percentages were decreased by 7.88% and 8.87%, respectively.
Some studies have shown that dopamine first undergoes oxidation to dopaminequinone under alkaline conditions, followed by intramolecular cyclization via 1,4 Michael-type addition to yield leu-codopaminechrome. Leucodopaminechrome further undergoes oxidization and rearrangement to form 5,6-dihydroxyindole, which is easily oxidized to 5,6-indolequinone28,29. These two reaction products are capable of undergoing branching reactions, leading to the formation of multiple isomers of dimers and higher oligomers, which self-assemble through the reverse dismutation reaction between catechol and o-quinone to yield the cross-linked polymer30. A number of supramolecular interactions, including π-stacking, chargetransfer, and hydrogen bonding, have been shown to be the prominent features of the polymer’s structures30. In addition, with the increasing time, the coverage of PDA on the α-AlH3 surface increases due to conjugation of PDA aggregates on the surface, accompanying by the growth of PDA aggregates, which result in the deposition of PDA particles onto α-AlH3 surface. Therefore, compact and uniform PDA coating on α-AlH3 was possibly due to these factors. Firstly, under ambient conditions, polydopamine was prone to diffusion on organic α-AlH3 surfaces through noncovalent binding interaction such as hydrogen bonding, or π-π stacking to yield an effective adhesion layer; secondly, reactions between catechol in dopamine molecule and oxidation product o-quinone yielded the cross-linked polymers; thirdly, the cross-linked polymers can further assemble to PDA aggregates and deposit on the α-AlH3 surfaces, resulting in the PDA layers.
To study whether a small amount of coating agent PDA can slow down the moisture absorption of α-AlH3, the moisture absorption rates of PDA@α-AlH3 and α-AlH3 were tested. The water contact angles of PDA@α-AlH3 and α-AlH3 are measured. These results are shown in Fig. 4. The results show that at room temperature and 93% relative humidity, the moisture absorption rate of α-AlH3 coated with PDA is significantly lower than that of α-AlH3. With increasing time, the moisture absorption rate of uncoated α-AlH3 increases rapidly with storage time and reaches the equilibrium point of moisture absorption after 12 days, which is as high as 13.3%. The moisture absorption rate of α-AlH3 after being coated with PDA is only 0.5%, which shows that the polydopamine film on the surface plays an important role in isolating moisture in the air.
Thermal stability is a key performance factor for novel materials. To investigate the thermal stability of the samples before and after modification, the thermal performance was determined by DSC. The test results of the samples are displayed in Fig. 5. The characteristic parameters are summarized in Table 3. As shown in Fig. 5, the DSC curve of α-AlH3 exhibits a single endothermic desorption process starting at and peaking at 181.2 °C, which is in accordance with the endothermic reaction being attributed to the dehydriding of α-AlH3. However, the thermal decomposition temperature of PDA@α-AlH3 is 191.1 °C, indicating that the thermal stability of α-AlH3 is greatly improved after PDA modification, which is due to the formation of organic PDA to improve its heat resistance. These results clearly demonstrate that PDA@α-AlH3 completely decomposed into Al is more difficult than pure α-AlH3.
According to the relevant literature, AlH3 will slowly decompose to hydrogen at room temperature. For this reason, the prepared samples are naturally placed in a desiccator, and after a period of time, the hydrogen content is tested, and the decomposition rate is indirectly calculated through the change in hydrogen content. The test results of samples α-AlH3 and PDA@α-AlH3 are shown in Table 4. Table 4 shows that the hydrogen content of the product is reduced due to the decomposition of. The initial hydrogen content of sample #1 is 9.745%. From the initial hydrogen content data of #2, #3 and #4, we can see that there are varying degrees of reduction in hydrogen about α-AlH3 modified by PDA. However, from the perspective of decomposition rate, the decomposition rate of α-AlH3 is the highest, and the decomposition rate is reduced after modification by PDA. The decomposition of PDA@α-AlH3 may be slowed due to the formation of the organic polymer PDA. The more organic content there was, the lower the decomposition rate.
To investigate the influence of PDA on the sensitivity of α-AlH3, different batches of prepared PDA@α-AlH3 samples were numbered, and the impact, friction and electrostatic sensitivity were tested to study the influence of PDA on the sensitivity of α-AlH3. The impact sensitivity was conducted according to the GJB772A-97 standard method 601.1. The friction sensitivity was conducted according to the GJB772A-97 standard method 602.1. The electrostatic sensitivity was evaluted according to the GJB5891.27-2006.18 The test results are shown in the Table 5.
Experimental batch number #1 is α-AlH3, and experimental batch numbers #2, #3, #4 and #5 are all PDA-modified materials. From Table 5, it can be seen that the α-AlH3 surface modification material polydopamine has an effect on α-AlH3. The impact sensitivity, friction sensitivity and electrostatic sensitivity have little effect. The reason is that PDA did not change the crystal morphology, structure and crystal surface energy of α-AlH3. After being modified by PDA, it can meet the requirements of later application indexes.
Conclusion
Polydopamine (PDA) was successfully generated on the surface of α-AlH3 by in situ dopamine (DA) polymerization, and the structure and morphology of the package were characterized by various test methods, such as XRD, XPS and SEM. The results showed that the coating layer was more uniform, the coating effect was better, and the crystal type of the material was not changed. The stability of the PDA@α-AlH3 composite at room temperature and 93% high humidity is significantly higher than that of uncoated α-AlH3. This research provides new ideas for the long-term storage performance of α-AlH3 and its application in propellants.
Methods and materials
α-AlH3 was typically synthesized following a wet chemical method. LiAlH4 and AlCl3 react in diethyl ether to produce an alane-ether complex. Then, α-AlH3 was crystallized from the crystallization solution by removing ether by heating the crystallization solution to a temperature ranging from approximately 80 ℃ to 90 °C; additionally, the crystallization additive was also added to the crystallization solution. α-AlH3 was synthesized in our institute. Dopamine hydrochloride was purchased from Sigma–Aldrich and used as received. The other chemicals were commercial, analytical grade and used without further purification.
Coating of α-AlH3 with PDA
A phosphate-buffered saline (PBS pH = 7 ~ 7.5) solution was first prepared after the as-prepared solution and α-AlH3 was dispersed to the PBS solution, with stirring at 300 rpm for 30 min. Then, dopamine was added to the above suspension, with stirring at 200 rpm for 4 h. During the polymerization process, the color of the solution changed from white to dark brown as a result of dopamine polymerization. The obtained dark brown solution was filtered. The samples were rinsed with distilled water and then dried in a vacuum oven at 60 °C. The entire operation process is shown in Fig. 6.
Hygroscopicity test
Static hygroscopicity refers to the GJB772A-97 method. Place the saturated solution of potassium nitrate in a desiccator. After equilibrium, a hygrometer was used to determine the relative humidity in the desiccator to be 93%. Combine α-AlH3 and PDA@α-AlH3 at the same time. Place it in a dry place, and use a Mettler analytical electronic analytical balance for weighing, with an accuracy of 0.0001. The weight change was recorded every 24 h, the change in moisture absorption rate was observed, and the moisture absorption rate was calculated as follows:
In the formula, Q is the moisture absorption rate, ΔG is the weight gain of the sample after moisture absorption, and m is the initial weight of the sample.
Characterization
Structural characterization of the α-AlH3 and PDA@α-AlH3 samples was performed by powder X-ray diffraction (XRD, DMAX2400 with Cu Ka radiation at λ = 1.5418 Å). The morphology of the samples was examined by field emission scanning electron microscopy (SEM, Quanta600FEG). The surface chemistry was analyzed using X-ray elemental analysis (XPS, Thermo Fisher spectrometer equipped with monochromatic Al Ka radiation (1486.6 eV)). The content of H element was analysised using organic element analyzer. The thermal analysis was studied by DSC (NETZSCH STA 449C). The samples were heated from room temperature to the set temperatures with a heating rate of 10 ℃/min under an Ar2 gas flow rate of 70 ml/min to prevent oxidation.
References
Graetz, J. et al. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloys Compd. 509, S517–S528 (2011).
Sandrock, G. et al. Accelerated thermal decomposition of AlH3 for hydrogen-fueld vehicles. Appl. Phys. A Mater. Sci. Processing. 80, 687–690 (2005).
Grew, K. N. et al. Assessment of Alane as a hydrogen storage media for portable fuel cell power sources. J. Power Sources. 217, 417–430 (2012).
Young, G. Aluminum hydride as a fuel supplement to nanothermites. J. Propuls. Powder 30, 70–77 (2014).
Maggi, F. et al. theoretical analysis of hydrides in solid and rocket propulsion. Int. J. Hydrogen Energy. 37, 1760–1769 (2012).
Deluca, I. T. et al. High-energy metal fuels for rocket propulsion: Characterization and performance. Chin. J. Explos. Propellants. 36, 1–14 (2013).
Volker, W. et al. On the oxidation and combustion of AIH3 a potential FueI for rocket propellants and gas generators. Prope, Explos, Pyrotech. 32, 213–221 (2007).
DeLuca, L. T. et al. Physical and ballistic characterization of AlH3-based space propellants. Aerosol Sci. Technol. 11, 18–25 (2007).
Duan, C. W., Hu, L. X. & Ma, J. L. Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nanocomposites. J. Mater. Chem. A. 6, 6309–6318 (2018).
Jeong, W., Lee, S. H. & Kim, J. Synthesis and hydrogen desorption properties of aluminum hydrides. J. Nanosci. Nanotechnol. 16, 2987–2991 (2016).
Graetz, J. & Reilly, J. J. Thermodynamics of the α, β and γ polymorphs of AlH3. J. Alloys Comp. 424, 262–265 (2006).
Saitoh, H. et al. Formation and crystal growth process of AlH3 Al-H system. J. Alloys Compd. 496, 25–28 (2010).
Zhu, Z. Y. et al. Effects of phase impurity on stability and security of aluminum hydrid. Chin. J Energ. Mater. 19, 637–640 (2011).
Ismail, I. M. K. & Hawkins, T. Kinetics of thermal decomposition of aluminum hydride I-nonisothermal decomposition under vacuum and in inert atmosphere (argon). Thermochim. Acta. 439, 32 (2005).
Bulychev, B. M. Nonsolvated aluminum hydride. Crystallization from diethyl ether-benzene solutions. Russ Chem Bull. 56, 1305 (2007).
Sandrock, G. et al. Alkali metal hydride doping of α-AlH3 for enhanced H2 desorption kinetics. J. Alloys Compd. 421, 185–189 (2006).
Petrie, M. A., et al. Preparation of α-Aluminum hydride polymorphs, particular stabilized α-AlH3. USP 6228338, (2001).
Cai, X. W. et al. liquid carbon dioxide as anti-solvent coating aluminum hydride. Propellants, Expolos. Pyrotech. 40, 914–919 (2015).
Jiang, Z. et al. Research progress in the stabilization of aluminum hydride. Chin. J. Explos. Propellans 43, 107–115 (2020).
Qin, M. N. et al. The α-AlH3 coated with stearic acid: preparation and its electrostatic sensitivity. Chin. J. Energetic Mater. 25, 59–62 (2017).
Xing, J. H. Xia,Y. & Wang, J.W. A method for improving thermal stability of aluminum hydride. CN 109019507A (2018).
Petrie, M. A. et al, Preparation of aluminum hydride polymorphs, particularly stabilized α-AlH3. US, 6228338 B1, (2001).
Chen, R. et al. Surface passivation of aluminum hydride particles via atomic layer deposition. J. Vac. Sci. Technol. Vac. Surf. Films. 35, 03111 (2017).
Xing, X. H. et al. Research progress in improving thermal stability of aluminum trihydride. Chem. Propellants Polymeric Mater. 16, 21–25 (2018).
Lee, H., Lee, B. & Messersmith, P. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 1, 338–341 (2007).
Maerten, C. T. et al. Morphogen electrochemically triggered self-construction of polymeric films based on mussel-inspired chemistry. Langmuir 31, 13385–13393 (2015).
Lee, H. S., Dellatore, W. & Messersmith, P. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Liu, Y., Ai, K. & Lu, L. H. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 114, 5057–5115 (2014).
Dreyer, D. R. et al. Perspectives on poly(dopamine). Chem. Sci. 4, 3796–3802 (2013).
Feiyan, G. et al. Mussel-inspired coating of energetic crystals: A compact core–shell structure with highly enhanced thermal stability. Chem. Eng. J. 309, 140–150 (2017).
Funding
The funding was provided by Youth Science Fund Project of National Science Foundation of China (Grant No. 22105158).
Author information
Authors and Affiliations
Contributions
M.Q., B.Y., Q.S. and W.T, led the research study and prepared the manuscript. S.C., T.G.,and W.W. characterized the samples under the supervision of Y.Z. and Z.G.. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, M., Yao, B., Shi, Q. et al. PDA modification and properties of α-AlH3. Sci Rep 12, 12348 (2022). https://doi.org/10.1038/s41598-022-16424-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16424-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.