Abstract
Vernalization, an acceleration of flowering after long-term winter cold, is an intensively studied flowering mechanism in winter annual plants. In Arabidopsis, Polycomb Repressive Complex 2 (PRC2)-mediated suppression of the strong floral repressor, FLOWERING LOCUS C (FLC), is critical for vernalization and a PHD finger domain protein, VERNALIZATION INSENSITIVE 3 (VIN3), recruits PRC2 on FLC chromatin. The level of VIN3 was found to gradually increase in proportion to the length of cold period during vernalization. However, how plants finely regulate VIN3 expression according to the cold environment has not been completely elucidated. As a result, we performed EMS mutagenesis using a transgenic line with a minimal promoter of VIN3 fused to the GUS reporter gene, and isolated a mutant, hyperactivation of VIN3 1 (hov1), which showed increased GUS signal and endogenous VIN3 transcript levels. Using positional cloning combined with whole-genome resequencing, we found that hov1 carries a nonsense mutation, leading to a premature stop codon on the HEAT SHOCK TRANSCRIPTION FACTOR B2b (HsfB2b), which encodes a repressive heat shock transcription factor. HsfB2b directly binds to the VIN3 promoter, and HsfB2b overexpression leads to reduced acceleration of flowering after vernalization. Collectively, our findings reveal a novel fine-tuning mechanism to regulate VIN3 for proper vernalization response.
Similar content being viewed by others

Introduction
As sessile organisms, plants evolve to adapt to their surrounding environment. As the transition from the vegetative to reproductive phase is usually irreversible, the proper decision of flowering time in response to the environment is one of the most important developmental processes in plants1. Vernalization, an acceleration of flowering after long-term winter cold, is one of the mechanisms that render plants to flower in a timely manner. Arabidopsis winter annuals exhibit a late-flowering phenotype but their flowering time is dramatically accelerated by vernalization. In contrast, summer annuals exhibit an early-flowering phenotype regardless of cold treatment2. Before winter, the winter annuals display strong expression of FLOWERING LOCUS C (FLC), a MADS-box transcription factor that represses precocious flowering, however, FLC is gradually suppressed according to the winter cold period, which allows plants to flower in the spring2,3,4. Thus, the molecular mechanism of vernalization in Arabidopsis involves the suppression of FLC by winter cold. Suppression of FLC by long-term cold exposure involves epigenetic silencing which undergoes three critical stages; stages before, during, and after cold. During stage before cold, the proteins containing plant specific B3 DNA-binding domain, VP1/ABI3-LIKE 1 (VAL1) and VP1/ABI3-LIKE 2 (VAL2) directly bind to the, so called, RY element in the 1st intron of FLC5,6. VAL1 and VAL2 establish the nucleation region for histone modification marks, which is a prerequisite for the next stage. In stage during cold, H3K27me3 mark, a repressive histone modification, is accumulated on the nucleation region of FLC chromatin by a protein complex called PHD-PRC2 complex. It includes the core components of PRC2, CURLY LEAF (CLF) and SWINGER (SWN), Arabidopsis homologues of H3K27 methyltransferase, VERNALIZATION 2 (VRN2), FERTILIZATION INDEPENDENT ENDOSPERM (FIE), the WD‐40 domain protein MSI1, and VERNALIZATION INSENSITIVE3 (VIN3), a protein bearing a Plant Homeo Domain (PHD) motif7,8,9,10. During stage after cold, accumulated H3K27me3 on the nucleation region spreads all over the gene body by LIKE HETEROCHROMATIN PROTEIN 1 (LHP1), which causes the suppression stabilized7,11.
Among the genes encoding the components of PHD-PRC2 complex, VIN3 is the only gene induced by vernalization. Until exposed to cold temperature, VIN3 is known to be expressed rarely and sparsely throughout the meristematic regions8. If plants are exposed to cold temperature, VIN3 is induced within few hours, and its expression is gradually increased in proportion to the length of the cold period8. However, such induction is transient such that the VIN3 level gets reverted to non-vernalized conditions if plants are returned to warm temperature8. The vin3 mutant fails to respond to vernalization treatment, while constitutive expression of VIN3 is not sufficient for vernalization response. Therefore, these results indicate that VIN3 is a factor required, but not sufficient for vernalization8. In addition to the cold exposure, there are many additional factors capable of inducing VIN3, such as hypoxic condition and nicotinamide treatment. But the molecular mechanism behind the VIN3 induction is still not known yet12,13.
There were many efforts to understand the molecular mechanism of the VIN3 regulation over decades. For example, epigenetic regulation has been found to be a molecular basis for gradual VIN3 expression over long-term cold exposure12,13. In detail, bivalent modification of active (H3Ac, H3K36me3) and repressive (H3K27me3) histone marks on the VIN3 chromatin is revealed as a molecular mechanism of VIN3 induction12,13. In addition, transcriptional regulators also have been reported recently. For example, NAC WITH TRANSMEMBRANE MOTIF 1-LIKE 8 (NTL8) has been identified as a direct regulator of VIN3 expression through its accumulation during long-term cold exposure14. Moreover, two circadian clock regulators, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), were identified as direct regulators of VIN3, which presumably render diurnal rhythms of VIN3 expression15. Such findings provide supportive explanations for VIN3 regulation under multiple thermosensory pathways which was previously constructed by mathematical modeling of VIN3 dynamics16,17. However, such findings are still insufficient to understand regulatory mechanism of VIN3.
Cellular proteins are easily damaged when exposed to various environmental stresses. To protect cellular proteins from such cellular stresses, most eukaryotic organisms, including plants, have evolved molecular chaperones18. The most well-studied molecular chaperones are heat shock proteins (HSPs), which are induced by myriads of cellular stresses as well as heat shock19. The activation of HSPs, a general stress response in most eukaryotic organisms, is induced by a family of transcription factors known as Heat Shock Factors (Hsfs). Hsfs act as components of signal transduction that induce the expression of HSPs in response to a broad range of abiotic stresses20. By binding to the cis-elements, called Heat Shock Elements (HSEs; inverted repeat of a basal element 5′-nGAAn-3′), which are conserved in the promoters of heat stress–inducible genes of all eukaryotes, Hsfs directly regulate the transcription of stress-responsive genes, including HSPs21,22,23. There are 21 Hsf genes in the Arabidopsis genome and are divided into three classes; A, B and C24,25. Class A contains the motif (AHA motif) with activation activity, which is characterized by aromatic, large hydrophobic, and acidic amino acid residues25. Class A proteins have been reported to act as positive regulators in response to a broad range of stress conditions in plants22,26,27. In contrast, class B and C proteins are considered transcriptional repressors, as they lack AHA motifs and contain the repressive R/KLFGV motif28. Among the five class B proteins, HsfB1 and HsfB2b have been reported to act as transcriptional repressors, but positively regulate redundantly the acquired thermotolerance, an enhanced thermotolerance by prior heat treatment29. Besides the acquired thermotolerance, HsfB1 and HsfB2b have been shown to negatively regulate pathogen resistance redundantly30, while HsfB2b alone has been shown to mediate abiotic stress responses of the circadian clock31.
In the present study, we identified HsfB2b as a novel repressor of VIN3. Further, we isolated one mutant, hov1, with hyperactive VIN3 from a mutant pool that originated from the pVIN3::GUS reporter lines. The mutant was identified to carry a nonsense mutation in exon 1 of HsfB2b. Overexpression of HsfB2b rescued hyperactive VIN3 in hov1, and HsfB2b was found to bind to the conserved HSEs located in the 5′-UTR of VIN3. Moreover, overexpression of HsfB2b in the late-flowering FRI Col background resulted in defects in the vernalization response, suggesting that HsfB2b negatively regulates the vernalization response.
Results
Isolation and characterization of the mutant showing hyperactivation of VIN3, hov1
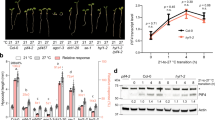
To identify upstream regulators of VIN3, the GUS reporter line (− 0.2 kb pVIN3_U_I::GUS)15 was mutagenized with ethyl methanesulfonate (EMS). A total of 3,412 M1 lines were generated and their seeds were harvested as M2 seeds. Approximately 25 M2 seedlings from each line were grown at room temperature for 10 days, and transferred to the cold chamber for 3 days, then analyzed for GUS staining. Throughout the screening, the first and second true leaves of the M2 seedlings were used for GUS staining when seedlings produced more than 5 leaves. One mutant showing the hyperactivation of GUS was identified and initially named hov1. Compared to the parental line, which showed a very weak GUS signal after 3 days of cold exposure (3V), hov1 showed an enhanced GUS signal with the same treatment (Fig. 1a). Consistent with the results of the GUS assay, endogenous VIN3 transcript levels were enhanced in the mutant after 3V (Fig. 1b). The mutant phenotype was not found in the F1 plants when backcrossed to the parental line, and the phenotype was segregated by approximately 3:1 (81 WT vs 29 mutants) in F2 population. Such results indicate that the mutant phenotype is completely recessive and is caused by a mutation in a single locus. Thereafter, we performed a time course analysis of VIN3 levels in hov1 for vernalization treatment. As shown in Fig. 1c, hov1 plants displayed higher levels of VIN3 than the controls without vernalization treatment, indicating that HOV1 is necessary to completely suppress VIN3 at room temperature. The hov1 plants also had higher levels of VIN3 throughout the vernalization time course and the mutant had higher levels of VIN3 than the control plants after returning to room temperature for 5 days (40VT5). These results indicate that HOV1 is required for the suppression of VIN3 under all conditions.
Isolation and characterization of the mutant, hov1, showing hyperactivation of VIN3. (a,b) Characterization of the hov1 mutant. Seedlings of the control (− 0.2 kb pVIN3_U_I::GUS) and hov1 were grown at room temperature for 10 days and analyzed after 3 days of cold treatment. (a) Images of representative seedlings after GUS staining. (Scale bars, 2 mm) (b) Endogenous VIN3 transcript levels in control and hov1. Data are presented as mean ± SEM of three biological replicates. Asterisks indicate significant difference compared with the control (Student’s t-test; ***P < 0.001). (c) Time-course analysis of VIN3 levels during vernalization treatment. NV, non-vernalized; 10V, 20V, 30V, and 40V, 10 d, 20 d, 30 d, and 40 d vernalized, respectively; 40VT7, 7 d grown at room temperature after 40V. Data are presented as mean ± SEM of three biological replicates. Asterisks indicate significant difference compared with the control (Student’s t-test; *P < 0.05). The inset in (c) is enlarged for NV. (d) Schematic structure of the HsfB2b gene. Black bars indicate exons, and white boxes and lines represent untranslated regions and introns, respectively. The mutations that occurred in the two alleles are shown: T-DNA insertion as a triangle and point mutation as an asterisk.
To identify the causative mutation, hov1 was crossed with Ler for positional cloning. A total of 156 F2 plants with enhanced GUS signals in the leaves were selected for mapping analysis. We mapped the mutation to the 590 kilobase pair interval on chromosome 4, which contained 142 genes (Supplementary Fig. S1). The genomes of hov1 and parental − 0.2 kb pVIN3_U_I::GUS were sequenced using the Illumina sequencing method for comparison. Analysis of the sequence data revealed nine potentially disruptive point mutations, including one mutation within the At4g11660 gene (G to A, causing a nonsense mutation from Trp89 to the stop codon) (Fig. 1d). At4g11660 encodes the class B heat shock transcription factor, HEAT SHOCK TRANSCRIPTION FACTOR B2b (HsfB2b).
HsfB2b acts as a transcriptional repressor of VIN3
To verify that HsfB2b is the causative gene of the upregulation of VIN3 level in hov1, we checked the VIN3 level in the T-DNA-inserted mutant, hsfb2b-1. As expected, hsfb2b-1 had higher levels of VIN3 than wild-type Col-0 under non-vernalized conditions (Fig. 2a). Previously, HsfB2b was reported to display functional redundancy with HsfB1 instead of HsfB2a for acquired thermotolerance, although the sequence of HsfB2b had higher homology with that of HsfB2a than HsfB129. To determine whether HsfB2b is functionally redundant with HsfB1 for the vernalization response, we compared VIN3 levels among the hsfb2b, hsfb1, and hsfb1 hsfb2b mutants. The hsfb1 mutant displayed similar levels of VIN3 to the wild-type under all conditions. However, the hsfb1 hsfb2b double mutant did not show any difference compared with hsfb2b in VIN3 levels (Fig. 2a). Such finding suggests that HsfB1 is not functionally redundant to HsfB2b, at least for VIN3 regulation.
HsfB2b acts as a transcriptional repressor of VIN3. (a) HsfB1 is not functionally redundant with HsfB2b in VIN3 regulation. VIN3 transcript levels in Col-0, hsfb1, hsfb2b and hsfb1 hsfb2b during vernalization treatment were determined using RT-qPCR. NV, non-vernalized; 3V, 10V, 40V, 3, 10, and 40 d vernalized; 40VT1, 1 d growth at room temperature after 40V. Transcript levels were normalized to those of PP2A. Data are presented as mean ± SEM of three biological replicates. Significant differences have been marked using different letters (a-l; P < 0.05; one-way ANOVA followed by Tukey’s post-hoc test). The inset in (a) is enlarged for the NV and 3V. (b,d) Complementation of hov1 with pHsfB2b::HsfB2b-myc. (b) GUS staining of NV or 3V seedlings of the parental line, hov1, and pHsfB2b::HsfB2b-myc hov1 transgenic line. Images of representative seedlings after GUS staining (c) HsfB2b or (d) VIN3 transcript levels in 3V Col-0, hov1, and two representative transgenic lines of pHsfB2b::HsfB2b-myc hov1. Transcript levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates. (e,f) Effects of hsfb2b mutation on VIN3 levels. VIN3 levels in Col-0 and hsfb2b, as determined by RT-qPCR after (e) short-term cold treatment (0, 1, 3, and 5 h) or (f) long-term cold exposure (NV, 10V, 20V, 40V, 40VT1, 40VT3). Transcript levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates.
Finally, we introduced HsfB2b::HsfB2b-myc into the hov1 mutant to determine whether HsfB2b can rescue the hov1 mutation. The transcript levels of HsfB2b were found to be overexpressed in all transgenic lines we obtained (Figs. 2c, 6a, and d). Here, we used two representative lines of HsfB2b:HsfB2b-myc hov1, #1, and #2. As expected, the phenotype of the GUS signal in hov1 was complemented by HsfB2b::HsfB2b-myc, such that the GUS signal was barely detected after 3 d of cold exposure (Fig. 2b). Moreover, the endogenous VIN3 transcript levels in both transgenic lines were lower than those in Col-0, as well as the hov1 mutant, whereas HsfB2b transcript levels in the transgenic lines were higher than those in Col-0 (Fig. 2c and d). Taken together, our results indicate that HsfB2b is a causative gene that reduces VIN3 level in hov1 and acts as a transcriptional repressor of VIN3.
In a previous report, VIN3 was found to gradually increase by long-term cold exposure from the first day of cold treatment13. Thus, we determined whether HsfB2b affects VIN3 expression during the initial stage of vernalization treatment (Fig. 2e and f). As shown, hsfb2b caused strong derepression of VIN3 from the initial phase, and the effect was strongest after 3 h of cold treatment. Of note, the derepression effect of hsfb2b is stronger at short-term cold and 40VT1 (1 d at room temperature after returning from 40 days of vernalization treatment) than during vernalization treatment (Fig. 2e and f).
Effects of vernalization on HsfB2b
Hsfb2b expression is well-known to be induced by heat shock treatment to suppress hyperactivated heat shock-responsive genes29. However, the effect of long-term cold treatment on Hsfb2b is unknown. Before vernalization treatment, the basal level of Hsfb2b was detected as previously reported (Fig. 3a). During the long-term cold treatment, such levels of Hsfb2b did not change significantly. In contrast, a slight increase of Hsfb2b was observed when returned to room temperature (Fig. 3a). We also checked HsfB2b protein levels using pHsfB2b::HsfB2b-eGFP transgenic lines during vernalization treatment (Fig. 3b). However, the protein levels were not found to be significantly affected by vernalization treatment. Nonetheless, vernalization treatment caused retarded migration of HsfB2b-eGFP proteins on polyacrylamide gel from the initial phase, suggesting that HsfB2b undergoes post-translational modifications, such as phosphorylation by cold (Fig. 3b). Such retardation in HsfB2b migration was also observed in the protein immunoblot result using pHsfB2b::HsfB2b-myc transgenic plants (Supplementary Fig. S2). After returning to room temperature, such modifications may have been rapidly erased as 40VT1 displayed the same protein pattern as NV.
Characterization of Hsfb2b during cold and vernalization treatments. (a) Effect of vernalization treatment on the transcript levels of HsfB2b. Col-0 seedlings were vernalized before total RNA extraction for RT-PCR analysis. Expression levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates. (b) Immunoblot analysis of the HsfB2b-eGFP protein extracted from vernalized seedlings of pHsfB2b::HsfB2b-eGFP. Rubisco was used as a loading control. NV, non-vernalized; 1V, 5V, 10V, 20V and 40V, 1 d, 5 d, 10 d, 20 d, and 40 d, respectively; 40VT1, 40VT3, 1 d, and 3 d, grown at room temperature after 40V, respectively. Original blots are presented in Supplementary Figure S7. (c,d) Confocal images of roots from NV or 5V plants expressing pHsfB2b::HsfB2b-eGFP. (Scale bars, 20 μm) Five days-old Arabidopsis seedlings, with or without 5 days of cold exposure, were harvested and counterstained with propidium iodide. (e) Effect of the early phase of vernalization on the rhythmic expression of HsfB2b in Col-0. Expression levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates. (f) Effect of the hsfb2b mutation on the rhythmic expression of VIN3. VIN3 levels during the early phase of vernalization were analyzed using seedlings of Col-0 and hsfb2b collected at 4-h intervals over 72 h in LD at 4 °C. The x-axis indicates the exposure time to cold. Data are presented as mean ± SD of three technical replicates.
To confirm whether the retarded migration of HsfB2b protein is due to the phosphorylation, phosphatase assay was conducted using total protein extracted from vernalized or non-vernalized pHsfB2b::HsfB2b-myc seedlings. Subsequent immunoblot assay showed that the retarded migration of HsfB2b-myc from vernalized seedlings was abolished by phosphatase treatment, while migration of HsfB2b-myc from non-vernalized seedling was not changed by the treatment (Supplementary Fig. S3). These results indicate that retarded migration of HsfB2b-myc from vernalized seedlings was due to the phosphorylation.
As the cellular localization of other Hsf is changed by post-translational modification32, we checked whether cold treatment can change that of HsfB2b (Fig. 3c and d). Using the pHsfB2b::HsfB2b-eGFP transgenic lines, we observed the root tissue before and after 5 days of cold. In both cases, GFP signals were observed in the nucleus, indicating that neither cold treatment nor protein modification altered the cellular localization of HsfB2b. This result is consistent with the fact that the protein sequence of HsfB2b has a nuclear localization signal (NLS), but lacks a nuclear export signal (NES) motif25. Taken together, HsfB2b is neither transcriptionally induced nor is the subcellular localization of the proteins altered by vernalization treatment.
VIN3 expression is reported to show a circadian rhythm and HsfB2b acts as a negative regulator of the circadian clock regulator, PSEUDO RESPONSE REGULATOR7 (PRR7)15,16,31. Thus, we verified whether the VIN3 rhythm was affected by hsfb2b during cold treatment. Although the amplitude of the circadian rhythm was increased by hsfb2b mutation due to the increase in VIN3 level, the rhythmic pattern was not significantly different (Fig. 3e and f). Therefore, HsfB2b seems to constitutively repress VIN3, and this repression is independent of the HsfB2b-regulated circadian clock.
HsfB2b directly regulates VIN3 repression
Heat shock transcription factors regulate a variety of genes by directly binding to the HSE in the promoters22,23. Consistently, HSE was detected near the VIN3 promoter, approximately 40-bp downstream of the transcription start site (Fig. 4a). In addition, the HSE was highly conserved among the VIN3 orthologs from Brassicaceae species (Arabidopsis thaliana, Arabidopsis lyrata, Boechera stricta, and Capsella rubella) (Supplementary Fig. S4). Therefore, we determined whether HsfB2b directly binds to the VIN3 promoter. In silico analyses using the DNA affinity purification (DAP)-seq database33 showed that several heat shock transcription factors bind to the 5′-UTR of VIN3, where HSE is located (Supplementary Fig. S5).
HsfB2b directly binds to the HSE on the VIN3 gene. (a) Schematic of the VIN3 gene with description of the HSEVIN3 sequence and PCR amplicons. A-D, amplicons used for ChIP-qPCR. Black bars indicate untranslated region, and grey boxes and lines represent exons and introns, respectively. (b) Yeast one-hybrid assay between heat shock factors and HSEVIN3 cis-element. As DNA baits, four tandem copies of the 24-bp sequences containing HSEVIN3 were inserted into the pHisI vector and used as the reporter construct. The CDS of HsfA1a, HsfA2, HsfA3, HsfA4a, HsfA6a, HsfA8, HsfB1, HsfB2b, and HsfC1 was cloned into pGADT7 and used as an effector construct. GAL4 AD alone (AD) was used as the control. The effector and reporter constructs were co-transformed into the yeast strain, AH109. Representative growth status of yeast cells is shown on synthetic defined (SD)-LUH medium, with or without 5 mM 3-AT. LUH, SD medium without Leu, Ura, His; − LUH + 5 mM 3-AT, SD medium without Leu, Ura, His but containing 5 mM 3-Amino-1,2,4-triazole. (c) ChIP-qPCR showing the enrichment of HsfB2b-eGFP. Chromatin of the transgenic line expressing pHsfB2b::HsfB2b-eGFP was immunoprecipitated using control beads or GFP-trap beads. Histograms show mean values ± SEM (n = 2 biological replicates, each biological replicate is an average value of three technical replicates) for enrichment calculated by percent input normalized against the control. Asterisks indicate significant differences compared with the control (Student’s t-test; ***P < 0.001). (d) In vitro binding of the recombinant MBP-HsfB2bDBD to HSEVIN3 sequence by EMSA. Purified recombinant MBP-HsfB2bDBD or MBP was incubated with Cy5-labeled 40-bp sequences including HSEVIN3 element as HSE probes. The same sequences with mutations in the HSEVIN3 was used as control probe (mHSE). Unlabeled competitor DNA (100 × molar excess) was added to each reaction, as indicated. Original gels are presented in Supplementary Figure S8.
We proceeded to assess whether HsfB2b bound to HSEVIN3 using yeast one-hybrid assay (Fig. 4b). Among the nine Hsf proteins analyzed, HsfA1, HsfA6a, HsfB1, HsfB2b, and HsfC1 were found to interact with HSEVIN3. To confirm in planta binding, we also performed chromatin immunoprecipitation-qPCR using transgenic pHsfB2B::HsfB2b-eGFP, grown under long days without cold treatment (Fig. 4a and c). HsfB2b-eGFP proteins were found to be enriched in the promoter region near the HSE location even without cold treatment, which is consistent with the elevated VIN3 level in hsfb2b. We also conducted electrophoretic mobility-shift assay (EMSA) to assess whether HsfB2b specifically binds to HSEVIN3 DNA element in vitro. The purified recombinant protein, MBP-HsfB2bDBD, DNA binding domain of HsfB2b fused with maltose binding protein (MBP), from E. coli indeed binds the HSEVIN3 probe but fails to bind the mutated version of HSEVIN3 probe (Fig. 4d). Taken together, these data strongly support the hypothesis that HsfB2b directly regulates VIN3 repression.
hsfb2b mutation does not change vernalization response under normal condition
To analyze the effect of hsfb2b on vernalization response, the hsfb2b mutation was introduced into FRI Col, a vernalization-sensitive line, by genetic cross34. As shown in Fig. 5, the flowering time of hsfb2b FRI was similar to that of FRI Col, although VIN3 levels were higher in hsfb2b FRI than in FRI Col throughout the time course of vernalization treatment (Fig. 5a–c). Consistently, the FLC levels were not significantly different between the two genotypes throughout vernalization treatment (Fig. 5d). Thus, the increased levels of VIN3 in hsfb2b may not alter the vernalization response under normal growth conditions. Such findings suggest that VIN3 levels in the FRI Col are sufficient for a proper vernalization response.
hsfb2b mutation does not change vernalization response under normal condition. (a) Images of FRI Col and hsfb2b FRI without (NV) or with 10 d (10V), 20 d (20V), 40 d (40V) of vernalization. (b) Flowering time of FRI Col and hsfb2b FRI after vernalization. Flowering time was measured by counting the number of primary rosette leaves formed when the first flower opened. Data are presented as mean ± SD. Significant differences have been marked using different letters (a-l; P < 0.05; one-way ANOVA followed by Tukey’s post-hoc test). (c,d) VIN3 or FLC transcript levels in FRI Col and hsfb2b FRI after vernalization treatment. The transcript levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates. Asterisks indicate significant difference compared with the control (Student’s t-test; *P < 0.05). n.s, not significant.
HsfB2b overexpression leads to hyposensitive response to vernalization
In our complementation analysis, all HsfB2b transgenic lines displayed overexpression of HsfB2b, although the transgenes were driven by the endogenous promoter. Thus, we analyzed the vernalization response in HsfB2b overexpressing lines. The hov1 mutant, containing a nonsense mutation in the first exon of HsfB2b, showed approximately threefold higher HsfB2b levels than Col-0, which might be due to the negative feedback regulation (Fig. 6a). When the transgenes, pHsfB2b::HsfB2b-myc or pHsfB2b::HsfB2b-eGFP, were introduced into the hov1 background, the HsfB2b levels were increased by 20–50-fold relative to that of Col-0, indicating that the transgenic lines were HsfB2b overexpressors (Fig. 6a). The GUS and endogenous VIN3 levels among the parental lines (− 0.2 kb pVIN3_U_I::GUS), hov1 (in − 0.2 kb pVIN3_U_I::GUS background), and pHsfB2b::HsfB2b-myc hov1 (Fig. 6b and c) were subsequently compared after 40 days of vernalization treatment. The overexpression of HsfB2b was found to markedly reduce VIN3 levels after 40 days of vernalization treatment. Such finding is consistent with the hypothesis that HsfB2b represses VIN3 transcription.
HsfB2b overexpression leads to hyposensitive response to vernalization. (a) Comparison of the HsfB2b transcript levels in Col-0, hov1, hsfb2b, and the transgenic lines expressing pHsfB2b::HsfB2b-eGFP or pHsfB2b::HsfB2b-myc in hov1. Transcript levels were normalized to those of PP2A. Fold changes relative to Col-0 were marked for comparison. Data are presented as mean ± SD of three technical replicates. (b) GUS staining for 40V seedlings of the parental lines (− 0.2 kb pVIN3_U_I::GUS), hov1, and pHsfB2b::HsfB2b-myc hov1. Images of representative seedlings after GUS staining are shown. (c) VIN3 levels in 40V seedlings of the parental line, hov1, and pHsfB2b::HsfB2b-myc hov1. Transcript levels were normalized to those of PP2A. Data are presented as mean ± SD of three technical replicates. (d) Transcript levels of HsfB2b, VIN3, and FLC in FRI Col, hsfb2b FRI, and transgenics expressing pHsfB2b::HsfB2b-eGFP in FRI Col after 20V. Transcript levels were normalized to those of UBC. Data are presented as mean ± SD of three technical replicates. (e) Photographs of WT (FRI Col), hsfb2b FRI, and transgenics expressing pHsfB2b::HsfB2b-eGFP in FRI Col after 20V. Images captured when 20V WT and hsfb2b plants were fully flowered. (f) Flowering time is presented as a box plot. Flowering time was measured by counting the number of primary rosette leaves formed when the first flower opened. The center lines indicate the medians. Box limits indicate the 25th and 75th percentiles. Significant differences have been marked using different letters (a–d; P < 0.05; one-way ANOVA followed by Tukey’s post-hoc test).
We proceeded to verify whether HsfB2b overexpression caused any changes in the vernalization response. Briefly, we introduced pHsfB2b::HsfB2b-eGFP into FRI Col by transformation. As expected, all 10 transgenic lines showed overexpression of HsfB2b (3 ~ 10 folds) based on the level after 20 days of vernalization (20V) (Fig. 6d). In these transgenic lines, VIN3 levels were lower than those in both FRI Col and hsfb2b FRI after 20V, which supports the hypothesis that HsfB2b overexpression causes the repression of VIN3 in FRI Col plants (Fig. 6d). Consistent with the fact that VIN3 is required for the suppression of FLC8, the FLC levels in pHsfB2b::HsfB2b-eGFP FRI lines were slightly higher than those in FRI Col after 20V (Fig. 6d). Finally, the flowering time of the transgenic lines, pHsfB2b::HsfB2b-eGFP FRI, was less accelerated than that of both FRI Col and hsfb2b FRI by 20V (Fig. 6e and f). Therefore, in contrast to hsfb2b mutation, HsfB2b overexpression causes defects in the vernalization response under normal growth conditions.
Discussion
VIN3 is required for proper vernalization in Arabidopsis, particularly winter annuals. However, the molecular mechanism by which VIN3 is finely regulated has not been fully elucidated. In this study, we isolated a mutant, hov1, that showed hyperactivation of VIN3. By map-based cloning combined with whole-genome resequencing, HsfB2b was defined as the causative gene for VIN3 derepression in hov1. Interestingly, hsfb2b exhibited higher VIN3 levels under all conditions, including before and after vernalization. Therefore, HsfB2b might act as a general repressor of VIN3, regardless of cold treatment. Nonetheless, the intensity of the derepression in hsfb2b was strongest at the initial stages of cold treatment and stronger at the phase of return to room temperature after 40V than during vernalization treatment. Taken together, HsfB2b might act in a fine-tuning mechanism, suppressing precocious VIN3 activation during the fall when temperature drops abruptly and suppressing VIN3 levels rapidly after spring.
Higher VIN3 levels in hsfb2b failed to show a stronger vernalization response in the late-flowering FRI Col background. This result is consistent with that of previous studies where ectopic expression of VIN3 was not found to alter the vernalization response, despite complementing vin3 mutation35,36. In contrast, the lower VIN3 levels in the HsfB2b overexpression lines caused a weak vernalization response in both the acceleration of flowering time and FLC suppression by 20 days of vernalization treatment. This result is also consistent with the fact that the vin3 mutation causes failure of the vernalization response7,8. Finally, HsfB2b was found to directly repress VIN3, a key factor in the vernalization process, by binding to the HSE on the 5′-UTR of VIN3 (Fig. 5). Of note, the HSEVIN3 sequences on the 5′-UTR of the VIN3 orthologues are highly conserved, whereas other regions of the 5′-UTR are relatively diversified among Brassicaceae species. As vernalization responses have been observed throughout Brassicaceae, conservation of such cis-elements suggests that VIN3 regulation by Hsfs may also be conserved across the Brassicaceae family37,38,39.
Although hsfb2b shows constitutively up-regulated VIN3 level, vernalization response is not much affected by the hsfb2b mutation. Similarly, vernalization response is not affected by the ectopic expression of VIN3, despite complementing vin3 mutation35,36. Previous report indicated that vernalization-mediated removal of H3K4me3 is a prerequisite for VIN3-PRC2 accumulation at the FLC nucleation region. In addition, the association of VAL1 and VAL2, two B3 domain transcription factors, to the nucleation region of FLC is also reported as a prerequisite for VIN3-containing PRC2 activity5,6. Such results explain why VIN3 alone is not sufficient to suppress FLC. In natural conditions, where daily temperature fluctuates largely, especially at late fall or early spring, it is beneficial to fine-tune vernalization response. In case the prerequisites mentioned above are fulfilled accidentally by sudden temperature drop, the fine-tuning of VIN3 expression by transcription repressor like HsfB2b may provide another layer of huddle to prevent hypersensitive vernalization responses.
Early works on vernalization have reported that the immediate treatment of heat above 30 °C after long-term cold can erase vernalization effect. It is called devernalization40,41. Since overexpression of HsfB2b causes reduced VIN3 expression, resulting in weak vernalization response, (Fig. 6) and VIN3 level in 40VT1 plant is elevated in hsfb2b compared to wild-type (Fig. 2f), it is possible that HsfB2b may mediate devernalization process. However, we could not observe any difference in devernalization effect between wild-type and hsfb2b mutant when heat (30 °C) treated right after long-term cold treatment (Supplementary Fig. S6). Therefore, it is not likely that HsfB2b is involved in erasing repressive epigenetic marks, such as H3K27me3, induced by vernalization on the FLC locus, which is a proposed molecular mechanism of devernalization42.
In Arabidopsis, Hsfs have been reported to regulate diverse stress responses, including responses to both biotic and abiotic stresses, such as bacterial infection, fungal infection, and heat and drought stresses43,44,45,46. During such responses, both class A and class B Hsfs are incorporated into complex and multi-layered regulatory systems, and different combinations of Hsfs seem to act on each stress response27. Although most of Hsfs are induced by heat stress, they usually show basal expression level without heat or cold stress similar to Hsfb2b. Such basal expression level may be required for the rapid response to diverse stresses. Here, HsfB1 was not functionally redundant with HsfB2b for VIN3 regulation (Fig. 2). However, several Hsfs, besides HsfB2b, including HsfB1, bound to the HSEVIN3 elements present in the 5′-UTR of VIN3 in the yeast one-hybrid assay (Fig. 5). Therefore, other Hsfs, recognizing HSEVIN3, may regulate VIN3 transcription in response to other stresses, such as low oxygen conditions at which VIN3 is induced47. This notion is consistent with the finding that Hsfs are required for a broad range of stress responses22,26,27. It would be interesting to determine whether VIN3 acts as a hub for the stress responses mediated by Hsfs.
Plants perceive winter cold as a signal for vernalization, but simultaneously perceive it as long-term cold stress. In Arabidopsis, several HSPs and factors are strongly induced by cold stress, and the roles of both HSPs and Hsfs in the cellular response to cold stress have been reported previously22,48,49,50. The HsfB2b protein displayed retarded migration on polyacrylamide gels during vernalization treatment. Such cold-induced post-translational modifications indicate that HsfB2b is involved in a subset of cold signal transduction (Fig. 3b). Previously, HsfB2b has been reported as a protein phosphorylated by SnRK2 kinases, which is activated by plant hormone abscisic acid (ABA)51. Considering that ABA is known to play a role in broad range of stress response including cold response52,53, the phosphorylation of HsfB2b is probably involved in stress responses triggered by ABA. Moreover, the transcript level of HsfB2b was slightly elevated after returning from cold temperatures to warm temperatures (Fig. 3a). Such observations may indicate that HsfB2b is required for sensing temperature changes, which are inevitable during vernalization treatment. Thus, VIN3 regulation by HsfB2b may have evolved from a mechanism that senses cold stress.
The circadian clock was previously demonstrated to be involved in the regulation of VIN3, and components of the circadian clock, CCA1 and LHY, directly regulate the diurnal rhythm of VIN3 during vernalization treatment15,16. One of the circadian clock regulators, PRR7, has also been reported to be a transcription factor repressed by HsfB2b31, which is required for proper abiotic stress responses. However, our data indicate that HsfB2b is not involved in regulating the diurnal rhythm of VIN3 under cold treatment, despite affecting the amplitude (Fig. 3e and f). As the circadian clock has rhythmic robustness due to multiple feedback loops consisting of diverse transcription factors, the defect in clock regulation by hsfb2b seems to be minor for the VIN3 rhythm54.
Under natural conditions, where environmental changes markedly occur, plants must avoid and distinguish between uncertain signals. For vernalization, plants must distinguish transient changes in temperature from winter cold. For example, plants often experience a sudden cold during late fall or a sudden warmth in early spring. Thus, plants must have an elaborate mechanism to regulate VIN3 expression in response to ever-changing environmental conditions. Consistently, VIN3 has been demonstrated to display dynamic expression patterns depending on fluctuating temperature16. For such elaborate regulation of VIN3, HsfB2b may provide a fine-tuning mechanism to prevent unintentional flowering from sudden cold.
Materials and methods
Plant materials and growth conditions
All Arabidopsis thaliana lines used were in the Columbia (Col-0) background except Ler ecotype used to generate mapping population for map-based gene cloning. The wild-type, Col:FRISf2 (FRI Col) have been previously described34. hsfb1, hsfb2b, and hsfb1 hsfb2b mutants have been previously described29.
To produce pHsfB2b::HsfB2b-eGFP construct, the genomic sequences including 2624 bp upstream of the promoter and the whole coding sequence of HsfB2b were amplified by polymerase chain reaction. The fragment was cloned into pCR2.1-TOPO vector, then fused in-frame to pCAMBIA1300 vector containing eGFP. The construct was transformed into the hov1 mutant. To produce pHsfB2b::Hsfb2b-myc, the 3 kb HsfB2b promoter and the HsfB2b-coding sequence were amplified and fused in-frame to pPZP221 vector containing 4xmyc (EQKLISEEDL). The construct was transformed into the indicated lines using Agrobacterium (Agrobacterium tumefaciens)-mediated Arabidopsis floral dip method55.
The plants were grown under 16 h/8 h light/dark cycle (long day) or 8 h/16 h light/dark cycle (short day) (22 °C/20 °C) in a controlled growth room with cool white fluorescent lights (125 μmol m−2 s−1). Vernalization treatments were done as previously described36. Nonvernalized seedlings were grown for 11 d. For 10V, 20V, 40V treatments, seedlings were grown for 10, 9, 7 d under short days respectively after germination, then transferred to vernalization chamber at 4 °C. After vernalization treatment, seedlings were sampled or transplanted to the soil. Flowering time was measured by counting the number of rosette leaves when the first flower opened using at least 20 plants.
Devernalization treatment was performed following the previously described method with some modifications56. Seeds were sown on a round plate containing half-strength Murashige and Skoog medium with 1% sucrose in 1% agar. The plates were wrapped with aluminum foils for complete darkness and placed at 4 °C for vernalization treatment. After 40 days of vernalization treatment, plates were transferred to heating incubator (30 °C) for additional 7 days, or placed at room temperature. Then, aluminum foils were peeled from the plates, and the plates were placed at room temperature. After 10 days of growth, seedlings were sampled or transplanted to the soil for further growth. All the plant materials and methods used in the current study were carried out following relevant institutional, national, and international guidelines and legislation.
EMS mutagenesis and positional cloning
EMS mutagenesis was performed as previously described57. For the positional cloning of the causative gene of hov1, F2 progenies were obtained by crossing hov1 to Ler. Mapping procedure was followed using 135 GUS-hypersensitive F2 plants and molecular makers described as previous reports58,59. After rough mapping, the genomes of hov1 and the parental − 0.2 kb pVIN3_U_I::GUS were sequenced and compared by illumina Hiseq2000 platform (illumina) sequencing to find mutant-specific SNPs in hov1 using BGI services.
Histochemical GUS staining
GUS staining was done following the standard methods that have been previously described60. Photographs were taken with a USB digital‐microscope Dimis‐M (Siwon Optical Technology, South Korea).
Quantitative PCR
For real-time quantitative PCR, total RNA was isolated using TRIzol solution (Sigma). Four micrograms of total RNA were treated with recombinant DNase I (TaKaRa, 2270A) to eliminate genomic DNA. cDNA was generated using the RNA with reverse transcriptase (Thermo scientific, EP0441) and oligo(dT). Quantitative PCR was performed using the 2 × SYBR Green SuperMix (Bio-Rad 170-8882) and monitored by the CFX96 real-time PCR detection system. The relative transcript level of each gene was determined by normalization of the resulting expression levels compared to that of UBC. The primer sequences used in real-time RT-PCR analyses were shown in Supplementary Table S1.
Immunoblotting
For immunoblot assay, the seedlings of pHsfB2b::HsfB2b-eGFP were harvested at each time point. Total proteins were prepared from 100 mg of harvested samples in protein extraction buffer (50 mM Tris–Cl pH 7.5, 150 mM NaCl, 10 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM 1,4-Dithiothreitol (DTT), 1Χ complete Mini, and EDTA-free protease inhibitor cocktail (Roche). Total proteins were separated by sodium-dodecyl sulfate (SDS)-PAGE. For phosphatase assay, total proteins were treated with or without alkaline phosphatase (Thermo scientific, EF0652) for 1 h at 37 °C, then separated by SDS-PAGE. The proteins were transferred to PVDF membranes (Amersham Biosciences) and probed with anti-GFP (Clontech, JL-8, 1:10,000 dilution) or anti-myc (Santa Cruz Biotechnology, sc-40, 1:10,000 dilution) antibodies overnight at 4 ℃. The samples were then probed with horseradish peroxidase-conjugated anti-mouse IgG (Cell Signaling, #7076, 1:10,000 dilution) antibodies at room temperature. The signals were detected using ImageQuant LAS 4000 (GE Healthcare) with WesternBrightTM Sirius ECL solution (Advansta).
Confocal laser-scanning microscopic (CLSM) analysis
For microscopic observations, 5-day-old pHsfB2b::HsfB2b-eGFP seedlings with or without 5 days of additional cold treatment were prepared. Seedlings were pre-stained with propidium iodide (PI), mounted on glass slides, and observed using confocal microscopy (LSM700, Zeiss) following the manufacturer's instructions.
Promoter analysis
The promoter sequences from plant species were downloaded from GBrowse at Phytozome (phytozome-next.jgi.doe.gov). The following VIN3 loci (At5g57380) were identified using BLAST Search: Arabidopsis lyrata (AL8G33360), Boechera stricta (Bostr.26833s0518) and Capsella rubella (Carub.0008s1790). The sequences were processed and aligned in T-coffee (tcoffee.crg.eu).
Yeast one-hybrid assay
Yeast one-hybrid assay was performed following the previously described method with some modifications15. For the reporter constructs used in the Y1H analysis, four tandem repeats containing HSEVIN3 (5′-TTAGAAACATCTAGAAAAAACAAA-3′) were cloned into the pHisi vector. For the effector, the coding sequences of HsfA1a, HsfA2, HsfA4a, HsfA6a, HsfA8, HsfB1, HsfB2b and HsfC1 were cloned in-frame with the sequences of the GAL4 activation domain into pGADT7. The Y1H assay was performed following the manufacturer’s instructions. In brief, the reporter construct and effector construct were transformed into yeast strain YM4271. The yeast cells were spotted on synthetic define (SD) medium lacking Leu, Ura, and His, with or without 5-mM 3-amino-1,2,4-triazole (3-AT).
Preparation of fusion protein and electrophoretic mobility shift assays (EMSA)
Coding sequence encoding DNA binding domain of HsfB2b was fused to pMAL-c2 vector. The MBP and MBP-HsfB2bDBD proteins were expressed in Escherichia coli BL21 strain according to the manufacturer’s instructions using the pMAL Protein Fusion and Purification System (#E8200; New England BioLabs) and purified using MBPtrap HP column (Cytiva) attached to ÄKTA FPLC system (Cytiva). The Cy5-labeled probes (HSE, 5′-Cy5- TTTCCTCCTTAGAAACATCTAGAAAAAACAAAAGGAGAGA-3′; mHSE, 5′-Cy5- TTTCCTCCTTAAAAACATTTAAAAAAAACAAAAGGAGAGA -3′) and unlabeled competitors were generated by annealing 40 bp-length oligonucleotides. 5 μM of purified proteins and 100 nM of Cy5-labeled probe were incubated at room temperature in binding buffer (10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 5% glycerol and 5 mM DTT). For competition assay, 100-fold molar excess of each competitor was added to the reaction mixture before incubation. The reaction mixtures were resolved by electrophoresis through 6% polyacrylamide gel in 0.5X Tris–borate EDTA buffer at 100 V. The Cy5 signals were detected using WSE-6200H LuminoGraph II (ATTO).
Chromatin Immunoprecipitation
Approximately 4 g of whole Arabidopsis seedlings were collected and cross-linked using 1% (v/v) formaldehyde for 10 min and quenched by 0.125 M glycine for 5 min under vacuum. Seedlings were rinsed with distilled water, frozen in liquid nitrogen, and grounded to fine powder. The powder was resuspended in Nuclei Isolation Buffer [1 M hexylene glycol, 20 mM PIPES-KOH (pH 7.6), 10 mM MgCl2, 15 mM NaCl, 1 mm EGTA, 1 mM PMSF, complete protease inhibitor mixture tablets (Roche)], and Arabidopsis nuclei were isolated by centrifugation, lysed by Nuclei Lysis Buffer [50 mM TRIS–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% SDS], and sonicated using a Branson sonifier to shear the DNA. Sheared chromatin solution was diluted tenfold with a ChIP Dilution Buffer [50 mM TRIS–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA]. The beads, chromatins and GFP-Trap A beads (gta-20, ChromoTek, Planegg, Germany) or Binding control agarose (bab-20) were mixed and incubated for overnight at 4 °C. Beads were washed with ChIP dilution buffer for 4 times and DNA extraction was performed using Chelex 100 resin following the manufacturer’s instruction. qPCR analysis was performed using 1% input and immunoprecipitated DNA.
Accession numbers
The Arabidopsis Genome Initiative locus identifiers for the genes discussed in this paper are as follows: VIN3 (At5g57380), FLC (At5g10140), FRI (At4g00650), HsfA1a (At4g17750), HsfA2 (At2g26150), HsfA3 (At5g03720), HsfA4a (At4g18880), HsfA6a (At5g43840), HsfA8 (At1g67970), HsfB1 (At4g36990), HsfB2a (At5g62020), HsfB2b (At4g11660), HsfC1 (At3g24520), CCA1 (At2g46830), LHY (At1g01060), and PP2A (At1g13320).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Chouard, P. Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 11, 191–238. https://doi.org/10.1146/annurev.pp.11.060160.001203 (1960).
Michaels, S. D. & Amasino, R. M. Memories of winter: Vernalization and the competence to flower. Plant Cell Environ. 23, 1145–1153. https://doi.org/10.1046/j.1365-3040.2000.00643.x (2000).
Sheldon, C. C. et al. The FLF MADS box gene: A repressor of flowering in arabidopsis regulated by vernalization and methylation. Plant Cell 11, 445–458. https://doi.org/10.1105/tpc.11.3.445 (1999).
Michaels, S. D. & Amasino, R. M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956. https://doi.org/10.1105/tpc.11.5.949 (1999).
Qüesta Julia, I., Song, J., Geraldo, N., An, H. & Dean, C. Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science 353, 485–488. https://doi.org/10.1126/science.aaf7354 (2016).
Yuan, W. et al. A cis cold memory element and a trans epigenome reader mediate Polycomb silencing of FLC by vernalization in Arabidopsis. Nat. Genet. 48, 1527–1534. https://doi.org/10.1038/ng.3712 (2016).
Yang, H. et al. Distinct phases of Polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science 357, 1142–1145. https://doi.org/10.1126/science.aan1121 (2017).
Sung, S. & Amasino, R. M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427, 159–164. https://doi.org/10.1038/nature02195 (2004).
De Lucia, F., Crevillen, P., Jones, A. M. E., Greb, T. & Dean, C. A PHD-Polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. 105, 16831. https://doi.org/10.1073/pnas.0808687105 (2008).
Wood, C. C. et al. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. 103, 14631–14636. https://doi.org/10.1073/pnas.0606385103 (2006).
Sung, S. et al. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 38, 706–710. https://doi.org/10.1038/ng1795 (2006).
Bond, D. M., Dennis, E. S., Pogson, B. J. & Finnegan, E. J. Histone acetylation, VERNALIZATION INSENSITIVE 3, FLOWERING LOCUS C, and the vernalization response. Mol. Plant 2, 724–737. https://doi.org/10.1093/mp/ssp021 (2009).
Bond, D. M., Wilson, I. W., Dennis, E. S., Pogson, B. J. & Jean Finnegan, E. VERNALIZATION INSENSITIVE 3 (VIN3) is required for the response of Arabidopsis thaliana seedlings exposed to low oxygen conditions. Plant J. 59, 576–587. https://doi.org/10.1111/j.1365-313X.2009.03891.x (2009).
Zhao, Y., Antoniou-Kourounioti, R. L., Calder, G., Dean, C. & Howard, M. Temperature-dependent growth contributes to long-term cold sensing. Nature 583, 825–829. https://doi.org/10.1038/s41586-020-2485-4 (2020).
Kyung, J. et al. The two clock proteins CCA1 and LHY activate VIN3 transcription during vernalization through the vernalization-responsive cis-element. Plant Cell https://doi.org/10.1093/plcell/koab304 (2021).
Hepworth, J. et al. Absence of warmth permits epigenetic memory of winter in Arabidopsis. Nat. Commun. 9, 639. https://doi.org/10.1038/s41467-018-03065-7 (2018).
Antoniou-Kourounioti, R. L. et al. Temperature sensing is distributed throughout the regulatory network that controls FLC epigenetic silencing in vernalization. Cell Syst. 7, 643-655.e649. https://doi.org/10.1016/j.cels.2018.10.011 (2018).
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 40, 253–266. https://doi.org/10.1016/j.molcel.2010.10.006 (2010).
Feder, M. E. & Hofmann, G. E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. https://doi.org/10.1146/annurev.physiol.61.1.243 (1999).
Wu, C. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11, 441–469. https://doi.org/10.1146/annurev.cb.11.110195.002301 (1995).
Enoki, Y. & Sakurai, H. Diversity in DNA recognition by heat shock transcription factors (HSFs) from model organisms. FEBS Lett. 585, 1293–1298. https://doi.org/10.1016/j.febslet.2011.04.014 (2011).
Busch, W., Wunderlich, M. & Schöffl, F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 41, 1–14. https://doi.org/10.1111/j.1365-313X.2004.02272.x (2005).
Amin, J., Ananthan, J. & Voellmy, R. Key features of heat shock regulatory elements. Mol. Cell Biol. 8, 3761–3769. https://doi.org/10.1128/mcb.8.9.3761-3769.1988 (1988).
Nover, L. et al. The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones 1, 215–223. https://doi.org/10.1379/1466-1268(1996)001 (1996).
Nover, L. et al. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need?. Cell Stress Chaperones 6, 177–189. https://doi.org/10.1379/1466-1268(2001)006 (2001).
von Koskull-Döring, P., Scharf, K.-D. & Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 12, 452–457. https://doi.org/10.1016/j.tplants.2007.08.014 (2007).
Andrási, N., Pettkó-Szandtner, A. & Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 72, 1558–1575. https://doi.org/10.1093/jxb/eraa576 (2021).
Ikeda, M. & Ohme-Takagi, M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 50, 970–975. https://doi.org/10.1093/pcp/pcp048 (2009).
Ikeda, M., Mitsuda, N. & Ohme-Takagi, M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs But positively regulate the acquired thermotolerance. Plant Physiol. 157, 1243–1254. https://doi.org/10.1104/pp.111.179036 (2011).
Kumar, M. et al. Heat shock factors HsfB1 and HsfB2b Are involved in the regulation of Pdf1.2 expression and pathogen resistance in arabidopsis. Mol. Plant 2, 152–165. https://doi.org/10.1093/mp/ssn095 (2009).
Kolmos, E., Chow, B. Y., Pruneda-Paz, J. L. & Kay, S. A. HsfB2b-mediated repression of PRR7 directs abiotic stress responses of the circadian clock. Proc. Natl. Acad. Sci. 111, 16172. https://doi.org/10.1073/pnas.1418483111 (2014).
Evrard, A. et al. Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. PeerJ 1, e59. https://doi.org/10.7717/peerj.59 (2013).
O’Malley, R. C. et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165, 1280–1292. https://doi.org/10.1016/j.cell.2016.04.038 (2016).
Lee, I., Michaels, S. D., Masshardt, A. S. & Amasino, R. M. The late-flowering phenotype of FRIGIDA and mutations in LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J. 6, 903–909. https://doi.org/10.1046/j.1365-313X.1994.6060903.x (1994).
Lee, J., Yun, J.-Y., Zhao, W., Shen, W.-H. & Amasino, R. A methyltransferase required for proper timing of the vernalization response in Arabidopsis. In Proceedings of the National Academy of Sciences of the United States of America 112, https://doi.org/10.1073/pnas.1423585112 (2015).
Kim, D.-H. & Sung, S. The binding specificity of the PHD-finger domain of VIN3 moderates vernalization response. Plant Physiol. 173, 1258–1268. https://doi.org/10.1104/pp.16.01320 (2017).
Kuittinen, H., Niittyvuopio, A., Rinne, P. & Savolainen, O. Natural Variation in Arabidopsis lyrata Vernalization Requirement Conferred by a FRIGIDA Indel polymorphism. Mol. Biol. Evol. 25, 319–329. https://doi.org/10.1093/molbev/msm257 (2008).
Anderson, J. T., Lee, C.-R. & Mitchell-Olds, T. Life-history QTLS and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution 65, 771–787. https://doi.org/10.1111/j.1558-5646.2010.01175.x (2011).
Guo, Y.-L., Todesco, M., Hagmann, J., Das, S. & Weigel, D. Independent FLC mutations as causes of flowering-time variation in Arabidopsis thaliana and Capsella rubella. Genetics 192, 729–739. https://doi.org/10.1534/genetics.112.143958 (2012).
Purvis, O. N. & Gregory, F. G. Devernalization by high temperature. Nature 155, 113–114. https://doi.org/10.1038/155113a0 (1945).
Gregory, F. G. & Purvis, O. N. Reversal of vernalization by high temperature. Nature 161, 859–860. https://doi.org/10.1038/161859a0 (1948).
Bouché, F., Detry, N. & Périlleux, C. Heat can erase epigenetic marks of vernalization in Arabidopsis. Plant Signal. Behav. 10, e990799. https://doi.org/10.4161/15592324.2014.990799 (2015).
Yoshida, T. et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genom. 286, 321–332. https://doi.org/10.1007/s00438-011-0647-7 (2011).
Bechtold, U. et al. Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and infection. J. Exp. Bot. 64, 3467–3481. https://doi.org/10.1093/jxb/ert185 (2013).
Nishizawa, A. et al. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 48, 535–547. https://doi.org/10.1111/j.1365-313X.2006.02889.x (2006).
Ogawa, D., Yamaguchi, K. & Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 58, 3373–3383. https://doi.org/10.1093/jxb/erm184 (2007).
Banti, V., Mafessoni, F., Loreti, E., Alpi, A. & Perata, P. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 152, 1471–1483. https://doi.org/10.1104/pp.109.149815 (2010).
Sabehat, A., Lurie, S. & Weiss, D. Expression of small heat-shock proteins at low temperatures. A possible role in protecting against chilling injuries. Plant Physiol. 117, 651–658. https://doi.org/10.1104/pp.117.2.651 (1998).
Ul Haq, S. et al. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20215321 (2019).
Swindell, W. R., Huebner, M. & Weber, A. P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 8, 125–125. https://doi.org/10.1186/1471-2164-8-125 (2007).
Minkoff, B. B., Stecker, K. E. & Sussman, M. R. Rapid phosphoproteomic effects of abscisic acid (ABA) on Wild-Type and ABA receptor-Deficient A. Thaliana Mutants. Mol. Cell Proteom. 14, 1169–1182. https://doi.org/10.1074/mcp.M114.043307 (2015).
Lång, V., Heino, P. & Palva, E. T. Low temperature acclimation and treatment with exogenous abscisic acid induce common polypeptides in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genetics 77, 729–734. https://doi.org/10.1007/BF00261251 (1989).
Heino, P., Sandman, G., Lång, V., Nordin, K. & Palva, E. T. Abscisic acid deficiency prevents development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genetics 79, 801–806. https://doi.org/10.1007/BF00224248 (1990).
Shalit-Kaneh, A., Kumimoto Roderick, W., Filkov, V. & Harmer Stacey, L. Multiple feedback loops of the Arabidopsis circadian clock provide rhythmic robustness across environmental conditions. In Proceedings of the National Academy of Sciences 115, 7147–7152, doi:https://doi.org/10.1073/pnas.1805524115 (2018).
Clough, S. J. & Bent, A. F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x (1998).
Périlleux, C. et al. A root chicory MADS box sequence and the Arabidopsis flowering repressor FLC share common features that suggest conserved function in vernalization and de-vernalization responses. Plant J. 75, 390–402. https://doi.org/10.1111/tpj.12208 (2013).
Kim, Y., Schumaker, K. S. & Zhu, J. K. EMS mutagenesis of Arabidopsis. Methods Mol. Biol. 323, 101–103. https://doi.org/10.1385/1-59745-003-0:101 (2006).
Lukowitz, W., Gillmor, C. S. & Scheible, W.-R.D. Positional cloning in arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol. 123, 795–806. https://doi.org/10.1104/pp.123.3.795 (2000).
Hou, X. et al. A platform of high-density INDEL/CAPS markers for map-based cloning in Arabidopsis. Plant J. 63, 880–888. https://doi.org/10.1111/j.1365-313X.2010.04277.x (2010).
Shin, J., Jeong, G., Park, J.-Y., Kim, H. & Lee, I. MUN (MERISTEM UNSTRUCTURED), encoding a SPC24 homolog of NDC80 kinetochore complex, affects development through cell division in Arabidopsis thaliana. Plant J. 93, 977–991. https://doi.org/10.1111/tpj.13823 (2018).
Acknowledgements
We would like to thank Dr Masaru Ohme-Takagi for providing the hsfb1, hsfb2b and hsfb1 hsfb2b seeds. This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development (No. PJ01315201 and PJ01315401), Rural Development Administration, Republic of Korea, and of the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. 2019R1A2C2004313). This work was also supported by NRF Grant funded by the Ministry of Science, Republic of Korea (NRF-2021R1A5A1032428). G. Jeong were supported by the Brain Korea 21 Plus Project of the NRF Grant and was also supported by the Stadelmann-Lee Scholarship Fund, Seoul National University, Seoul, Korea.
Author information
Authors and Affiliations
Contributions
G.J., J.S. and I.L. conceived and designed the experiments; J.S. and G.J. performed the experiments. G.J., M.J., J.S. and I.L. performed data analysis. G.J. and I.L. prepared the manuscript. All authors have contributed in writing the manuscript and have read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, G., Jeon, M., Shin, J. et al. HEAT SHOCK TRANSCRIPTION FACTOR B2b acts as a transcriptional repressor of VIN3, a gene induced by long-term cold for flowering. Sci Rep 12, 10963 (2022). https://doi.org/10.1038/s41598-022-15052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15052-6
This article is cited by
-
Mining genomic regions associated with agronomic and biochemical traits in quinoa through GWAS
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.