Abstract
Multiple heavy metal pollution in mangrove wetlands is serious. Kandelia obovata seedlings were cultured in pots in which lead (Pb), zinc (Zn) and copper (Cu) were added separately and in combinations. The results showed that heavy metal stress improved the rate of root oxygen leakage, enhanced root activity, and reduced root porosity. The root under single heavy metal stress was impacted by the addition of other heavy metals, demonstrating antagonistic or synergistic effects. Iron plaque (IP) formation was improved under single Zn or Cu stress, and inhibited in binary stress of Pb + Cu. The adsorptions of IP on heavy metals in combined stress (Pb, 62–116 μg g−1; Zn, 194–207 μg g−1; Cu, 35–52 μg g−1) were higher than that in single stress (Pb, 18 μg g−1; Zn, 163 μg g−1; Cu, 22 μg g−1). K. obovata accumulated higher levels of heavy metals in root (Pb, 7–200 μg g−1; Cu, 4–78 μg g−1), compared with IP (Pb, 18–116 μg g−1; Cu, 22–52 μg g−1), stem (Pb, 3–7 μg g−1; Cu, 9–17 μg g−1), and leaf (Pb, 2–4 μg g−1; Cu, 4–7 μg g−1). Correlation analysis showed that single and binary stresses affected K. obovata, with more significant effect of trinary stress. Regression path analysis showed that multiple heavy metal stress firstly affected root, then indirectly contributed to IP formation, as well as heavy metal in IP and root; at last, heavy metal in IP directly contributed to heavy metal bioaccumulations in root.
Similar content being viewed by others
Introduction
Mangrove is important for maintaining ecological balance and biodiversity in coastal zone, being mainly featured with low tide current velocity, high organic matter deposition, and reducibility in sediment1,2. To overcome the harsh hypoxia intertidal environment, aerenchyma (root porosity) is developed in mangrove plant to transfer oxygen from aboveground part to root, and release oxygen into the rhizosphere environment, being known as radial oxygen loss (ROL). ROL can affect nutrient uptake and heavy metal tolerance, alter microbial activity and chemical process in the rhizosphere environment, changing the bioavailability of heavy metal through iron plaque (IP) formation on the root surface3,4. IP formation acts as “barrier” or “reservoir” to regulate the transfers of heavy metals in plants, being attributed to the status of IP formation5,6. On the other hand, IP formation would be affected by heavy metal stress through the following ways: (1) causing oxidative stress and production of peroxide free radical which oxidizes Fe2+ to Fe3+7; (2) altering the ROL and anatomical structure of root (thickened exodermis, enhanced lignification, and reduced aerenchyma)8; (3) affecting sediment physic-chemical properties and microbial community to alter the availability of Fe2+ in IP formation9.
The responses of root and IP formation in terms of single heavy metal stress have been widely reported10,11,12. In actual field environment, several heavy metals coexist normally, and single heavy metal could not really reflect the true occurrence characteristics and biological toxicity of multiple heavy metals due to their complex interactions13. Furthermore, combined pollution of heavy metals and other substances could impact the iron plaque formation and heavy metal absorption in plants (Table S1). Owing to urbanization, coastal landfill, and aquaculture, mangroves have inevitably suffered from ubiquitous heavy metals such as Pb, Zn and Cu14,15,16. As for combined heavy metal stress of Pb, Zn, and Cu, the metal tolerance of mangrove species is ascribed to lignification/suberization deposition within root exodermis, which reduces ROL, and delays the uptake of heavy metals in mangrove root17. Our previous studies have found that trinary combined Pb, Zn, and Cu could affect physiological characteristics, improve IP formation and heavy metal deposition on the root surface of Kandelia obovata, without exploring the interactions among various heavy metals18,19. The effects of single, binary, and trinary stress of Pb, Zn and Cu on physiological responses of K. obovata are also intensively studied, with synergy and antagonism to be detected20. However, the internal influences of apparent responses are not included, especially for heavy metal bioaccumulation. In fact, Zn has the same family, as well as similar ion radius and chemical property with Cd, being more mobile than that of Pb and Cu. Nowadays, the interactive effects of multiple heavy metals in modulating root response, IP formation, and metal adsorption are still ambiguous. It is hypothesized that multiple heavy metals would demonstrate more significant effect than a single metal in affecting root response, thus changing IP formation and heavy metal bioaccumulations. Thus, systematic exploration is conducted on K. obovata in regards to single, binary, and trinary metal stresses. This study aims to (1) explore the characteristics of root responses, IP formation and heavy metal bioaccumulation under multiple heavy metals; (2) clarify the interrelations among single, binary, and trinary stresses, as well as the inner links among root responses, IP formation and heavy metal bioaccumulation.
Results
Root characteristics of Kandelia obovata
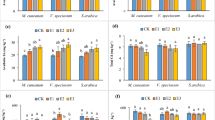
In this study, RROL did not change significantly in single stress compared to the control (P > 0.05, Fig. 1A). Higher level of RROL was detected in Pb + Cu binary stress compared to single Cu/Pb stress (P < 0.05). While, RROL reduced significantly in trinary stress compared to binary stresses of Pb + Cu and Zn + Cu (P < 0.05). In Fig. 1B, no significant change of root activity was detected in single stress (P > 0.05), with improved root activity under binary stresses (P < 0.05). However, root activity reduced significantly in trinary stress compared to binary stresses of Pb + Cu and Zn + Cu (P < 0.05). In Fig. 1C, the root porosity reduced from 44.37 to 34.44% in single Cu stress (P < 0.05). Binary stresses of Pb + Zn and Pb + Cu reduced root porosity compared to single Pb stress (P < 0.05), with no significant reduce for single Zn or Cu stress (P > 0.05). Moreover, no significant change of root porosity was detected in trinary stress compared to binary stresses (P > 0.05).
IP formation and heavy metal immobilizations in Kandelia obovata
IP increased significantly under single Zn or Cu stress (P < 0.05, Fig. 2A), and binary Zn + Cu stress reduced Fe concentration significantly (P < 0.05). Moreover, binary stresses of Pb + Cu and Pb + Zn significantly reduced Fe concentration in single Cu and Zn stress, respectively (P < 0.05). Trinary stress significantly improved Fe concentration compared to binary Pb + Cu stress (P < 0.05). In Fig. 2B–D, heavy metal stress improved the immobilizations of Pb, Zn, and Cu in IP (P < 0.05); No significant change of heavy metal in IP was detected among single and binary stresses (P > 0.05); Trinary stress significantly improved the immobilizations of Pb and Cu in IP (P < 0.05). In combined stress, heavy metals on IP (Pb, 62–116 μg g−1; Zn, 194–207 μg g−1; Cu, 35–52 μg g−1) were higher than that in single stress (Pb, 18 μg g−1; Zn, 163 μg g−1; Cu, 22 μg g−1).
Bioaccumulation and transfer of heavy metals in Kandelia obovata
As shown in Fig. 3, the bioaccumulations of heavy metals in roots were higher than that in stem and leaf (P < 0.05). K. obovata mainly accumulated heavy metals in root (Pb, 7–200 μg g−1; Cu, 4–78 μg g−1), compared with IP (Pb, 18–116 μg g−1; Cu, 22–52 μg g−1), stem (Pb, 3–7 μg g−1; Cu, 9–17 μg g−1), and leaf (Pb, 2–4 μg g−1; Cu, 4–7 μg g−1). As for Pb in root, Zn reduced Pb concentration in single Pb stress (P < 0.05); while, significant improvements were detected in trinary stress compared to binary stresses of Pb + Zn and Pb + Cu (P < 0.05). As for Zn in roots, Pb or Cu reduced Zn concentration in single Zn stress (P < 0.05), and trinary stress improved Zn concentration in binary Zn + Cu stress (P < 0.05). As for Cu in roots, no significant difference was detected among multiple heavy metal stresses (P > 0.05). Thus, single heavy metal in K. obovata root was affected by the presence of a second or third heavy metal, leading to the inhibited or increased accumulation of heavy metals.
Furthermore, the transfers of heavy metals from IP to root (TFroot) and from root to leaf (TFleaf) indicated that the interactions of heavy metals on TFroot levels were limited (P > 0.05, Table 1). As for TFleaf, though no significant changes of interactive effects were detected for TFleaf levels of Pb and Cu (P > 0.05), the presence of Cu in binary and trinary stresses improved TFleaf levels of Zn compared to single Zn stress (P < 0.05).
Interactions of root response, IP formation, and metal bioaccumulation
In order to explore interactions of plant responses, pearson correlation analysis was performed (Table 2). In this study, IP formation showed negative correlations with root activity (r = − 0.643, P < 0.01) and RROL(r = − 0.531, P < 0.05), indicating their indirect even reverse interaction relationships. While, positive correlation was detected among IP formation and root porosity though not significant. Root activity was positively and negatively correlated with root ROL (r = 0.758, P < 0.01) and porosity (r = − 0.536, P < 0.01), respectively.
Furthermore, three-way ANOVA analysis indicated that single heavy metal stress affected plant response, except for root ROL, and stem biomass (Table 3). Root activity, root ROL, and IP formation were significantly affected by Pb + Zn (P < 0.01). The effects of Pb + Zn, Zn + Cu, and Pb + Cu on heavy metals in IP, root, stem and leaf were significant (P < 0.01), except for heavy metal in root under Pb + Cu treatment. There were interactive effects of Pb + Zn + Cu on root porosity, root activity, root ROL, IP formation, root biomass, and heavy metals in IP, root, and stem (P < 0.01).
As shown in Fig. 4, the RPA process was conducted among root responses, IP formation and heavy metal bioaccumulation. The results showed that root activity was the main parameter influencing root oxygen leakage (P < 0.01). Root porosity and root oxygen leakage did not have direct impact on IP formation, which also did not significantly affect heavy metal in IP and root (P > 0.05). Heavy metal in IP significantly affected heavy metals in root (P < 0.05), which negatively affected heavy metals in stem (P < 0.05).
Discussion
Root responses under heavy metal stress
Plant biomass acts as a good indicator for the health of K. obovata under heavy metal stress. In the present study, no toxicity symptom in the plant (such as necrosis) was found, indicating higher tolerance of K. obovata in responding to multiple heavy metals. Furthermore, the growths of root and stem were not significantly reduced by heavy metals, with leaf to be improved (especially for binary stress of Pb + Zn), leading to higher root/shoot under heavy metal stress (Figure S1). Under multiple heavy metal stress, K. obovata was expected to spend extra energy for leaf growth and produce more O2, which would be transferred back to the belowground roots to cope with the heavy metal-related effects in rhizosphere environment. In order to explore the interactions of heavy metals on root, various indicators were explored, including root ROL, root activity, root porosity, IP formation, and heavy metal accumulation.
Mangrove plants have evolved aerenchyma in root (being featured with porosity) to adapt to the harsh tidal environment21,22. Generally speaking, root porosity in plant (originated from aerenchyma tissue) was regulated by various stimuli, including ethylene and hypoxia23,24. Under heavy metal stress, root porosity of mangrove plants would reduce regardless of single or combined heavy metals25. In Fig. 1C, root porosity was mainly reduced under heavy metal stress, and no significant interactions were detected among different heavy metal stresses, except for antagonistic effect with binary Pb + Zn stress compared to single Pb stress. Previous study found that root porosity and root ROL were positively correlated in Bruguiera gymnorrhiza, and higher level of root porosity facilitated the loss of oxygen from the root to the rhizosphere environment17. However, in this study, root porosity of K. obovata did not have a similar trend with that of root ROL, and reduced under heavy metal stress, especially for the lowest level in Pb + Cu treatment (Fig. 1A,C). Thus, root porosity was affected not only by single heavy metal application, but also by their combinations.
As for mangrove plants Avicennia marina, Aegiceras corniculatum, and B. gymnorrhiza, the combined stress of Pb, Zn and Cu inhibited seedling growths and reduced root ROL26. Furthermore, under combined stress of Pb, Zn and Cu, the reduced root ROL in B. gymnorrhiza coexisted with metal-induced inhibition of growth and root aeration, which may be, at least partly, related to the damage to root tissues such as aerenchyma17. However, the improved levels of root ROL were detected under binary stresses of Pb + Cu and Zn + Cu (Fig. 1), demonstrating plant species-specific characteristics in response to heavy metal stresses. As for root ROL and root activity, there were synergistic effects with binary stress of Pb + Cu compared to corresponding single metal stress, with the same effect for root activity under binary stress of Zn + Cu; while, antagonistic effects were detected in trinary stress of Pb + Cu + Zn compared to binary stresses of Pb + Cu and Zn + Cu (Fig. 1). The improved root ROL under heavy metal stress was expected to be beneficial for maintaining the oxidative condition, reducing the combination among metal and sulfur, and increasing the bioavailability of heavy metals. Under heavy metal stress, K. obovata would mainly promote root activity to increase root ROL, not by increasing root porosity (Fig. 1). The possible explanation may be that at least part of oxygen was transported to belowground roots for aerobic metabolism instead of spreading into the rhizosphere environment, which deserved further investigation.
IP formation under heavy metal stress
Generally speaking, IP formation is an adaptive behavior of plants to stressful environment11,27. Previous studies have found that ROL in plant root was important in affecting IP formation, and heavy metal stress inhibited oxygen release from roots to reduce IP formation10,28. Heavy metals affected IP formation in wetland plants, with single Cd to improve IP formation in K. obovata and Avicennia marina4,7. In this study, Pb reduced IP formation under single Zn or Cu stress, and Zn improved IP formation in binary stress of Pb + Cu (Fig. 2A). Thus, the interactions of heavy metals on IP formation were heavy metal type and combination-specific to some extent. On the other hand, the response of IP formation was not similar to that of root ROL, root activity, and root porosity (Fig. 1), which was also verified by their insignificant (r = 0.297, P > 0.05) or negative (r = − 0.643, − 0.531, P < 0.01) correlations (Table 2). In particular, the negative correlation between IP formation and root ROL in this study may be due to the formation of oxygen permeation barrier affecting root ROL29. Yang et al. (2012) also found that the highest level of root ROL and lowest thickness of IP coexisted in Veronica serpyllifolia30. Thus, it was expected that the roles of root ROL, root activity, and root porosity on IP formation of K. obovata were limited, and some other factors should be investigated further. In fact, apart from root ROL, there were also some biological and abiotic factors affecting IP formation, including microorganism, root exudate, Fe2+ activity, and soil moisture31,32.
Heavy metal bioaccumulation under heavy metal stress
Nowadays, the role of IP on heavy metal absorption and transfer in plants is inconclusive, with IP to promote or prevent the absorption of heavy metals in rhizosphere environment5,12,33. This study showed that IP formation was only improved under single Zn or Cu stress, the heavy metals immobilized in IP were all improved, and trinary stress improved the immobilizations of Pb and Cu (not Zn) compared to binary stress (Fig. 2). Huang et al. (2012) also reported that IP formation on plant root especially for rice would age/decompose during the whole growth process34. As for wetland plants, low IP formation coexisted with high root activity35. Root activity was negatively correlated with IP formation (Table 2). Thus, it was not always that more IP formation coexisted with more heavy metal immobilization, which would be affected by IP vitality, especially for higher root activity under binary stresses of Pb + Cu and Zn + Cu.
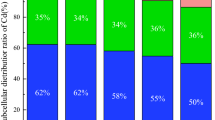
IP can combine with heavy metals and nutrients by adsorption and co-precipitation, affecting their distribution and accumulation in plants5,19,36. As showed in Fig. 3 and Table 1, heavy metals were mainly distributed in roots (especially for Pb and Cu), which were also verified by their lower levels of TFleaf. The phytostabilization of Zn in root was limited, with more distribution to be detected in stem and leaf compared to Pb and Cu (Fig. 3), which was also verified by higher TFleaf of Zn (Table 1). In fact, the similar physic-chemical properties of Zn with highly mobile Cd (such as valence state and iron radius) resulted to the absorption and transfer of Zn into the aboveground parts13,37,38. Under combined heavy metal stress, IP formation affected the transfer of Pb from sediment to rice, instead of Cd or Cu39. As for Pb and Zn in roots (Fig. 2), binary stress demonstrated antagonistic effect compared with single stress, and trinary stress resumed heavy metal accumulation compared to binary stress. While, such trends were not significant for Cu accumulated in root. As for allocation strategies of heavy metals between IP and root (Table 1), Cu and Pb were mainly distributed in roots with high TFroot levels (> 1), and Zn mainly accumulated in IP with lower TFroot levels (< 1). Thus, the interactions of heavy metals on allocation strategies between IP and root were limited.
In order to fully explore the interactive effects of single, binary, and trinary heavy metals, pearson correlation analysis was performed (Table 2). The results indicated that single and binary stresses had impact on plant responses to some extent, with more significant effect of trinary stress. Furthermore, RPA process was conducted to comprehensively explore root responses, and variations of IP formation and heavy metal bioaccumulations caused by multiple heavy metals (Fig. 4). PRA process was widely applied in exploring relationships among various parameters from an overall perspective40,41,42. In this study, multiple heavy metal stress firstly affected root responses, which indirectly contributed to IP formation on root, as well as heavy metal accumulations in IP and root; while heavy metals in IP directly contributed to their bioaccumulations in root, reducing their transfers to aboveground parts.
Conclusions
This study mainly explored the interactions of Pb, Zn, and Cu on root growth, IP formation, and heavy metal bioaccumulation in Kandelia obovata. The results showed that the root ROL, root activity, root porosity, and IP formation under heavy metal stress were affected with the presence of other metals. Furthermore, the changes of root ROL and root activity were not inhibited under the presence of heavy metals, being different from the reducing root porosity. Thus, heavy metal stress would change root ROL through affecting root activity of K. obovata. The adsorption of IP on heavy metals in combined stress was higher than that in single stress. Most heavy metals were stabilized in belowground parts (especially for Pb and Cu), with limited interactions of heavy metals on their allocations between root and IP. Furthermore, the phytoextraction of Zn in leaf was higher than that of Pb and Cu, which was improved with the presence of Cu in binary and trinary stresses. Single and binary heavy metal stresses affected plant responses, with more significant effect to be detected in trinary stress. Overall, multiple heavy metals affected root responses, indirectly impacted IP formation, and heavy metals in IP and root, and directly impacted on heavy metal in plant, especially for root.
Methods
Materials preparation and treatments
Materials preparation
The propagules of K. obovata and sediment for plant culture were collected from mangrove wetlands in Futian National Nature Reserve, Shenzhen, China (114° 00′–114° 02′ E, 22° 30′–22° 32′ N). In details, propagules (20 cm tall with no fungi infections and insect damages) were planted into the seedbed (50 × 40 × 15 cm, length, width, height) filled with clean sand. The seedbed was irrigated with 1/2-strength Hoagland’s nutrient solution (500 mL, 5‰ NaCl, pH 6.5). During the experiment, the deionized water was provided to ensure the moist every morning as previous reports19,20. After 2 months, the seedlings were prepared for pot experiment with combined heavy metal stresses.
Experiment design
The sediment matrix was mixed fully and separated into 8 groups (Table 4), including control, Pb, Zn, Cu, Pb + Zn, Pb + Cu, Zn + Cu, and Pb + Zn + Cu. The background physicochemical properties of sediment matrix were: moisture, 61.2%; Eh, –204.2 mV; pH, 6.7; salinity, 13‰; TOC, 5.5%; EC, 21.3 mS cm–1; Pb, 88.55 μg g−1; Zn, 216.03 μg g−1; Cu, 65.53 μg g−119,20. PbCl2, ZnCl2, and CuCl2 were applied into sediments based on the actual heavy metal pollution status in mangrove wetlands17,43. Chemical reagents (guaranteed reagent, GR) were firstly dissolved in deionized water, and then homogenized with the sediment matrix. The concentrations of Pb, Zn and Cu in different treated sediment matrix were presented in Table 4. Furthermore, the sediment matrix was kept fresh by irrigating deionized water and mixed every week19,20. After 2 months, the sediment matrix was used for pot experiments. A total of 32 pots were used (19.0 × 18.0 cm, diameter, height). In each pot, air-dried sediment (3 kg) was filled in and a nylon net (16.0 × 16.0 cm length, width; 500 mesh) was installed. Three uniform seedlings of K. obovata were transplanted into the nylon net installed in the pot. The nylon net could restrict root growth and help the roots to be sampled from sediment44.
Management
All the experimental pots were placed outdoors randomly and were protected from the rain by a transparent canopy. The temperatures in the summer and autumn were 26–32 °C and 22–31 °C, respectively. The pots were irrigated with deionized water to compensate for evaporation loss of water every morning19,20. The plant culture and experimental implementation were shown in Figure S2.
Sampling and determination
After 5 months, the seedlings of K. obovata were collected, which were firstly separated (roots, stems, and leaves) and then dried at 70 °C for two days to obtain the constant weight. Some fresh roots were used to determine root ROL, root activity, root porosity, and IP formation. The root activity was determined by triphenyl tetrazolium chloride (TTC), which was commonly used in studies on plant stress45.
Determination of root ROL
ROL was determined colorimetrically22,46, which can be expressed as follows:
where rate of ROL (RROL) is the rate of radial oxygen loss (μmol O2 kg−1 root d.w. h−1); c is the initial volume of Ti3+-citrate added to each tube (L); y is the concentration of Ti3+-citrate solution of control (without plants) (μmol Ti3+ L−1); z is the concentration of Ti3+-citrate solution (μmol Ti3+ L−1); g is root dry weight after drying at 70 °C for 72 h (in kg).
Determination of root porosity
Root porosity (% gas volume/root volume) was measured for the entire lateral roots by a pycnometer method47,48, which can be expressed as follows:
where FA is the mass of pycnometer with water and vacuumed roots; FB is the mass of pycnometer with water and fresh roots in g; FW is the mass of water-filled pycnometer in g; TW is the mass of fresh roots in g.
Determination of iron plaque and heavy metals
The cold dithionite–citrate–bicarbonate (DCB) solution was used to extract IP from the root surface within 24 h after harvesting22,49. The dry roots, stems, and leaves were grounded and digested with HNO3/HClO4 (10:1, v/v, USEPA, 1996)50. IP was determined using an atomic absorption spectrophotometer (TAS-990, China), with Pb, Zn, and Cu immobilized in IP, plants, and sediments to be determined using inductively coupled plasma-atomic emission spectrometry (Optima 2000 DV, Perkin Elmer, USA). IP was expressed as 0.1591 × [Fen+] in the extraction/root dry weight (mg g−1 root d‧wt). The detection limits for Fe, Pb, Zn, and Cu were 0.002, 0.001, 0.003, and 0.003 μg mL−1, respectively. The reagents with no sample addition were regarded as blank. Furthermore, internal standard method was used to test the recovery of heavy metals in samples. In this study, the recoveries of heavy metals ranged from 94.45% to 107.21%. The translocation factors of heavy metals in plant were expressed as: TFroot = Croot/CDCB, TFleaf = Cleaf/Croot. Croot, Cleaf, and CDCB are heavy metal concentrations in root, leaf, and DCB extract, respectively.
Statistical analysis
The data were shown as means ± standard deviation (S.D.) with triplicates. One-way ANOVA and Duncan test were conducted to determine the significant difference among different groups. A three-way ANOVA was conducted to assess interactive effects of different heavy metal stress on root growth, iron plaque formation, and metal bioaccumulation. Pearson correlation analysis was performed to explore the relationships among different growth parameters. Regression path analysis (RPA) was employed to identify relationships among root response, IP formation, and heavy metal bioaccumulations. In this study, SPSS Version 20.0 (IBM Inc, USA) was used to perform all statistical analysis.
Research statement
Experimental research on plants complies with relevant institutional, national, and international guidelines and legislation. Appropriate permission was obtained as plant samples were collected in this study.
References
MacFarlane, G. R., Koller, C. E. & Blomberg, S. P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 69, 1454–1464 (2007).
Krauss, K. W. & Osland, M. J. Tropical cyclones and the organization of mangrove forests: A review. Ann. Bot. 125, 213–234 (2020).
Kirk, G. J. D. & Krinzucker, H. J. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: A modeling study. Ann. Bot. 96, 639–646 (2005).
Wu, C., Huang, L., Xue, S. G. & Pan, W. S. Oxic and anoxic conditions affect arsenic (As) accumulation and arsenite transporter expression in rice. Chemosphere 168, 969–975 (2017).
Tripathi, R. D. et al. Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics 6, 1789–1800 (2014).
Xiao, W. et al. Continuous flooding stimulates root iron plaque formation and reduces chromium accumulation in rice (Oryza sativa L.). Sci. Total Environ. 788, 147786 (2021).
Lee, C. H., Hsieh, Y. C., Lin, T. H. & Lee, D. Y. Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant Soil 363, 231–241 (2013).
Dai, M. Y. et al. Phosphorus effects on radial oxygen loss, root porosity and iron plaque in two mangrove seedlings under cadmium stress. Mar. Pollut. Bull. 119, 262–269 (2017).
Liu, C. Y., Chen, C. L., Gong, X. F., Zhou, W. B. & Yang, J. Y. Progress in research of iron plaque on root surface of wetland plants. Acta Ecol. Sin. 34, 2470–2480 (2014).
Li, J., Liu, J. C., Yan, C. L., Du, D. L. & Li, H. L. The alleviation effect of iron on cadmium phytotoxicity on mangrove A. marina. Alleviation effect of rion on cadmium phytotoxicity in mangrove Avicennia marina (Forsk.) Vierh. Chemosphere 226, 413–420 (2019).
Zhang, J. Y. et al. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 260, 113970 (2020).
Farhat, Y. A., Kim, S. H., Seyfferth, A. L., Zhang, L. & Neumann, R. B. Altered arsenic availability, uptake, and allocation in rice under elevated temperature. Sci. Total Environ. 763, 143049 (2021).
Wu, X. Y. et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. R. 23, 8244–8259 (2016).
Abubakar, U. S., Zulkifli, S. Z. & Ismail, A. Heavy metals bioavailability and pollution indices evaluation in the mangrove surface sediment of Sungai Puloh Malaysia. Environ. Earth. Sci. 77, 225 (2018).
Kulkarni, R., Deobagkar, D. & Zinjarde, S. Metals in mangrove ecosystems and associated biota: A global perspective. Ecotox. Environ. Safe. 153, 215–228 (2018).
Shi, C., Ding, H., Zan, Q. J. & Li, R. L. Spatial variation and ecological risk assessment of heavy metals in mangrove sediments across China. Mar. Pollut. Bull. 143, 115–124 (2019).
Cheng, H. et al. Mixture of Pb, Zn and Cu on root permeability and radial oxygen loss in the mangrove Bruguiera gymnorrhiza. Ecotoxicology 29, 691–697 (2020).
Cheng, S. S. et al. Temporal variations in physiological responses of kandelia obovata seedlings exposed to multiple heavy metals. Mar. Pollut. Bull. 124, 1089–1095 (2017).
Shen, X. X. et al. Does combined heavy metal stress enhance iron plaque formation and heavy metal bioaccumulation in Kandelia obovata?. Environ. Exp. Bot. 186, 104463 (2021).
Shen, X. X. et al. Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandelia obovata seedlings. Environ. Geochem. Hlth. 41, 135–148 (2019).
Youssef, T. & Saenger, P. Anatomical adaptive strategies to flooding and rhizosphere oxidation in mangrove seedlings. Aust. J. Bot. 44, 297–313 (1996).
Cheng, H., Wang, Y. S., Fei, J., Jiang, Z. Y. & Ye, Z. H. Differences in root aeration, iron plaque formation and waterlogging tolerance in six mangroves along a continues tidal gradient. Ecotoxicology 24, 1659–1667 (2015).
Takahashi, H., Yamauchi, T., Colmer, T. D. & Nakazono, M. Aerenchyma formation in plants. In Low-Oxygen Stress in Plants (eds van Dongen, J. T. & Licausi, F.) 247–265 (Springer, 2014).
Yamauchi, T., Colmer, T. D., Pedersen, O. & Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 176, 1118–1130 (2018).
Cheng, H. et al. The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environ. Pollut. 158, 1189–1196 (2010).
Liu, Y. et al. Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Mar. Pollut. Bull. 58, 1843–1849 (2009).
Wu, C., Li, H., Ye, Z., Wu, F. & Wong, M. H. Effects of As levels on radial oxygen loss and as speciation in rice. Environ. Sci. Pollut R. 20, 8334–8341 (2013).
Mendellshn, A., Kleiss, B. A. & Wakeley, J. S. Factors controlling the formation of oxidized root channels—a review. Wetlands 15, 37–46 (1995).
Moller, C. L. & Sand-Jesen, K. Iron plaques improve the oxygen supply to root meristems of the freshwater plant Lobelia dortmanna. New Phytol. 179, 848–856 (2008).
Yang, J. X., Liu, Y. & Ye, Z. H. Root-induced changes of pH, eh, Fe (II) and fractions of Pb and Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere 22, 518–527 (2012).
Hu, M., Li, F., Liu, C. & Wu, W. The diversity and abundance of as (III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci. Rep. 5, 13611 (2015).
Huang, G. X., Ding, C. F., Li, Y. S., Zhang, T. L. & Wang, X. X. Selenium enhances iron plaque formation by elevating the radial oxygen loss of roots to reduce cadmium accumulation in rice (Oryza sativa L.). J. Hazard. Mater. 398, 122860 (2020).
Thakur, S. et al. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 188, 206–212 (2016).
Huang, H., Zhu, Y., Chen, Z., Yin, X. & Sun, G. Arsenic mobilization and speciation during iron plaque decomposition in a paddy soil. J. Soil. Sediment. 12, 402–410 (2012).
Zhong, S. Q. Effect of iron plaque on root growth and activity of two wetland plants. J. Hydroecol. 36, 74–79 (2015).
Khan, N. et al. Root iron plaque on wetland plants as a dynamic pool of nutrients and contaminants. Adv. Agron. 138, 1–96 (2016).
Ma, H. H. et al. Formation of iron plaque on roots of Iris pseudacorus and its consequence for cadmium immobilization is impacted by zinc concentration. Ecotox. Environ. Safe. 193, 110306 (2020).
Martinez, S., Sáenz, M. E., Alberdi, J. L. & Di Marzio, W. D. Comparative ecotoxicity of single and binary mixtures exposures of cadmium and zinc on growth and biomarkers of Lemna gibba. Ecotoxicology 29, 571–583 (2020).
Huang, Y. Z., Hu, Y. & Liu, Y. X. Heavy metal accumulation in iron plaque and growth of rice plants upon exposure to single and combined contamination by copper, cadmium and lead. Acta Ecol. Sin. 29, 320–326 (2009).
Deraison, H., Badenhausser, I., Börger, L. & Gross, N. Herbivore effect traits and their impact on plant community biomass: An experimental test using grasshoppers. Funct. Ecol. 29, 650–661 (2015).
Wang, F., Wang, X. & Song, N. Polythylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 784, 147133 (2021).
Yu, H. et al. Microplastic residues in wetland ecosystems: Do they truly threaten the plant-microbe-soil system?. Environ. Int. 156, 106708 (2021).
He, B., Li, R. L., Chai, M. W. & Qiu, G. Y. Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environ. Geochem. Hlth. 36, 467–476 (2014).
Du, J. N., Yan, C. L. & Li, Z. D. Formation of iron plaque on mangrove Kandalar. Obovata (S.L.) Root surfaces and its role in cadmium uptake and translocation. Mar. Pollut. Bull. 74, 105–109 (2013).
Hao, Z. B., Cang, J. & Xu, Z. Plant Physiology Experiment (Harbin Institute of Technology Press, 2004).
Kludze, H. K., Delaune, R. D. & Patrick, W. H. A colorimetric method for assaying dissolved oxygen loss from container-grown rice roots. Agron. J. 86, 483–487 (1994).
Kludze, H. K., Delaune, R. D. & Patrick, W. H. Aerenchyma formation and methane and oxygen-exchange in rice. Soil Sci. Soc. Am. J. 57, 386–391 (1993).
Mei, X. Q., Yang, Y., Tam, N. F. Y., Wang, Y. W. & Li, L. Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res. 50, 147–159 (2014).
Taylor, G. J. & Crowder, A. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am. J. Bot. 70, 1254–1257 (1983).
USEPA (United States Environmental Protection Agency). Method 3052: microwave assisted acid digestion of siliceous and organically based matrices SW-846. DC: Washington (1996).
Acknowledgements
This work was financially supported by the Program of Science and Technology of Shenzhen (JCYJ20200109140605948) and Research Grants Council of the Hong Kong Special Administrative Region, China (UGC/FDS16/M07/19).
Author information
Authors and Affiliations
Contributions
M.W.C.: conceptualization, methodology, investigation, writing-original draft. R.L.L.: investigation, supervision, writing-review & editing. X.X.S.: software, formula analysis, writing-review & editing. L.Y.Y.: resource, supervision, software. J.H.: supervision, writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chai, M., Li, R., Shen, X. et al. Multiple heavy metals affect root response, iron plaque formation, and metal bioaccumulation of Kandelia obovata. Sci Rep 12, 14389 (2022). https://doi.org/10.1038/s41598-022-14867-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14867-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.