Abstract
The association between non-alcoholic fatty liver disease (NAFLD) and the risk of pancreatic cancer in the general population remains unclear. This nationwide cohort study included 8,120,674 adults who underwent a national health screening in 2009 from the Korean National Health Insurance Service database. Participants were followed-up until December 2017 for the development of pancreatic cancer. NAFLD was assessed using the fatty liver index: ≥ 60, NAFLD and < 30, no NAFLD. Multivariable Cox proportional hazards regression was performed. During the follow-up of 59.1 million person-years, 10,470 participants were newly diagnosed with pancreatic cancer. NAFLD was significantly associated with an increased risk of pancreatic cancer compared to no NAFLD (adjusted hazard ratio [aHR], 1.17; 95% CI 1.09–1.26). This association was significant in both the obese (aHR, 1.14; 95% CI 1.05–1.23) and non-obese groups (aHR, 1.14; 95% CI 1.003–1.29). Individuals with fatty liver index 30–59 also had an increased risk (aHR, 1.10; 95% CI 1.05–1.16). The risk of pancreatic cancer increased with increasing fatty liver index scores (P for trend < 0.001). This study demonstrated that NAFLD was independently associated with an increased risk of pancreatic cancer, regardless of obesity. Our finding suggests that NAFLD may be a modifiable risk factor for pancreatic cancer.
Similar content being viewed by others
Introduction
Pancreatic cancer is one of the most lethal diseases, with an overall 5-year survival rate of approximately 9%1. Approximately 60,000 new cases of pancreatic cancer are diagnosed annually in the United States2. The incidence of pancreatic cancer is increasing, and it is predicted to become the second leading cause of cancer-related deaths by 20303,4. However, evidence supporting the utility of pancreatic cancer screening is still lacking5. Thus, identifying modifiable risk factors for pancreatic cancer should be prioritized to reduce the burden of pancreatic cancer worldwide1,3,5.
Non-alcoholic fatty liver disease (NAFLD) refers to excessive fat accumulation in the liver with no heavy alcohol consumption or other secondary causes of steatosis6. The incidence and prevalence of NAFLD are rapidly increasing worldwide7. Recent evidence has revealed that NAFLD is a risk factor for several cancers, such as hepatocellular carcinoma8,9,10 and colorectal cancer10.
However, the association between NAFLD and the risk of pancreatic cancer remains unclear. Only a few small hospital-based studies were conducted, and these studies showed inconsistent results of positive11,12,13,14 and null associations15. Moreover, these studies did not adjust for known risk factors related to pancreatic cancer such as pancreatitis11,12,13,14,15, body mass index (BMI)11,12,13,14,15, and smoking11,12,14. Notably, no study has assessed the effect of NAFLD on the risk of pancreatic cancer in the general population.
To assess NAFLD, liver biopsy; imaging studies; and non-invasive biomarkers, including the fatty liver index, have been used10,16,17,18,19,20,21. However, performing a liver biopsy or imaging studies is not feasible in the asymptomatic general population. As recommended by the recent international guidelines from the European Association for the Study of the Liver, non-invasive serum biomarkers are the preferred assessment tool for NAFLD in large-scale population-based studies10.
Therefore, we conducted this nationwide, population-based cohort study of over 8 million adults to investigate the association between NAFLD and pancreatic cancer risk using the fatty liver index in the general Korean population.
Materials and methods
Data source
We used the data from the national health screenings and the Korean National Health Insurance Service (KNHIS), which is a mandatory national health insurance system managed by the government. The KNHIS covers approximately 97% of the total population. The remaining 3% of the population is covered by the Medical Aid Program, where their claims data are also reviewed by the KNHIS. Therefore, the KNHIS database covers the entire Korean population.
The KNHIS provides a standardized biannual national health screening program for citizens ≥ 20 years of age. The national health screening data includes anthropometric measurements, laboratory test findings, past medical history, and health-related behaviors. Using the KNHIS database, we obtained clinical information, including demographics, diagnostic codes, medical treatment-related data (prescriptions, hospital admissions, and procedures), and results of the national health screenings22. The KNHIS claims database uses the International Classification of Diseases-10th Revision-Clinical Modification (ICD-10-CM) codes.
In 2006, the KNHIS also performed a registration program with special reimbursement codes to lower the copayment rate to 5% for cancers (V codes). All patients with such diseases are required to have their diagnosis certified by a physician to receive the payment benefits for cancer-related management. Thus, V codes based on national registration data for cancer patients are reliable.
The present study was approved by the Institutional Review Board of the Samsung Medical Center (#SMC2019-08-106) and the KNHIS Big Data Steering Department (NHIS-2019-1-499) and was exempted from informed consent requirements. The study was conducted in accordance with the principles of the 1964 Declaration of Helsinki. The KNHIS data were obtained from the Korea National Health Insurance Sharing Service after receiving permission from the Institutional Data Access/Ethics Committee.
Study population
Figure 1 shows the selection process of the study population. We included 10,490,491 adults aged ≥ 20 years who underwent a health examination provided by the KNHIS between January 1 and December 31, 2009. To define NAFLD, we excluded patients with alcoholic liver cirrhosis (ICD-10-CM Code K703; n = 81,916), patients with hepatitis (ICD-10-CM code K746; n = 911,558), and those with heavy alcohol consumption (≥ 30 g of alcohol per occasion; n = 683,534)23. We excluded individuals with a previous diagnosis of cancer (n = 118,756). To avoid potential reverse causality, we further excluded participants who developed pancreatic cancer or died within the first year of entering the cohort (n = 63,128). We also excluded participants with missing variables (n = 510,925). Finally, 8,120,674 participants were included in the study and followed-up until the date of pancreatic cancer development, death, or December 31, 2017, whichever came first.
Clinical variables and biochemical analysis
A trained clinician measured the patients’ height, weight, and waist circumference. The BMI was calculated by dividing the weight by height squared (kg/m2). Obesity was defined as a BMI ≥ 25 kg/m2 according to Asian standards24.
Blood samples were obtained after overnight fasting to measure the serum levels of glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, total cholesterol, γ-glutamyl transferase (GGT), alanine aminotransferase, and aspartate aminotransferase.
Lifestyle-associated covariates, including smoking history (none, ex-smoker, or current smoker), alcohol consumption (none, mild consumption [< 30 g of alcohol per day], heavy alcohol consumption [≥ 30 g of alcohol per day])25, and physical activity (high-intensity activity ≥ three times/week or moderate-intensity activity ≥ five times/week), were also evaluated using standardized self-administered questionnaires. Income level was dichotomized into the lowest quartile (25%).
Comorbidities were defined as follows: pancreatitis (ICD-10-CM codes K85, K86.0, and K86.1); diabetes mellitus (fasting blood glucose levels ≥ 126 mg/dL measured during the health screening or ≥ one claim per year for ICD-10-CM codes E10–E14 and a prescription for an antidiabetic drug); dyslipidemia (fasting blood total cholesterol levels ≥ 200 mg/dL measured during the health screening or ≥ one claim per year for ICD-10 Code E78 and a prescription for lipid-lowering medications); and hypertension (systolic/diastolic blood pressure ≥ 140/90 mmHg during the health screening or the presence of at least one claim per year for ICD-10-CM codes I10-13, I15, and a prescription for an antihypertensive agent).
Assessment of NAFLD and pancreatic cancer
NAFLD was assessed using fatty liver index, one of the best-validated fatty-liver-prediction models10,26. The 2016 European guidelines recommend non-invasive biomarkers as the preferred diagnostic tools for hepatic steatosis in large-scale population-based studies, as the availability and cost of liver biopsies and imaging substantially impact study feasibility10. The fatty liver index, ranging from zero to 100, was calculated as follows; (e0.953 × Ln(triglyceride) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × waist circumference ± 15.745)/ (1 + e0.953 × Ln (triglyceride) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × waist circumference ± 15.745) × 10026. The participants were then categorized into three groups based on criteria used in previous studies: ≥ 60, NAFLD; 30–59, intermediate score; and < 30, no NAFLD18,21,23,26,27,28,29,30,31,32.
The primary outcome was newly diagnosed pancreatic cancer, which was identified based on hospitalization with the ICD-10-CM code for pancreatic cancer (C25) and a reimbursement code for cancer in the national registration data (V193) between January 2009 and December 2017.
Statistical analysis
Baseline characteristics were analyzed using a one-way analysis of variance to compare continuous variables and χ2 tests for categorical variables. The incidence rates of pancreatic cancer were estimated by dividing the number of incident cases per 1000 person-years. We used multivariable Cox proportional hazards regression models to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between NAFLD and pancreatic cancer risk. Model 1 was adjusted for age and sex, and Model 2 was further adjusted for smoking, alcohol consumption, physical activity, income, BMI, diabetes, and pancreatitis. In addition, we conducted a sensitivity analysis using an inverse probability-weighted analysis, a type of propensity score analysis, to control for confounding by observed covariates33,34. We performed subgroup analyses and reported P-values for interactions. We also calculated P-values for linear trends of pancreatic cancer risk across the fatty liver index categories. All statistical tests were two-sided, and significance was set at a P-value of < 0.05. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
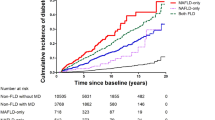
During the mean follow-up period of 7.2 years, 10,470 individuals were newly diagnosed with pancreatic cancer. Table 1 shows the baseline characteristics of the study population. The mean age of the participants was 46.7 ± 14.1 years, and 52.1% of the participants were male. Those with NAFLD were more likely to be male, mild drinkers, current smokers, and physically active than those without NAFLD. In addition, those with NAFLD had higher mean values for BMI, fasting glucose, blood pressure, and total cholesterol levels than those without NAFLD (all P < 0.001).
Association between NAFLD and risk of pancreatic cancer
Table 2 presents the risk of pancreatic cancer according to NAFLD status. NAFLD was associated with an increased risk of pancreatic cancer compared to no NAFLD after adjusting for age and sex (in Model 1; HR, 1.36; 95% CI 1.29–1.44). The association persisted even after adjusting for age, sex, alcohol consumption, smoking, physical activity, income, BMI, diabetes, and pancreatitis in Model 2 (hazard ratio [HR], 1.17; 95% CI 1.09–1.26). Intermediate fatty liver index was also significantly associated with an increased risk of pancreatic cancer in all models (HR; 95% CIs: [1.19; 1.14–1.24] and [1.10; 1.05–1.16] for Models 1 and 2, respectively). The adjusted HRs of pancreatic cancer tended to increase progressively with increasing fatty liver index (P for trend < 0.001). Even after applying inverse probability weights, a significant association between NAFLD and pancreatic cancer was consistently observed (Supplementary Table 1).
Subgroup analyses
Figure 2 presents the adjusted HRs of pancreatic cancer and P for interactions after adjusting for multiple confounders in each subgroup. There were no significant interactions between NAFLD and stratified variables, except for diabetes (P = 0.009). The association between NAFLD and pancreatic cancer risk did not differ according to age, sex, smoking, physical activity, hypertension, dyslipidemia, or obesity (all P > 0.05; Fig. 2). The association between NAFLD and the risk of pancreatic cancer was significant in both obese (HR, 1.14; 95% CI 1.05–1.23) and non-obese groups (HR, 1.14; 95% CI 1.003–1.29).
Association between non-alcoholic fatty liver disease and the risk of pancreatic cancer by subgroup. Forrest plots of hazard ratios (HRs) and 95% confidential intervals (CIs) adjusted for age, sex, smoking, alcohol consumption, physical activity, income level, body mass index, diabetes, and pancreatitis according to subgroups.
Combined effects of NAFLD and smoking on pancreatic cancer risk
Table 3 shows the risk of pancreatic cancer according to the combination of NAFLD and smoking after adjusting for multiple confounders. Compared to non-smokers without NAFLD, non-smokers with NAFLD and smokers without NAFLD showed an increased risk of pancreatic cancer (HR, 1.12; 95% CI 1.04–1.21; and HR, 1.38; 95% CI 1.29–1.47, respectively). The combined effects of NAFLD and smoking further increased the risk of pancreatic cancer by 42% (HR 1.42; 95% CI 1.28–1.58).
Discussion
This nationwide cohort study, including over 8 million individuals, demonstrated that NAFLD was independently associated with an increased risk of pancreatic cancer after adjusting for potential confounders. The significant association between NAFLD and the risk of pancreatic cancer was consistent in both the obese and non-obese groups. The risk of pancreatic cancer tended to increase as the fatty liver index increased. To the best of our knowledge, the present study is the first nationwide cohort study to demonstrate an association between NAFLD and the risk of pancreatic cancer in the general population, regardless of obesity.
Limited data are available on the association between NAFLD and pancreatic cancer. Only a few hospital-based studies have examined the association between NAFLD and pancreatic cancer risk. However, previous studies showed inconsistent results with positive11,12,13,14 or null associations15. Furthermore, previous studies have possible limitations (Supplemental Table 2). First, all previous studies were performed in hospitals, even though NAFLD is generally asymptomatic; thus, these studies may not include a substantial proportion of asymptomatic patients with NAFLD and included a greater proportion of NAFLD patients with other confounding comorbidities11,12,13,14,15. Therefore, these studies might have a selection bias and were not representative of the general population. Second, previous studies included a limited number of pancreatic cancer cases. These studies analyzed pancreatic cancer risk using sample sizes ≤ 188 cases, which can also lead to undercoverage bias. Third, previous studies did not consider significant confounders related to pancreatic cancer, such as pancreatitis11,12,13,14,15, BMI11,12,13,14,15, and smoking11,12,14. The results of previous studies are summarized in Supplemental Table 2. Recently, beyond the concept of NAFLD, evidence for an association between metabolic dysfunction–associated fatty liver disease (MAFLD) and pancreatic cancer risk has been suggested35. MAFLD is defined as a fatty liver disease with metabolic abnormalities, such as overweight, diabetes, and metabolic syndrome.
Several potential biological mechanisms underlie the increased pancreatic cancer risk in patients with fatty liver disease. First, systemic release of proinflammatory cytokines in hepatic steatosis can induce chronic, low-grade systemic inflammation36,37. Inflammation has diverse tumor-promoting effects, such as cell proliferation and inhibition of adaptive immunity, in cancers, including pancreatic cancer38,39. Several studies have reported that diverse inflammatory signaling pathways, such as NF-κB, the IL-6-STAT3 axis, and TGF-β, promote the carcinogenesis of pancreatic cancer40,41. Second, the altered microbiome in patients with hepatic steatosis can increase pancreatic cancer risk42,43. Recently, the role of gut microbiota in the pathophysiology of various human diseases, such as metabolic diseases, inflammatory diseases, and cancers, has been revealed. Gut dysbiosis is significantly involved in hepatic steatosis progression and carcinogenesis in different cancers, including pancreatic cancer, via the gut-liver axis42,43. Third, insulin resistance is a key factor in the pathophysiology of hepatic steatosis. Insulin resistance increases cell proliferation, cellular mobility, angiogenesis, and damage to DNA molecules by active forms of oxygen37,44. Last, insulin-like growth factor-1, which is associated with hepatic steatosis, inhibits apoptosis and promotes progression through the cell cycle, and may play a role in pancreatic carcinogenesis.
It can be argued that the association between NAFLD and pancreatic cancer risk may be due to shared risk factors, such as obesity. However, regardless of obesity, the significant association between NAFLD and pancreatic cancer risk was consistent after adjusting for potential confounders. The current concept of “non-obese fatty liver disease” or “lean NAFLD” supports the hypothesis of an independent effect of NAFLD on the development of pancreatic cancer in the absence of obesity45,46,47.
This study also showed that a combination of NAFLD and smoking further increased the risk of pancreatic cancer. Our findings demonstrate that the combined effect of these two modifiable risk factors has significant clinical implications for lowering the risk of pancreatic cancer.
This cohort study has several strengths. First, the KNHIS data covers the entire Korean population, and all medical records are tracked in the database. Second, this cohort study used systematically and longitudinally collected measurements and clinical data at an individual level prior to the incidence of pancreatic cancer in a vast population of over 8 million people. The recall bias was considered to be minimal in our study. Third, to achieve a high diagnostic accuracy for pancreatic cancer, we used both diagnostic and special reimbursement codes (C and V codes). Last, we obtained both health screening data and KNHIS claim data; hence, we could adjust for confounding factors for pancreatic cancer, including pancreatitis, BMI, diabetes, physical activity, and smoking.
Our study has several limitations. First, we tried to minimize reverse causality by excluding those who had a cancer diagnosis before or were diagnosed with pancreatic cancer or died within 1 year after cohort entry. Nonetheless, there may be a possibility of reverse causality. Second, the information on the pathological subtype of pancreatic cancer was not obtained. However, pancreatic adenocarcinoma accounts for approximately 90% of all pancreatic cancers, and pancreatic neuroendocrine tumors are rare, accounting for less than 5% of all cases32. Last, although we adjusted for multiple confounders, including pancreatitis, smoking, and BMI, the possibility of residual confounding cannot be excluded.
In conclusion, this nationwide cohort study demonstrated that NAFLD was significantly associated with an increased risk of pancreatic cancer in the general Korean population. The association was significant regardless of obesity status. In addition, the combined effects of NAFLD and smoking further increased the risk of pancreatic cancer. Our findings suggest that NAFLD is a modifiable risk factor for pancreatic cancer. Further studies are needed to investigate the effects of NAFLD management on reducing the risk of pancreatic cancer.
Data availability
The Korean National Health Insurance Service (KNHIS) permits researchers to access the KNHIS data after reviewing the research topic. Requests for access to the data can be made on the KNHIS website.
References
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155 (2014).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA: Cancer J. Clin. 71, 7–33. https://doi.org/10.3322/caac.21654 (2021).
GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 4, 934–947. https://doi.org/10.1016/S2468-1253(19)30347-4 (2019).
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: A review. JAMA 326, 851–862. https://doi.org/10.1001/jama.2021.13027 (2021).
Henrikson, N. B. et al. Screening for pancreatic cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA 322, 445–454. https://doi.org/10.1001/jama.2019.6190 (2019).
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 158, 1851–1864. https://doi.org/10.1053/j.gastro.2020.01.052 (2020).
Younossi, Z. et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20. https://doi.org/10.1038/nrgastro.2017.109 (2018).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American gastroenterological association, American association for the study of liver diseases, and american college of gastroenterology. Gastroenterology 142, 1592–1609. https://doi.org/10.1053/j.gastro.2012.04.001 (2012).
Benson, A. B. et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 19, 541–565. https://doi.org/10.6004/jnccn.2021.0022 (2021).
European Association for the Study of the, L., European Association for the Study of, D. & European association for the study of, O. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64, 1388–1402. https://doi.org/10.1016/j.jhep.2015.11.004 (2016).
Sorensen, H. T. et al. Risk of cancer in patients hospitalized with fatty liver: A Danish cohort study. J. Clin. Gastroenterol. 36, 356–359. https://doi.org/10.1097/00004836-200304000-00015 (2003).
Allen, A. M., Hicks, S. B., Mara, K. C., Larson, J. J. & Therneau, T. M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity: A longitudinal cohort study. J. Hepatol. 71, 1229–1236. https://doi.org/10.1016/j.jhep.2019.08.018 (2019).
Chang, C. F. et al. Exploring the relationship between nonalcoholic fatty liver disease and pancreatic cancer by computed tomographic survey. Intern. Emerg. Med. 13, 191–197. https://doi.org/10.1007/s11739-017-1774-x (2018).
Simon, T. G. et al. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: A population-based cohort study. Hepatology 74, 2410–2423. https://doi.org/10.1002/hep.31845 (2021).
Kim, G. A. et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 68, 140–146. https://doi.org/10.1016/j.jhep.2017.09.012 (2017).
Wong, V. W., Adams, L. A., de Ledinghen, V., Wong, G. L. & Sookoian, S. Noninvasive biomarkers in NAFLD and NASH: Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 15, 461–478. https://doi.org/10.1038/s41575-018-0014-9 (2018).
Koehler, E. M. et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 11, 1201–1204. https://doi.org/10.1016/j.cgh.2012.12.031 (2013).
Castera, L., Friedrich-Rust, M. & Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1264–1281. https://doi.org/10.1053/j.gastro.2018.12.036 (2019).
Aberg, F. et al. Risks of light and moderate alcohol use in fatty liver disease: Follow-up of population cohorts. Hepatology 71, 835–848. https://doi.org/10.1002/hep.30864 (2020).
Kim, D., Vazquez-Montesino, L. M., Li, A. A., Cholankeril, G. & Ahmed, A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 72, 1556–1568. https://doi.org/10.1002/hep.31158 (2020).
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272. https://doi.org/10.1038/s41586-020-1961-1 (2020).
Lee, Y. H. et al. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab. J. 40, 79–82. https://doi.org/10.4093/dmj.2016.40.1.79 (2016).
Gastaldelli, A. et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 49, 1537–1544. https://doi.org/10.1002/hep.22845 (2009).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 (2009).
Agarwal, D. P. Cardioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol Alcohol. 37, 409–415. https://doi.org/10.1093/alcalc/37.5.409 (2002).
Bedogni, G. et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33. https://doi.org/10.1186/1471-230X-6-33 (2006).
Lee, Y. H. et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 63, 776–786. https://doi.org/10.1002/hep.28376 (2016).
Lee, Y. H. et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: Development, validation and comparison with other scores. PLoS One 9, e107584. https://doi.org/10.1371/journal.pone.0107584 (2014).
Cuthbertson, D. J. et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur. J. Endocrinol. 171, 561–569. https://doi.org/10.1530/EJE-14-0112 (2014).
Zelber-Sagi, S. et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J. Gastroenterol. 19, 57–64. https://doi.org/10.3748/wjg.v19.i1.57 (2013).
Jiang, Z. Y. et al. Fatty liver index correlates with non-alcoholic fatty liver disease, but not with newly diagnosed coronary artery atherosclerotic disease in Chinese patients. BMC Gastroenterol. 13, 110. https://doi.org/10.1186/1471-230X-13-110 (2013).
Hidalgo, M. et al. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 15, 8–18. https://doi.org/10.1016/j.pan.2014.10.001 (2015).
Chesnaye, N. C. et al. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 15, 14–20. https://doi.org/10.1093/ckj/sfab158 (2022).
Allan, V. et al. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J. Comp. Effect. Res. 9, 603–614. https://doi.org/10.2217/cer-2020-0013 (2020).
Liu, Z. et al. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metab. Clin. Exp. 127, 154955. https://doi.org/10.1016/j.metabol.2021.154955 (2022).
Byrne, C. D. & Targher, G. NAFLD: A multisystem disease. J. Hepatol. 62, S47–S64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Gehrke, N. & Schattenberg, J. M. Metabolic inflammation-a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease?. Gastroenterology 158, 1929–1947. https://doi.org/10.1053/j.gastro.2020.02.020 (2020).
Colotta, F., Allavena, P., Sica, A., Garlanda, C. & Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 30, 1073–1081. https://doi.org/10.1093/carcin/bgp127 (2009).
Ren, R. et al. Inflammation promotes progression of pancreatic cancer through WNT/beta-catenin pathway-dependent manner. Pancreas 48, 1003–1014. https://doi.org/10.1097/MPA.0000000000001386 (2019).
Hausmann, S., Kong, B., Michalski, C., Erkan, M. & Friess, H. The role of inflammation in pancreatic cancer. Adv. Exp. Med. Biol. 816, 129–151. https://doi.org/10.1007/978-3-0348-0837-8_6 (2014).
Villanueva, A. et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene 17, 1969–1978. https://doi.org/10.1038/sj.onc.1202118 (1998).
Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425. https://doi.org/10.1038/nrgastro.2016.85 (2016).
Wei, M. Y. et al. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol. Cancer 18, 97. https://doi.org/10.1186/s12943-019-1008-0 (2019).
Bugianesi, E., Moscatiello, S., Ciaravella, M. F. & Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 16, 1941–1951. https://doi.org/10.2174/138161210791208875 (2010).
Kim, D. & Kim, W. R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 15, 474–485. https://doi.org/10.1016/j.cgh.2016.08.028 (2017).
Wood, N. J. Liver: Nonobese individuals in the developing world are at risk of nonalcoholic fatty liver and liver disease. Nat. Rev. Gastroenterol. Hepatol. 7, 357. https://doi.org/10.1038/nrgastro.2010.95 (2010).
Das, K. et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 51, 1593–1602. https://doi.org/10.1002/hep.23567 (2010).
Funding
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Republic of Korea (2020R1I1A1A01067400). The funders played no role in the study’s design, conduct, and reporting.
Author information
Authors and Affiliations
Contributions
J.-H.P.: Conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. J.Y.H.: Conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. K.H.: Conceptualization, data curation, formal analysis, investigation, methodology writing—review and editing. W.K.: Conceptualization, formal analysis, investigation, methodology supervision, writing—review and editing. J.K.P.: Conceptualization, formal analysis, investigation, methodology, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, JH., Hong, J.Y., Han, K. et al. Increased risk of pancreatic cancer in individuals with non-alcoholic fatty liver disease. Sci Rep 12, 10681 (2022). https://doi.org/10.1038/s41598-022-14856-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14856-w
This article is cited by
-
Fatty liver index (FLI): more than a marker of hepatic steatosis
Journal of Physiology and Biochemistry (2024)
-
A niche-mimicking polymer hydrogel-based approach to identify molecular targets for tackling human pancreatic cancer stem cells
Inflammation and Regeneration (2023)
-
Comparative analysis of the relationship between four hepatic steatosis indices and muscle mass
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.