Abstract
Vegetables cultivated on contaminated agricultural soils are being consumed by the public, and consequently cause serious health concerns due to contaminants' dietary intake. The current study examines the safety and sustainability of eating eggplant (Solanum melongena) by looking into the possibility of heavy metals translocation from polluted soils to the edible sections, as well as the health hazards that come with it. Soil and eggplant samples were taken from three contaminated and other three uncontaminated farms to estimate their chemical constituents and plant growth properties. Based on the pollution load index data, the contaminated soils were highly polluted with Fe, Cu, Pb, and Zn; and relatively polluted with Cr, Mn, Cd, Mn, Co, and V. Under contamination stress, the fresh biomass, dry biomass, and production of eggplant were significantly reduced by 41.2, 44.6, and 52.1%, respectively. Likewise, chlorophyll a and b were significantly reduced from 1.51 to 0.69 mg g−1 and 1.36 to 0.64 mg g−1, respectively. The uncontaminated plant shoots had the highest quantities of N, P, and proteins (1.98, 2.08, and 12.40%, respectively), while the roots of the same plants had the highest K content (44.70 mg kg−1). Because eggplant maintained most tested heavy elements (excluding Zn and Pb) in the root, it is a good candidate for these metals' phytostabilization. However, it had the potential to translocate Mn and Zn to its shoot and Pb, Cr, Mn, and Zn to the edible fruits indicating its possibility to be a phytoextractor and accumulator of these metals. Cd, Cu, Ni, Pb, Mn, and Co quantity in the edible sections of eggplant grown in contaminated soils exceeded the permissible level for normal plants, posing health hazards to adults and children. For safety issues and food sustainability, our investigation strongly recommends avoiding, possibly, the cultivation of eggplant in contaminated agricultural lands due to their toxic effects even in the long run.
Similar content being viewed by others
Introduction
Food sustainability and safety is a global public issue that has drawn scientists' attention to the health risks connected with consuming tainted foodstuffs1. Unprecedented population growth and urbanization generate tremendous quantities of wastewater worldwide; their safe disposal is one of the common environmental concerns2. In developing countries, wastewater is an alternative irrigation source commonly used to maximize the yield of high-quality food crops in urban areas3. Wastewater is the main contributor to soil contamination; and thus, irrigating food crops with such water is a serious issue as these effluents are heavily loaded with toxic heavy metals4,5. Although fertilizers and pesticides are important for crop growth and production, their extensive use may leave residues on food products, which can potentially cause human health risks6.
Various pollutants are widely spread in the environment; heavy metals are of particular interest because of their bioaccumulation potential and harmful impacts on ecosystems7. Heavy metals are the most important concern in terms of vegetable growth and yield in contaminated agricultural fields all over the world8. They degrade soil, reducing vegetable quality, output, and food safety, thereby making vegetable cultivation unsustainable9. The most serious and pervasive agricultural concerns in emerging countries are heavy metal-contaminated soils10. Their accumulation in crop plants through contaminated wastewater irrigation can cause devastating impacts on consumer health11. The increased level of heavy metals because of natural processes or anthropogenic activity has exacerbated the food security threats for the world's increasing population12,13. Cultivating vegetables in polluted agricultural fields increases the danger of toxic metal poisoning, which can be harmful to human health14.
Vegetables grown with wastewater irrigation from sewage and industrial effluents are consumed by the general people, posing serious health risks because of heavy metal accumulation through dietary consumption of these polluted food plants15,16. After tomato, potato, and pepper, Solanum melongena L. (eggplant) is one of the common commercially economic solanaceous crops17. It is a high-yielding and biomass-productive fruit crop, which is well-adapted to hot and wet environments18. Eggplant has numerous health benefits, including its function in preventing chronic diseases19, as well as the effects of its phenolic components on human health, such as cardioprotective, anti-carcinogenic, antioxidant, anti-inflammatory, and anti-diabetic qualities20. Worldwide, more than 1,800,000 ha produce about 50 million tons of eggplant21, Accessed 03.08.2017). The production of eggplant is common in a few countries; 29.5 million tons in China, which is the top producer, followed by 13.5 million tons in India, 1.2 million tons in Egypt, 0.85 million tons in Iran, and 0.82 million tons in Turkey20.
In most developing countries, water scarcity is the primary worry22. According to the International Water Management Institute, 1.8 billion people would face severe water shortages by 202523. Thus, using nonconventional irrigation (e.g., wastewater) is an indispensable resource of irrigation water to mitigate the pressure on freshwater24. In this context, we hypothesize that the cultivation of vegetable crops in wastewater-irrigated agricultural soil may threaten food safety and human health. Consequently, investigating plant-heavy metals content is crucial to mitigate their concentration in crop plants and avoid their toxicity25. Therefore, the goal of this study was to assess the extent of heavy metals translocation from wastewater-irrigated soil to the edible sections of Solanum melongena (eggplant) to determine its health safety and sustainability for the public consumption.
Materials and methods
Study sites
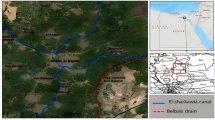
The study area lies in an urbanized region in Greater Cairo and comprised two sites; one of them is contaminated and receives industrial and municipal wastewater, and agricultural drainage (Fig. 1). It is situated on the eastern bank of the Nile in the Ekhsas neighborhood (29° 47′ 29.31′′ N–31° 18′ 38.05′′ E), south Cairo Province, and houses 42.6 percent of Greater Cairo's 18.29 million consumers26. The soils of this site suffer from high water levels, which may lead to desertification. However, another site is uncontaminated (control) and irrigated from the River Nile water through Al-Ebrahemia canal, which in turn receives no discharge of industrial or municipal wastes. It was located on the River Nile's western side et al.-Belada village (29° 42′ 11.06′′ N–31° 15′ 52.51′′ E), south Giza Province. The study sites extend along the Nile Valley, and thus their soils are alluvial. The research area's climate was defined by a mean annual temperature of 21.08 degrees Celsius, a mean annual relative humidity of 52.68 to 56.08 percent, and a yearly average rainfall of 1.67–2.13 mm per year.
Plant sampling and growth measurements
Samples of eggplant plants were taken from 6 different farms (about 2 acres each) and equally distributed in the contaminated and uncontaminated sites. Collected plants were the same cultivar and in the same growth stage (fruiting) and grown in the same conditions of climate and soil type. Voucher specimens were identified by the author (Tarek M. Galal) and deposited in Helwan University Herbarium, Cairo, Egypt. At each cultivated farm, 10 quadrats (2 × 2 m each) were randomly chosen, to collect the eggplant samples. By counting the number of individuals in each quadrat, plant density, as the number of individuals per 100 m2, was obtained, and plants were then collected and transported to the laboratory for further analysis. Plant samples were cleaned twice with distilled water and once with tap water to eliminate any dirt or dust. After measuring the length of the roots and shoots, as well as the count of the leaves per individual, the samples were sorted into roots, shoots, and fruits (edible parts). To measure fresh and dry biomass (weight/unit area), the separated shoots were weighed before and after oven drying at 105 °C until constant weight. Besides, the fruit's fresh weight was used to estimate fruit production.
Plant analysis
Chemical analysis
For chemical analysis, three composite oven-dried eggplant roots, shoots, and fruits (N = 54) were gathered from each of the contaminated and uncontaminated farms and processed into a powder in a metal-free plastic mill. Kjeldahl and molybdenum blue procedures were used to estimate total N and total P, respectively, while to estimate K, a flame photometer (CORNING M410) was used. Umbriet et al.27 and Lowry et al.28 procedures were used to calculate total soluble sugars (carbohydrates) and total soluble proteins, respectively. The acid digestion method was used to estimate the heavy metals concentration of sampled plant parts29. A ground sample of 1 g was digested in 20 mL of a tri-acid mixture of HNO3:H2SO4:HClO4 (5:1:1, v/v/v) until a transparent color appeared, then the digested plant was filtered and diluted with double-distilled water to 25 mL. Shimadzu AA-6300 (Shimadzu Co. Ltd., Kyoto, Japan) Spectrophotometer was used to estimate the amounts of Co, Fe, Cu, Ni, Mn, Pb, Cd, Zn, Cr, and V29. The detection limits for heavy metals were 9.0 for Co, 5.0 for Fe, 1.5 for Cu, 6.0 for Ni, 0.8 for Cd, Mn, and Zn, 15.0 for Pb, 3.0 for Cr, and 2.0 for V (in µg/l). The confidence level for all detection thresholds was 95% (3 standard deviations).
Quality control and assurance
A recognized reference sample (SRM 1573a, tomato leaves) was utilized to assess heavy metals accuracy, with recovery rates ranging from 94.8 to 103.7%. The digestion and analysis of the certified reference material were carried out with the same methods applied to the eggplant tissues. Three replicates were used for heavy metals digestion and determination. The measured heavy metals concentration was compared with those of the certified value to estimate the accuracy percentage.
Pigment content
From each quadrat in the contaminated and uncontaminated areas, three eggplant leaves were combined to generate 3 composite samples (N = 18) for each farm. Chlorophyll a, b, and carotenoids were prepared from 2 g fresh leaves by immersing them in 50 percent (v/v) acetone overnight at 4 °C in full darkness. The extract was then compared to aqueous acetone, as a blank, spectrophotometrically at three wavelengths: 663, 644, and 453 nm. Each pigment's content (mg g−1 fresh wt.) was calculated as follows29:
where A represents the absorbance.
Soil analysis
From each farm in the uncontaminated and contaminated areas, three composite sub-surface soil samples (N = 18) were taken from the eggplant rhizosphere. The electrical conductivity (EC) and pH value of 1:5 w/v soil–water extracts were determined using a conductivity meter 60 Sensor Operating Instruction Corning and a glass electrode pH meter (Model 9107 BN, ORION type), respectively. Nutrient elements (N, P, and K) and heavy metals (Co, Fe, Cu, Ni, Mn, Pb, Cd, Zn, Cr, and V) concentrations were determined using the same procedures in plant analysis29.
Data analysis
The pollution load index (PLI)
The PLI rates each heavy metal's soil pollution as follows:
where Cc and Cu are the heavy metals content in contaminated and uncontaminated soils, respectively30. The soil was categorized according to the PLI following Lu et al.31: uncontaminated: PLI = 0–1; uncontaminated- relatively contaminated: PLI = 1–2; relatively contaminated: PLI = 2–3; relatively -highly contaminated: PLI = 3–4; highly contaminated: PLI = 4–5; and very highly contaminated: PLI > 5.
Transfer factors (TF)
The transfer factor (TF) is a good indicator to represent heavy metal transmission from the soil to the various plant organs. It assumes a linear link between heavy metal concentrations in soil and plants. The TF is used to look at a plant's ability to acquire heavy metals in its underground roots and then translocate them to its aboveground shoot. According to Eid et al.32, it was computed as follows:
where, Cso, Cro, Csh, and Cfr are the element concentration (mg kg−1) in the soil, root, shoot, and fruit, respectively.
Health risk assessment
The daily intake of metals (DIM) for both children and adults was calculated following33
where C is the plant concentration of heavy metal (mg kg−1), F is a factor (0.085) for converting fresh to dry weight34, D is the daily vegetable requirement (0.345 and 0.232 kg/person/day) for children and adults, respectively, and B is the mean body weight (32.7 and 55.9 kg) for children and adults22. Furthermore, the estimated crop exposure / the reference oral dose ratio was used to calculate the health risk index (HRI) for local consumers of contaminated plants32. When the HRI exceeded one, it implies that the consumers' health is at risk35.
Statistical analysis
A paired-sample t-test was used to compare the differences in soil and plant characteristics between uncontaminated and contaminated areas. However, using SPSS software36, ANOVA 1 was used to estimate the considerable nutrients and heavy metals variation among the different plant organs. Duncan’s multiple range test was used to assess significant differences (at p < 0.05) between means.
Declaration
We declare that plant materials were collected from private farms and permissions were taken from the owners following the relevant institutional, national, and international guidelines and legislation.
Results
Soil properties
The soil chemical investigation showed highly significant differences (P < 0.001) in all chemical variables between contaminated and uncontaminated eggplant farms (Table 1). Soil pH, EC, and heavy metals were greater in the contaminated than in the uncontaminated soils, which contributed significantly higher macronutrients (N, P, and K) content. The heavy element's content of the contaminated soil was in the following order: Fe > Zn > Mn > Pb > Cu > Cd > Cr > Ni > Co > V. Furthermore, the PLI of the estimated heavy elements was greater than one, with Pb having the greatest value (248.9) and Ni having the lowest (1.0).
Growth measurements
In heavy metals-contaminated farms, eggplant growth measures revealed significant reductions in the root and shoot length, as well as the number of leaves (Table 2). The shoot length was significantly reduced from 94.1 ± 13.5 to 44.7 ± 9.7 cm, while the root length from 11.74 ± 1.75 to 6.4 ± 0.9 cm, and the number of leaves was from 44.7 ± 3.5 to 31.8 ± 2.7 leaves/individual under contamination conditions. In the same context, the dry and fresh biomass, and production of eggplants showed significant differences (P < 0.01) between uncontaminated and contaminated farms (Fig. 2). The mean fresh biomass, dry biomass, and production were significantly reduced from 16.9 ± 2.1, 3.2 ± 0.4, and 10.4 ± 1.9 to 9.9 ± 3.2, 1.8 ± 0.3, and 5.0 ± 0.8 t ha−1, respectively under contamination conditions with reduction percentages of 41.2, 44.6, and 52.1%, respectively.
Plant analysis
Plant pigments
Chlorophyll a and b levels in eggplant leaves differed significantly between uncontaminated and contaminated plants (Fig. 3). Under the contaminated circumstances, chlorophyll a and b concentrations were declined from 1.51 ± 0.32 to 0.69 ± 0.10 mg g−1 and from 1.36 ± 0.26 to 0.64 ± 0.09 mg g−1, respectively. Meanwhile, the carotenoid content in the infected plants did not significantly increase from 0.24 ± 0.06 to 0.35 ± 0.10 mg g−1.
Plant nutrients
The nutrient analysis revealed considerable variations (P < 0.05) among the various organs of eggplants from contaminated and uncontaminated fields (Table 3). The largest amounts of N, P, and proteins (1.98 ± 0.11, 2.08 ± 0.34, and 12.40 ± 0.67%) were observed in the shoots of uncontaminated plants, while the roots of the same plants had the highest K content (44.70 ± 0.88 mg kg−1) and the lowest P and carbohydrates (1.10 ± 0.13 and 13.90 ± 1.58%). Furthermore, the roots of the contaminated eggplants had the highest carbohydrate content (17.83 ± 1.90%) and the lowest N, K, and protein contents (1.15 ± 0.21%, 18.86 ± 1.14 mg kg−1, and 7.19 ± 1.31%).
Plant heavy metals
The examination of heavy metals in plant tissues revealed considerable variance (P < 0.05) among the various organs of eggplants, with all analyzed metal contents being markedly higher in contaminated farms than in uncontaminated farms (Table 4). Furthermore, the belowground roots acquired larger metal concentrations than the aboveground shoots. The highest quantities of the studied heavy metals were found in the roots of contaminated plants, while the lowest contents of all metals were found in the shoots of uncontaminated eggplants, except for Zn (4.52 ± 1.03 mg kg−1). The contaminated plant roots accumulated heavy metals content (mg kg−1) in the order: Fe (2076.33 ± 43.02) > Mn (158.17 ± 13.84) > Zn (110.83 ± 15.69) > Ni (46.33 ± 10.61) > Cu (42.00 ± 12.56) > Cr (34.33 ± 9.29) > Cd (23.00 ± 4.27) > Pb (16.85 ± 1.22) > Co (4.53 ± 0.55) > V (0.42 ± 0.03), while the contaminated shoots was Fe (1836.83 ± 58.18) > Mn (67.67 ± 10.41) > Zn (49.00 ± 3.77) > Ni (26.21 ± 8.78) > Cr (19.17 ± 2.10) > Cd (15.33 ± 0.76) > Pb (14.00 ± 3.28) > Cu (13.67 ± 2.02) > Co (2.93 ± 0.13) > V (0.40 ± 0.05).
Regarding the heavy metals’ analysis of the edible fruits of the eggplant, it was observed that all investigated metals were considerably (p < 0.01) higher in the contaminated than uncontaminated plants (Table 5). The order of heavy metals concentration accumulated by the contaminated fruits was Fe (2051.67 ± 2.89) > Mn (76.83 ± 0.29) > Zn (54.42 ± 0.08) > Ni (45.00 ± 0.5) > Cr (30.83 ± 0.29) > Pb (28.33 ± 0.76) > Cd (23.83 ± 0.29) > Cu (19.67 ± 0.29) > Co (2.93 ± 0.03) > V (0.18 ± 0.01), while that of the uncontaminated fruits was Fe (95.47 ± 7.08) > Mn (25.12 ± 3.59) > Cr (10.29 ± 1.28) > Zn (5.36 ± 0.13) > Pb (4.91 ± 0.14) > Ni (3.13 ± 0.11) > Co (1.17 ± 0.29) > Cu (0.48 ± 0.01) > Cd (0.31 ± 0.01) > V (0.03 ± 0.01).
Transfer factors (TF)
The eggplants' ability to accumulate heavy elements in polluted soils was revealed by the descriptive statistical test represented by the transfer factor (Table 6). The TF of all examined heavy metals (excluding Pb and Zn) from soil to plant root exceeded 1, with Ni having the greatest value (201.43). It was arranged as Ni > Cr > Cd > Fe > Co > Cu > V > Mn > Pb > Zn. On the other side, the TF of all determined metals, except Mn and Zn (1.16 and 4.52), from the plant root to the shoot was less than 1. Moreover, the TF of Pb, Cr, Mn, and Zn (1.02, 5.21, 1.65, and 3.11) from the plant root to the fruits of the eggplant was greater than 1, while that of the other investigated metals did not exceed 1.
Health risk assessment
The safety of eggplant consumption was assessed using the health risk index (HRI) as the heavy metals safety index, which requires at first the determination of the DIM for children and adults consumers (Table 7). The DIM of the estimated heavy elements (except Mn in contaminated farms) by consuming eggplants was less than one for both adults and children. For instance, the DIM of Mn was 1.08 and 1.24 mg day−1 for adults and children, respectively. Moreover, the results of the health risk assessment using the HRI showed the presence of health risks from consuming eggplants in the uncontaminated sites due to Pb (HRI: 2.57) for adults and Pb and Mn (2.96 and 1.08) for children. Besides, there are health risks from consuming the contaminated eggplants due to the high HRI of Pb, Cd, Ni, Fe, and Mn for adults (14.86, 12.50, 1.18, 1.54, and 2.88, respectively), and children (17.09, 14.37, 1.36, 1.77, and 3.31, respectively).
Discussion
Heavy metals contamination of the agricultural soils is a severe worry for food safety and a potential health hazard, in addition to its impact on the soil ecosystem37,38. The contaminated soils had higher pH, EC, and heavy metal values than the uncontaminated soils, resulting in significantly higher macronutrient content. These results agreed with those of Galal39, Eid et al.40, and Shehata and Galal25. The elevated heavy metal contents in the contaminated sites are the result of the industrial effluents, municipal wastewater, and the excessive use of pesticides and fertilizers. Galal et al.41 reported similar findings in south Greater Cairo. According to WHO/FAO42, soil Pb, Cd, Fe, Mn, Zn, Co, and V of the contaminated sites and soil Cd, Fe, Co, and V of the uncontaminated site exceeded the permissible limits for normal soils. Moreover, the PLI data revealed that the contaminated soils were highly polluted (PLI > 5) with Pb, Cu, Fe, and Zn; moderately polluted (PLI = 1–4) with Cd, Cr, Mn, Mn, Co, and V; and unpolluted (PLI < 1) with Ni31. Similar output was postulated by Galal et al.16,41,43.
The growth properties of the eggplant recognized significant reductions in the measured parameters except for plant density in heavy metals-contaminated farms. Under contamination, the length of the shoot and root, besides the number of leaves, declined by 52.5, 45.2, and 29.0 percent, respectively. These findings corroborated those of Chaturvedi et al.44 and Ai et al.45, who found that eggplant growth prevention is a prevalent indication of heavy metal stress. Additionally, Ekmekçi et al.46 reported a 19.2% reduction in the eggplant height under heavy metals stress. The growth reduction of the eggplant may be due to its uptake of high contents of heavy elements such as Ni, Cu, Fe, Mn, Cr, Cd, and Pb in its different tissues. Plant growth reduction due to heavy metals was reported by several researchers16,25,40,43,47, especially Pb and Cd, which significantly reduce root and stem length beside the number of leaves of the eggplant48. Besides, Farahat et al.49 and Ghazi et al.50 reported that Co and Cr ions inhibit shoot and root length, shoot and root biomass, and the number of leaves, and attributed this inhibition to the reduction in cell elongation and cell division, photosynthetic pigments, and photosynthetic activity. Moreover, the eggplant responds to heavy metals, especially Cu stress through the prevention of the shoot and/or root growth44,51. Plants may withstand heavy metal stress by accumulating these metals in their roots and preventing their transfer to other sections of the plant52. Consequently, heavy metals stored in the root inhibits its growth rather than affecting other organs.
Generally, the present study observed a considerable decrease in the eggplant biomass and production in heavy metals-contaminated farms. Under contamination conditions with significant heavy metals concentrations, the eggplant's fresh and dry biomass and production were reduced by 41.2, 44.6, and 52.1%, respectively. The results of Ai et al.53 recorded a 35.84% reduction in eggplant production in wastewater-irrigated soils. Vecchia et al.54 attributed the decrease in plant growth to the prevention of mitotic division caused by Pb and Cd heavy metals. Additionally, Rizwan et al.55 reported a negative impact of Cd on the biomass and yield of various vegetables. Also, essential elements (such as Zn and Cu) are necessary for plant development, but excessive amounts have a negative impact56. According to Ai et al.53, a combination of Cu, Zn, Pb, and Cd significantly affects eggplant biomass and production. On the other side, the high biomass and production of the eggplant in the uncontaminated farms may be attributed to the high nutrients (N, P, and K) content. This result coincided with Eid et al.40 who attributed the high yield of kidney beans to the higher contents of soil macronutrients and organic matter. Moreover, increasing N content can promote the growth of aboveground shoots resulting in high biomass and production45.
Chlorophyll a and b are required for photosynthesis and are very susceptible to heavy metal pollution stress46. Plants with high metal toxicity have a variety of growth disorders, including photosynthesis51. The chlorophyll a and b levels in plants cultivated in contaminated soil decreased significantly, which could be due to eggplant's high absorption capability for heavy metals that impede chlorophyll production32,41. Additionally, the high salinity of the contaminated sites enhances chlorophyllase activity, which degrades plant chlorophylls16. Chaturvedi et al.44 found a negative relationship between heavy metals quantity and pigments content in eggplant leaves. Furthermore, Piotrowska et al.57 and Singh et al.58 hypothesized that heavy metals like Pb could impede the biosynthesis of chlorophyll by blocking the intake of necessary components for photosynthetic pigments (e.g., Mg, K, Ca, and Fe). This may interpret the lower contents of macronutrients in the tissues of eggplant cultivated in the contaminated sites. According to Collado-González et al.59, macronutrients such as N, P, and K enhance the production of cauliflower curds. However, Kumar et al.11 stated that the high content of macro-and micro-nutrients may induce crop yield but may also negatively impacts biological activity. Moreover, the eggplant highly uptake K because it is a principal nutrient for its development17.
Compared to normal soils, vegetable plants absorb and store more heavy metals from contaminated soils60. In a similar trend, the current study found that eggplants grown in contaminated farms collected much greater levels of heavy metals than eggplants grown in uncontaminated fields. In the same context, Galal et al.16 and ur Rehman et al.61 stated that crop plants uptake higher heavy metal concentrations from wastewater-irrigated than freshwater-irrigated soils. Besides, the belowground roots accumulated higher concentrations of all investigated metals than the aboveground shoots. Tolerant plants, such as eggplant, tend to reduce soil-root and root-shoot transfer, resulting in less biomass accumulation41. The heavy metal contents in eggplant fruits were lower than in green vegetables like cabbage and common mallow16,43. According to Chowdhury et al.7, heavy element deposition in leafy vegetables is greater and easier than in fruit or grain crops, even though ingestion of these plants is the principal avenue of human exposure to heavy metals. It’s worth noting that the quantities of Cu, Ni, Pb, Cd, and Co in the shoots and edible fruits of eggplant cultivated in contaminated soils, as well as shoot Cr and fruit Mn, were above the permissible range for normal plants29,52,62. As reported by Ai et al.45, solanaceous edible fruits were mostly polluted with Pb, while the Cd concentration surpassed the standard level, exclusively in eggplant. They also found that Cu, Zn, and Pb levels in eggplant fruits were higher than the National Food Safety Standard for Contaminants in Foods (GB2762-2017). The heavy metal contents in the edible sections of eggplant grown in contaminated fields were higher than those found by Jolly et al.63, Zhou et al.64, and Chaturvedi et al.44 on the same plant grown in contaminated soils.
Heavy metals translocation is a metabolic pathway that is primarily affected by chemical and physical factors44. They could be taken up by eggplants from the soil, accumulated in their roots, and possibly transferred to their edible fruits. The transfer potential of eggplants revealed that, except for Pb and Zn, they primarily retain the heavy metals studied in their subterranean organs rather than translocating them to the aerial parts. This result can be related to the poor mobility of these metals from below-ground to above-ground components, making this plant a good choice for the phytostabilization of these metals. In the contaminated farms, the TF of the studied heavy elements from soil to eggplant roots fell in the order: Ni > Cr > Cd > Fe > Co > Cu > V > Mn > Pb > Zn, which is like that reported by Ai et al.53 on eggplant, Shehata and Galal25 on cucumbers, Galal et al.41 on cauliflower, and Galal et al.16,43 on cabbage and common mallow, respectively. On the other hand, the eggplants had the potential to translocate Mn and Zn to their shoot and Pb, Cr, Mn, and Zn to the edible fruits. These results indicate the possibility of eggplant being an accumulator and phytoextractor of these metals. Chaturvedi et al.44 recorded that Pb can be translocated to the shoots, but not to the fruits, while Cd was stored in the roots of eggplant. The translocation and accumulation of these metals in the cauliflower’s edible section will expose the public consumers to high health risks.
The nutritional quality of vegetables is a great concern since vegetables are a main component of the human diet8. Heavy metals accumulation in the edible organs of vegetable plants is a common hazard to the consumers’ health61. The DIM for the studied heavy metals (except Mn in contaminated farms) by consuming eggplants was less than one for both children and adults. For instance, the DIM of Mn was 1.24 and 1.08 mg day−1 for children and adults, respectively. These results coincided with those of Chaturvedi et al.44 on contaminated eggplant, Rehman et al.65, and ur Rehman et al.61 on wastewater-irrigated cauliflower. Moreover, the HRI showed the presence of health hazards from consuming eggplants in the uncontaminated sites due to Pb for adults and Pb and Mn for children. Besides, there are health risks from consuming the contaminated eggplants due to the high HRI (> 1) of Ni, Fe, Mn, Cd, and Pb for children and adults. In a corresponding study, Saeedifar et al.66 and Ai et al.45 found that the accumulation of Pb and Cd negatively affects the safety of eggplant fruits. According to the US-EPA35, the HRI > 1 of these metals shows that consumers are exposed to health risks because of the eggplant's large proportion in local populations' diets, posing a greater danger to human health. Galal et al.41 also observed health problems from eating contaminated cauliflower due to excessive dietary Pb, Cd, Mn, Fe, and Ni levels. Khanal et al.67 and Ma et al.13 recorded similar findings when it came to the health effects of these metals when eating contaminated cauliflower.
Conclusion
The contaminated soils supporting eggplant growth were highly polluted with Fe, Cu, Pb, and Zn, and relatively polluted with Cd, Cr, Mn, Mn, Co, and V. Cultivation of eggplant in contaminated soils can result in high amounts of Cu, Co, Ni, Mn, Cd, and Pb in its edible sections, exceeding the permissible level for normal plants, and exposing public consumers to significant health risks. The heavy metal contamination stress lowered morphological measures and photosynthetic pigments, which in turn affected eggplant crop growth and production. The eggplant retained most investigated heavy metals (except Pb and Zn) in its root due to their low mobility, and consequently, this plant is an appropriate candidate for these metals’ phytostabilization. However, the study plant had the potential to translocate Mn and Zn to its shoot and Pb, Cr, Mn, and Zn to the edible fruits indicating its possibility to be a phytoextractor and accumulator of these metals. The present study indicated health risks from consuming the contaminated eggplants due to the dietary intake of Fe, Mn, Cd, Ni, and Pb for adults and children. Therefore, environmental protection laws must be applied to control industrial and municipal wastes discharged into the agricultural lands and hence avoid the entrance of toxic metals into the food chain. Besides, washing eggplant with water and various household chemical solutions can significantly remove heavy metals. For safety issues and food sustainability, our investigation strongly recommends the cultivation of eggplant in uncontaminated areas and avoid, as possible, its cultivation in contaminated agricultural lands due to its toxic effects even in the long run.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Eid, E. M., Galal, T. M. & El-Bebany, A. F. Prediction models for monitoring heavy metals accumulation by wheat (Triticum aestivum L.) plants grown in soil amended with different rates of sewage sludge. Int. J. Phytoremediation 22(10), 1000–1008 (2020).
Singh, R. P. & Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 36(7), 969–972 (2010).
Wu, N., Su-mei, L., Gui-ling, Z. & Hong-mei, Z. Anthropogenic impacts on nutrient variability in the lower yellow river. Sci. Tot. Environ. 755, 142488 (2021).
Jacob, J. M. et al. Biological approaches to tackle heavy metal pollution: a survey of literature. J. Environ. Manag. 217, 56–70 (2018).
Gharib, F. A., Mansour, K. H., Ahmed, E. Z. & Galal, T. M. Heavy metals concentration, and antioxidant activity of the essential oil of the wild mint (Mentha longifolia L.) in the Egyptian watercourses. Int. J. Phytoremediation 23(6), 641–651 (2020).
Dai, T. et al. Dynamics of coastal bacterial community average ribosomal RNA operon copy number reflect its response and sensitivity to ammonium and phosphate. Environ. Pollut. 260, 113971 (2020).
Chowdhury, A. H., Chowdhury, T. & Rahman, A. Heavy metal accumulation in tomato and cabbage grown in some industrially contaminated soils of Bangladesh. J. Bangladesh Agric. Univ. 17(3), 288–294 (2019).
Hu, S., Liu, L., Zuo, S., Ali, M. & Wang, Z. Soil salinity control and cauliflower quality promotion by intercropping with five turfgrass species. J. Clean Prod. 266, 121991 (2020).
Hu, W. Y., Zhang, Y. X., Huang, B. & Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: current status and management strategies. Chemosphere 170, 183–195 (2017).
Qin, S. et al. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2), 168–180 (2020).
Kumar, V., Thakur, K. R. & Kumar, P. Assessment of heavy metals uptake by cauliflower (Brassica oleracea Var. botrytis) grown in integrated industrial effluent irrigated soils: a prediction modeling study. Sci. Hortic. 257, 108682 (2019).
Paltseva, A., Cheng, Z., Egendorf, S. & Groffman, P. Remediation of an urban garden with elevated levels of soil contamination. Sci Total Environ. 722, 137965 (2020).
Ma, L., Liu, Y., Wu, Y., Wang, Q. & Zhou, Q. The effects and health risk assessment of cauliflower co-cropping with Sedum alfredii in cadmium contaminated vegetable field. Environ. Pollut. 268, 115869 (2021).
Dong, Q., Fei, L., Wang, C., Hu, S. & Wang, Z. Cadmium excretion via leaf hydathodes in tall fescue and its phytoremediation potential. Environ. Pollut. 252, 1406–1411 (2019).
Perveen, S., Ihsanullah, I., Shah, Z., Shah, W. S. S. & Shah, H. H. Study on accumulation of heavy metals in vegetables receiving sewage water. J. Chem. Soc. Pak. 33, 220 (2011).
Galal, T. M., Sheded, Z. A. & Hassan, L. M. Hazards assessment of the intake of trace metals by common mallow (Malva parviflora L.) growing in polluted soils. Int. J. Phytoremediation 21(14), 1397–1406 (2019).
Ogundeji, A. O. et al. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium Wilt. Appl. Soil Ecol. 163, 103912 (2021).
Mauro, R. P. et al. Recovery of eggplant field waste as a source of phytochemicals. Sci. Hort. 261, 109023 (2020).
Martini, S., Conte, A., Cattivelli, A. & Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 341, 128298 (2021).
Gürbüz, N., Uluişik, S., Frary, A., Frary, A. & Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 268, 602–610 (2018).
FAOSTAT. Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/567/default.aspx#ancor (2014).
Asgari, K. & Cornelis, W. M. Heavy metal accumulation in soils and grains, and health risks associated with use of treated municipal wastewater in subsurface drip irrigation. Environ. Monit. Assess. 187, 410 (2015).
IWMI. (International Water Management Institute) Global water scarcity study. From http://www.iwmi.cgiar.org/home/wsmap.htm (2000).
Oweis, T. & Hachum, A. Water harvesting for improved rainfed agriculture in the dry environments. In Rainfed Agriculture: Unlocking the Potential (eds Wani, S. P. et al.) 164–181 (CABI, 2009).
Shehata, H. S. & Galal, T. M. Trace metal concentration in planted cucumber (Cucumis sativus L.) from contaminated soils and its associated health risks. J. Cons. Prot. Food Safe. 15, 205–217 (2020).
Elawa, O. E. Impact assessment of industrial pollution on some economic plants south of Cairo Province, Egypt. M.Sc. Thesis, Helwan Uni Cairo, Egypt 194 pp (2015).
Umbriet, W. W. et al. Monometric Technique, A Manual Description Method, Applicable to Study of Desiring Metabolism 239 (Burgess Publishing Company, 1959).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Allen, S. E. Chemical Analysis of Ecological Materials (Blackwell Scientific Publications, 1989).
Liu, R. L., Li, S. T., Wang, X. B. & Wang, M. Contents of heavy metal in commercial organic fertilizers and organic wastes. J. Agro-Environ. Sci. 24, 392–397 (2005).
Lu, X. W. et al. Risk assessment of toxic metals in street dust from a medium-sized industrial city of China. Ecotoxicol. Environ. Safe. 106, 154–163 (2014).
Eid, E. M., Alrumman, S. A., Galal, T. M. & El-Bebany, A. F. Regression models for monitoring trace metal accumulations by Faba sativa Bernh. plants grown in soils amended with different rates of sewage sludge. Sci. Rep. 9, 5443 (2019).
Khan, S. et al. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 152, 686–692 (2008).
Rattan, R. K., Datta, S. P., Chhonkar, P. K., Suribabu, K. & Singh, A. K. Long-term impact of irrigation with sewage effluents on heavy metals content in soils, crops and groundwater: a case study. Agric. Ecosyst. Environ. 109, 310–322 (2005).
US-EPA. Environmental Protection Agency, Region 9, Preliminary remediation goals (2012).
SPSS. SPSS Version 20.0. SPSS Inc, 233 S Wacker Drive, Chicago, Illinois (2012).
Al-jaboobi, M. et al. Evaluation of heavy metals pollution in groundwater, soil and some vegetables irrigated with wastewater in the Skhirat Region, Morocco. J. Mater Environ. Sci. 5(3), 961–966 (2014).
Rangamani, T., Rao, K. V. & Srinivas, T. Evaluation of heavy metals in water, soil and cauliflower in Penamaluru Mandal in Vijayawada. Int. J. Multidiscip. Adv. Res. Trends 1(3), 242–248 (2017).
Galal, T. M. Health hazards and heavy metals accumulation by summer squash (Cucurbita pepo L.) cultivated in contaminated soils. Environ. Monit. Assess. 188(7), 134 (2016).
Eid, E. M. et al. Evaluation of newly reclaimed areas in Abha, Saudi Arabia, for cultivation of the Phaseolus vulgaris leguminous crop under sewage sludge amendment. J. Cons. Prot. Food. Safe. https://doi.org/10.1007/s00003-020-01311-z (2021).
Galal, T. M., Hassan, L. M. & Elawa, O. E. Impact Assessment of Industrial Pollution on Important Food Crops (Lambert Academic Publishing Gmbh&Co.KG, 2019).
WHO/FAO (2013) Guidelines for the Safe Use of Wastewater and foodstuff; 2(1), 14 pp
Galal, T. M., Khalafallah, A. A., Elawa, O. E. & Hassan, L. M. Human health risks from consuming cabbage (Brassica oleracea L. var. capitata) grown on wastewater irrigated soil. Int. J. Phytoremediation 20(10), 1007–1016 (2018).
Chaturvedi, R., Favas, P., Pratas, J., Varun, M. & Paul, M. S. Ecotoxicology and environmental safety assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal (Loid) contaminated soil. Ecotoxicol. Environ. Safe. 148, 318–326 (2018).
Ai, P., Jin, K., Alengebawy, A., Elsayed, M. & Meng, L. Effect of application of different biogas fertilizer on eggplant production: analysis of fertilizer value and risk assessment. Environ. Technol. Innov. 19(13), 101019 (2020).
Ekmekçi, Y., Tanyolaç, D. & Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. Plant Physiol. 165, 600–611 (2008).
Hadi, F., Bano, A. & Fuller, M. P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80, 457–462 (2010).
Li, G. et al. A multi-level analysis of China’s phosphorus flows to identify options for improved management in agriculture. Agric. Syst. 144, 87–100 (2016).
Farahat, E. A., Galal, T. M., Elawa, O. E. & Hassan, L. M. Health risk assessment and growth characteristics of wheat and maize crops irrigated with contaminated wastewater. Environ. Monit. Assess. 189, 535 (2017).
Ghazi, S. M., Galal, T. M. & Husein, K. H. Monitoring Water Pollution in the Egyptian Watercourses: A Phytoremediation Approach (Lap Lambert Academic Publishing, 2019).
Körpe, D. A. & Aras, S. Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Mutat. Res. 719, 29–34 (2011).
Nagajyoti, P., Lee, K. & Sreekanth, T. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 8, 199–216 (2010).
Ai, S. et al. Temporal variations and spatial distributions of heavy metals in a wastewater-irrigated soil-eggplant system and associated influencing factors. Ecotoxicol. Environ. Safe. 153, 204–214 (2018).
Vecchia, F. D. et al. Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci. 168, 329–338 (2005).
Rizwan, M. et al. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 182, 90–105 (2017).
Kupper, H. & Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 8, 269–285 (2016).
Piotrowska, A., Bajguz, A., Godlewska, B., Czerpak, R. & Kaminska, M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lamnaceae). Environ. Exp. Bot. 66, 507–513 (2009).
Singh, R. et al. Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour. Technol. 101, 3025–3032 (2010).
Collado-González, J., Piñero, M. C., Otálora, G. & López-Marín, J. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hortic. 277, 109818 (2021).
Al Jassir, M., Shaker, A. & Khaliq, M. Deposition of heavy metals on green leafy vegetables sold on roadsides of Riyadh City, Saudi Arabia. Bull. Environ. Contam. Toxicol. 75, 1020–1027 (2005).
ur Rehman, K. et al. Ecological risk assessment of heavy metals in vegetables irrigated with groundwater and wastewater: the particular case of Sahiwal District in Pakistan. Agric. Water Manag. 226, 105816 (2019).
Misra, S. G. & Mani, D. Soil pollution (Ashish Publishing House, 1991).
Jolly, Y. N., Islam, A. & Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. Springerplus 2, 385 (2013).
Zhou, H. et al. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 13(3), 289 (2016).
Rehman, Z., Hussain, R. A., Jabeen, S. & Ali, S. Heavy metals (Cd, Pb & Zn) accumulation in cauliflower (Brassica oleracea var. botyris) and associated health risks assessment in three districts of Punjab Pakistan. Biol. Pak. 64(1), 105–110 (2018).
Saeedifar, F., Ziarati, P. & Ramezan, Y. Nitrate and heavy metal contents in eggplant (Solanum melongena) cultivated in the farmlands in the south of Tehran-Iran. Int. J. Farm Allied Sci. 3, 60–65 (2014).
Khanal, B. R., Shah, S. C., Sah, S. K. & Shriwastav, C. P. Heavy metals accumulation in cauliflower (Brassica oleracea L. var. botrytis) grown in brewery sludge amended sandy loam soil. Int. J. Agric. Sci. Technol. 2(3), 87–92 (2014).
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant No. (G: 134-662-1442). The authors, therefore, acknowledge with thanks DSR for technical and financial support. They also should present their deepest gratitude to Prof. Loutfy M. Hassan and Mr. Omar Elawa, Helwan University, for their kind help in the experimental work.
Author information
Authors and Affiliations
Contributions
The two authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by K.A. and T.G. The first draft of the manuscript was written by K.A. and T.G. commented on previous versions of the manuscript. The two authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alamer, K.H., Galal, T.M. Safety assessment and sustainability of consuming eggplant (Solanum melongena L.) grown in wastewater-contaminated agricultural soils. Sci Rep 12, 9768 (2022). https://doi.org/10.1038/s41598-022-13992-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13992-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.