Abstract
Himalayan ecosystem is characterized by its fragile climate with rich repositories of biodiversity. Waste collection and disposal are becoming increasingly difficult due to topographical variations. Aporrectodea caligenosa, a versatile psychrophillic soil dweller, is a useful biocatalyst with potent bio-augmented capability for waste treatment at low temperatures. Microcosm experiments were conducted to elucidate the comprehensive nature of biogenic nitrogen transformation to NH4+ and NO3− produced by coupling of earthworm-microbes. Higher biogenic recovery of NH4+-N from coprolites of garden soil (47.73 ± 1.16%) and Himalayan goat manure (86.32 ± 0.92%) with an increment of 14.12 and 47.21% respectively over their respective control (without earthworms) with a linear decline beyond 4th week of incubation was reported. NO3–-N recovery progressively sustained in garden soil and goat manure coprolites during entire incubation with highest 81.81 ± 0.45 and 87.20 ± 1.08 µg-N g−1dry weight recorded in 6th and 5th week of incubation respectively and peak increments as 38.58 and 53.71% relative to respective control (without earthworms). Declined NH4+–N in coprolites at low temperature (15.0 ± 2.0 °C) evidenced increased nitrification rates by taking over the process by abundant nitrifying microbes. Steady de-nitrification with progressive incubation on an average was 16.95 ± 0.46 ng-N g−1 per week and 21.08 ± 0.87 ng-N g−1 per week compared to 14.03 ± 0.58 ng-N g−1 per week and 4.50 ± 0.31 ng-N g−1 per week in respective control treatments. Simultaneous heterotrophic nitrification and aerobic denitrification (SHNAD) was found to be a prominent bioprocess at low temperature that resulted in high and stable total nitrogen and nitrate accumulation from garden soil and goat manure with relative recovery efficiency of 11.12%, 14.97% and 14.20%; 19.34%. A. caligenosa shows promising prospects for mass applicability in biogenic N removal from manure of Himalayan goat.
Similar content being viewed by others
Introduction
Potential psychrophillic class of soil macrofauna are earthworms (Endogeic and Anecic species) that exploited deeper mineral soil beds, and thereby remodel and restructure nutrient pools in uppermost soil layers (hereinafter: geo-engineering earthworms)1. Aporrectodea caliginosa (Endogeic) is a non-permanent horizontal burrower and lives in the uppermost soil horizons2,3, ingesting enormous load of soil, rich in organic matter, leaving the casting as a key input into the soil and contributing biogeochemical cycles. Such earthworms have a direct impact on soil nutrient status and microbial population (GAPs i.e., gut associated processes) or an indirect impact (CAPs i.e. coprolites associated processes)4,5. They have the capacity to transform as well as restructure nitrogen (N) pools6,7 and geo-engineering by earthworms may have considerable effects on arctic soils1. Earthworms may influence the entire soil food web8,9 with significant influence on soil structure through horizontal and vertical drillosphere burrows and castings10 escalating the distribution and community composition of the temperate soil micro flora11,12,13. In temperate ecosystems earthworm cast production can range from 36 to 250 tons ha−1 year−114. Earthworms represent biocatalysts of class oligochaeta that stimulate N mineralization15,16,17 and can bring about augmented nitrification18,19 or denitrification owing to their gut microbial community20,21. Earthworms stimulate microbial processes for recovery of N from organic compounds22. Moreover, microorganisms thrive in earthworm galleries because of transit through the worm gut and presence of lavish mucus and casts23,24. Biodegradable or perishable organic wastes could be converted into stable humus-like substances by way of vermicomposting25 as an end product which is a good organic fertilizer26 containing abundant humic acids and beneficial microbes27. Vermicoprolites have been observed to encompass elevated levels of NH4+, NO3−, Mg, P and K relative to surrounding soil13,28,29. The nitrogen transformation is an important component of waste conversion during composting and vermicomposting processes30,31,32 and processes like nitrification and denitrification are the key activities contributing to nitrogen cycle, playing an important role in nutrient supply, (NH4+-N), volatilization and greenhouse gas (GHG) emissions for various ecosystems30,33. Most of the reported heterotrophic nitrifying-aerobic bacteria are mesophilic bacteria, carrying out nitrification and denitrification at temperatures ranging from 15 to 37 °C34,35. Temperate microbes have obvious physiological advantages36 and unique ability to perform nitrification and denitrification synchronously37 and improve the overall nitrogen removing efficacy at lower temperatures. Guraz valley, a fragile cold ecosystem, located in high Himalayas with prominent features of hill agriculture and diverse habitats rich in biodiversity. The disparate topographic features offer microhabitat for a variety of organic herbal crop species to grow in the main cultivated area while woody Betula pendula, Pinus roxburghii, Quercus robur and Cedrus deodara grow in the surrounding forests38. All the three ethnic Himalayan communities (nomadic Bakerwals, seminomadic Gujjars and semi-sedentary Kashmiri shepherds) depend on natural resources38. Since, there is very insufficient and scanty information regarding wastes recycling and recovery of nitrogen nutrient in the Himalayan ecosystem. Nitrogen recovery from waste material has now become a pivotal issue globally (Wang et al. 2015; Chen et al. 2016). Open dumping of bio-wastes is considered a potent source of nitrogen pollution in different forms viz; NH3 and N2O to air and N-NO3− to ground and surface water resources39,40. Changes in transformation/emission rates are affected by the soil temperature, because it affects the activity of urease, nitrifier communities, and nitrification rate in the soil. Transition in NH4+-N, NO3−-N and total nitrogen in the interim of vermicomposting had been reported, however, divergence and succession of N-transformation process through involving functional psychrophillic microbes and earthworms were seldom reported. Therefore, the information on cold tolerant earthworms and associated nitrifying and denitrifying microbial species can furnish novel insights into the N transformation during the vermicomposting process in cold habitats. Moreover, the positive priming through the endogeic geophagous earthworm’s influence is expected to foster the recycling of nutrients, especially organic carbon, nitrogen and phosphorus41,42. The present study, which is perhaps the first of its kind under cold habitat of Guraz valley in Kashmir region, will pledge cognition and contribute to sound understanding of mineral N dynamics using psychrophillic A. caligenosa indigenous to the Guraz valley, with the goal as (i) o determine the involvement of coprolite associated microorganisms in bioconversion of N, (ii) to enumerate the changes in N-NH4+, N-NO3− and (iii) specifically, recycle and evaluate the impact of locally available garden soil and Himalayan goat manure on physico-chemical flux’s in coprolites resulting in minimizing nitrogen pollution in temperate ecosystems.
Results and discussion
Ammonium and nitrate dynamics
The analysis performed on coprolites of Aporrectodea caliginosa evidenced that ammonium (NH4+-N) concentration after one week of incubation were significantly affected by the source of food (Fig. 1). As evident from Fig. 1 the concentration of NH4+-N in all the earthworm released coprolites increased steadily upto the 4th week of the vermicomposting process, however, coprolites of goat manure (GM) shows continuous increase in NH4+-N content upto the 5th week. The NH4+-N concentration in GM coprolites was significantly (p < 0.05) higher than GM(control) concentration upto 5th week of incubation, after which the NH4+-N concentration began to fall steadily until the 6th week of incubation. Further, all treatments showed decline in the NH4+-N concentration after 4th week continued upto the 6th week of incubation. It was observed that after 6 weeks of vermicomposting the NH4+-N concentration in the coprolites was in the order; GM(control) (24 µg NH4+-N g-1 DW) > GS(Control) (35 µg NH4+-N g–1 DW) > GS (38 µgNH4+-Ng−1 DW) > GM (80 µg NH4+-N g−1 DW). Our results reflect that the gross transformation rate of N-NH4+ responded differently based on substrate type and feeding preference of A. caliginosa. GM aids gut associated microbes and was found to accelerate the mineralization of N to NH4+. The elevated NH4+-N levels in coprolites might be due to various nitrogen-containing compounds released by earthworm metabolism through muco proteins and urine. Previous studies also suggest that adult earthworms enhance ammonification43,44 by breakdown of large volumes of organic residues with the aid of gut associated bacteria thereby increases biogenic NH4+31,45. The steady increase of NH4+ concentration in GM and GS coprolites is related to the initial carbon and nitrogen content of the substrate. However, subsequent decrease with incubation time was reflected by the conversion of part of NH4+-N to NO3–-N, influenced by the dominance of nitrifying chemolithotrops at low temperatures (16.5–2 °C) (Fig. 1). Earlier research also suggests that ammonification is a temperature sensitive microbial mediated mineralization process46,47,48 and an increase in temperature upto 25 °C will significantly increase the transformation rate of N49. The trend for nitrate (NO3−N) concentration varied between 8.37 and 81.88 µg NO3–N g–1DW in coprolites influenced by different treatments. The NO3–-N concentration of coprolites from earthworms fed on GM and GS was found higher. NO3–-N concentration showed a linear increase from the 1st to 6th week of vermicomposting from coprolites of both GM and GS with the highest values recorded as 90.2 µg NO3–N g–1 DW and 81.81 µg NO3–N g–1 DW respectively. The usefulness of earthworm’s gut microbes inflates the process of nitrification compared to the situation without earthworms43,50. The NO3–-N removal from the GM was exceptionally high and ranged between 22.03 to 90.20 µg- NO3 g–1DW with an average value of 64.24 µg- NO3 g–1 DW during the entire incubation period (Fig. 2). Similarly, for GS NO3–-N recovery ranges from 8.37 to 81.81 µgNO3 g−1 DW. Further, our findings reveal a linear increase in NO3–-N concentration from GM coprolites along the duration of incubation, which is assumed to be correlated with a higher concentration of NH4+-N in coprolites, which is a primary source for the nitrification process. Our results also suggest that continuous escalation in NO3–-N could be due to the involvement of heterotrophic bacteria and fungi in coprolites (Table 1) preferring a moderate temperature between 15 and 25 °C which also shows that heterotrophic nitrification process exceeds autotrophic nitrification. Previous research findings suggest that earthworm gut and castings tend to have more active heterotrophic microbes that positively influence the amounts of extractable NO3–-N in upper layer of soil51,52,53 moreover, increased NO3− concentration in coprolites is a good indicator of the nitrification process and abundance of nitrifiers23,25,54,55,56. It is also reported that the heterotrophic nitrification has an optimum temperature requirement of 15 °C57 while, it exceeds over autotrophic nitrification if the temperature is increased to 35 °C49. Further, we confirm that the heterotrophic nitrification is an ecosystem dependent process which is directly dependent on substrate type, microbial diversity and abundance. The samples of study material yielded a large number of indigenous heterotrophic nitrifiers, which were determined to have high nitrogen removal abilities. Temperature stimuli, NH4+-N and NO3–- N content in substrates influenced the overall performance of heterotrophic nitrification process. Our previous research in the cold arid Ladakh and Kashmir valley backs up this argument. The influence of low temperature on heterotrophic nitrification at the experimental site (Guraz valley) is consistent with the previous findings, which demonstrated that an optimal temperature, substrate type and ecology conditions favor heterotrophic nitrification process13,58,59. Low concentration of NO3−N is related to low microbial dominance (nitrifying bacteria) in coprolites from control treatments of both the substrates suggesting the loss of potency in coprolites which inhibited the nitrification process. Lower functional redundancy in the nitrification process is due to the lack of earthworms in the control treatments which could not either enhance biogeochemical stability of organic matter or stimulate microbial activity (Table 1). Thus, the results clearly elucidated an amicable relationship between earthworm and heterotrophs which favors the heterotrophic nitrification. Previous studies support the increased mineralization of nutrients in earthworm coprolites relative to surrounding garden soil (without earthworm), which is associated with enrichment in liable compounds due to various factors such as, increased activity of nitrifying microbes60, digestion of earthworm could influence gut microbiome61 and earthworm-microbe association produces enzymes that are reported to increase NO3− N and NH4+-N content in coprolites by 31 and 14% respectively62,63,64,65.
Denitrification/ N2O emission during incubation
The interaction between earthworms and denitrifers directly affect the nitrogen dynamics via nitrous oxide (N2O) fluxes. Our study reveals that denitrification predominantly turned out to be limited by low temperature and C supply. N2O emission rates from mesocosm surface coprolites (soil fed earthworms) ranged from 5.90 ± 0.20 ng-N g−1 (1st week) to 16.6 ± 0.48 ng-N g−1 (6th week) with an average of 16.95 ng-N g−1 (Fig. 3). However, the emission rates of N2O from the control treatments, on the hand were relatively low ranging from 4.05 ± 0.29 ng-N g−1 (1st week) to 14.60 ± 0.22 n g-N g−1 (6th week) with an average of 14.0 ng-N g−1 per week. N2O emissions showed a steady increase along with the incubation period except from the 6th week onwards, there was a downward trend, with an average increase of 17.03% in emission from coprolites of GS compared to GS(control). Earlier research has shown that A. caliginous is capable to emit significant amounts of N2O emissions from the soil through different activities66,67,68. Earthworms have the potential to dramatically regulate the physico-chemical properties of their habitats and thereupon affecting the production of GHG20 however, denitrification is affected by a variety of environmental factors including availability of dissolved oxygen (DO), carbon (C), pH, temperature, denitrifying bacterial population and congregations of NO3−, NO2− and S2−31,69,70.
Under mesocosm condition, incubation of earthworm with GM stimulates the denitrification process, which showed a favorable relationship with N2O emission from manure coprolites ranged from 7.26 ± 1.32 ng-N g−1 (1st week) to 20.19 ± 1.03 ng-N g−1 (6th week) (Fig. 4). On an average N2O emission from coprolites of GM was 78.65% higher compared to GM(control), suggesting that A. caliginosa is playing a critical role in conversion of excess NO3− to N2O. It might be attributed to the higher initial availability of carbon in goat manure (Fig. 5), which constituted 70% of the total mass in this substrate, led to faster degradation and enhanced mineralization of N to NO3−. Limited reports are available about the mechanism involved in the combined relationship between the nitrifying and denitrifying microbes that help towards better understanding of the N-cycle in terrestrial ecosystem71,72. Bioconversion of cow dung, duck manure, kitchen waste by earthworms is reported to induce N2O emissions54,73; however, emission rates are significantly lower than thermophiles composting74,75. Vermicomposting under high moisture conditions were reported to decrease N2O and CH4 emissions by 25–36 and 22–26% compared to thermal composting74. Increased N2O emission in GM coprolite must be owing to the activities of denitrifying bacteria, as evidenced by significant (p < 0.05) difference in N2O emission between samples from GM and GM(control) treatments. The research also found a link between nitrification and denitrification (Figs. 4, 8), and demonstrate how a heterogeneous microbial population and function may coexist. Previous research also found that potential denitrification rates were positively correlated with coexistence of aerobic and anaerobic microbes (denitrifers)76,77,78,79,80. In our study, low N2O accumulation was found in headspace of bottles with no C2H2 or with low concentration for both control treatments of GS(control) and GM(control). However, significant (p < 0.05) difference in N2O emission was evidenced between the two control treatments GS(control) and GM(control). On an average, N2O produced from the coprolites fed on GM were 19.57% higher compared to castings obtained from GS.
Physico-chemical analysis of worm casts
Coprolites produced from GS and GM were rich in nutrients and A. caliginous had a substantial impact on the analyzed parameters. Significantly (p < 0.05) higher pH was observed in coprolites from GS and GM treatments relative to the GS(control) and GM(control) (Fig. 5, 6). The final pH values of both the coprolites from GS and GM were slightly acidic to alkaline attributed to gut microbial activity, indicating that these coprolites could be useful to remediate soil reaction. Vermicomposting of fruit and vegetable wastes81,82; seaweeds, sugarcane trash, coir pith amended with cow dung83; and flowering plants (Lantana camara)84 was also reported to produce vermicompost with a pH close to neutral. Analyses of variance for total organic carbon indicated significant (p < 0.05) differences between the coprolites from GS (18.24%) and GM (27.18%) with their controls GS (control) (29.42%) and GM(control) (32.02%) respectively. A linear decline in total organic carbon from GS and GM coprolites is attributable to utilization of carbon by microbes during the entire process (Fig. 7). Previous reports have also mentioned the loss of 19–67% of carbon during the process of vermicomposting85,86 where dehydrogenase activity plays a key role in the hydrolysis of cellobiose and other disaccharides during vermicomposting process82. It has been also reported that the chief mechanism for the carbon loss from the substrates could be attributed to the respiration of earthworms and microorganisms during the decomposition and transformation of substrates87,88. The Fig. 7 depicts a significant (p < 0.05) increase in NO3− by 15 and 39% in coprolites of GS and GM respectively, when compared to GS(control) and GM(control). Increased NO3- content in coprolite is attributed to the significant influence of gut associated nitrifying microbes in the production of NH4+ which is a primary substrate for NO3− yield, in addition, earthworm mucus and nitrogen-rich excretory secretions also contributed to NO3− content. The NH4+ concentration is also correlated with the initial N content of waste substrates, which was 1.85 ± 0.04 and 2.14 ± 0.05% in GS(control) and GM(control), respectively (Fig. 7). Previous research have noted that earthworms may ameliorate the castings as a consequence of N transformation from wastes by associated microbes through bio-waste mineralization and gut N-fixation21,89,90,91. Total N concentration in the coprolites significantly (p < 0.05) increased and could be interpreted due to the factors such as: initial N content of the substrate; bioconversion efficiency; possible death of a few baby worms; secretions of mucus, addition of nitrogenous substances during the entire process. Gut and skin of earthworm can secrete nitrogenous compounds which is also one of the reasons for enriched N content in the end product of the process86,92. At the end of vermicomposting process, the total phosphorus (TP) recovery was found 6.15% in GM and 8.19% GS from the respective substrates (Fig. 7). Increased P concentration in the GM castings could be due to secretion of various organic acids by related microbes and decomposition of substrates by earthworm, as corroborated with previous studies93,94. Table 1 shows that the bacterial population in both coprolites from GS and GM increased that includes Aerobacter sp., Bacillus sp., Citrobacter sp., Escherichia sp., Klebsiella sp., Pseudomonas sp., Proteus sp., Serratia sp. and Staphylococcus sp. The coprolites of GS and GM demonstrated significantly (p < 0.05) higher bacterial and fungal density compared to respective controls GS(control) and GM (control).
The earthworm amended treatments demonstrated significantly (p < 0.05) higher bacterial density than GM(control). Similarly, higher fungal density was also observed in earthworm inoculated coprolites of GS and GM. The fungal species included Aspergillus sp., Fusarium sp., Penicillium sp. and Saccharomyces sp. The presence of more bacteria and fungi in earthworm amended coprolites indicate that earthworms could favor and compliment the microbial communities during conversion of substrates. This is in accordance with earlier results of using mill waste95,96, forest litter waste97, sewage sludge and rice straw31 as substrates in vermicomposting which favors the microbial population. It has also been reported that presence of the earthworms in vermicomposting enhances the beneficial microflora and suppresses harmful pathogenic microbes98, that later enhance plant growth via the production of plant growth promoting compounds. Thus, the higher population of microbes in the earthworm castings could be due to the abundance of beneficial microbes in the earthworm gut. This concept was also supported by the findings of previous research99,100. The potential availability of nutrients in coprolites was revealed by regression analysis between the indices of physico-chemical properties of GM (Table 2). The Fig. 8 shows that the selected parameters were appropriate to determine the stabilization of coprolites in the current investigation. With a cumulative variance 93.10% and high rate of loading from first and second principal component, parameters MC, TN, TP, K, and Zn are strongly correlated with Fe, CN ratio, TOC in the first main component which reflects mineralization and breakdown potential of GM (goat manure). Nitrates and pH are negatively correlated with the second component. It can be inferred that A. caligenosa may have good impact on stabilization of coprolites with positive correlation to initial nutrient status of GM. It implies that coprolites produced from GM are superior to GS as a soil fertilizer. The loading of the variables on the components are presented in Table 3. Which indicates score data of extracted eigenvectors with a corresponding loading of 56.90 and 36.20% for principal component-1 and principal component-2 these loadings in have been computed from the correlation coefficients between these variables.
Nitrification potential
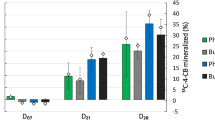
Replicated sampling analysis at intervals revealed an increase in potential nitrification rates, demonstrating a continuous nitrification process in coprolites in all the treatments except control (Fig. 9). The nitrification sampling in triplicates was done at three different stages of vermicomposting; nitrification-I (2nd week), nitrification-II (4th week) and nitrification-III (6th week) to estimate NO3− levels. Nitrification rates increased over ammonification, as performed by the involvement of both nitrifying bacteria and fungi (Table 1). The mean potential nitrification rates of GS and GM coprolites at three stages of the nitrification process were 4.63, 43.46, 51.21 µN g−1DW and 6.48, 130.43 135.71 µN g−1DW respectively that were significantly (p < 0.05) different from the potential nitrification rates of the respective GS(control) & GM(control) (Fig. 9). Abundances of nitrifying bacteria and nitrifying fungi increased nitrification during the entire incubation period, apart from that nitrification potential by earthworm, it also depends on the initial N content of organic substances used by earthworms as a source of food. The study evidenced that unlike other microbial processes, nitrification progressed with a steady increase in NO3− concentration at all the three stages of nitrification process. In contrast, the net rate of nitrification was comparatively low in the GS(control) & GM(control). Previous studies indicate that earthworm castings have strong associated activity for nitrification101,102,103. Our findings, support the hypothesis that earthworm activities with consumption of N-rich food material could increase the nitrification process in coprolites which is also supported by earlier research44,91,104.
Materials and methods
Site description and cold tolerant earthworms
Geophagous endogeic earthworms Aporrectodea caliginosa (A. caliginosa) were obtained from the Mountain Agricultural Research and Extension Station—Izmarg, Guraz valley (74.73° E, 34.64° N; altitude 2580 masl) of District Bandipora, Jammu and Kashmir-India (Fig. 10). The annual mean temperature and precipitation at experimental site are 15.6 °C and 2200 mm, respectively.
Study area map of Guraz valley in Kashmir region-India (Microsoft power point 2013; https://worldmapblank.org/wordpress/wp-content/uploads/2021/08/Location-of-India-on-the-World-map.pdf).
Experiment
The present study used A. caliginosa, which is found in the garden and coniferous forest soils of Guraz valley105. Garden soil (GS) from a field planted with maize + beans and goat manure (GM) from Bakerwal (Himalayan shepherds with rearing Himalayan goats) were used as substrate treatments in triplicates with corresponding control as designated in Table 4. Earthworms were placed in 12-mesocosms (polyvinylchloride–PVC) with a diameter of 550 cm3 filled with substrates separately (Fig. 11). The activity of nitrogen dynamics from the earthworm coprolites was sampled three times in a week. Mesocosms were filled with the same feeding material and inoculated with ten (10) non-clitellated young worms for 42–45 days. Surface coprolites samples were shifted to petri dishes with moistened filter paper for further analysis. The mesocosms were placed under ambient light, with an average air temperature and relative humidity of 16.8 ± 1.5 °C and 67 ± 4% respectively, to ensure similar microclimatic conditions during the course of the experiment.
Measurements
Surface coprolites of A. caliginosa were collected three times a week from each mesocosm, oven dried at 40 °C for 36 h and weighed for further examination. In the first week, a few dead earthworms were found on substrate surface and promptly replaced with new living specimens. Coprolites were analyzed for ammonium (NH4+) and nitrate (NO3−) concentration by extracting with 2 M KCl with KCl: coprolites ratio of 4:1. The mixture obtained was mechanically shaken for 3 h at 24 °C followed by filtration. NH4+ was determined by the indophenol blue method (IBM) (Keeney and Nelson 1982) using an automated unit (Sysmex BX4000) while NO3− by the cadmium reduction method (CRM)106. Triplicate surface coprolites (3 cm diameter) from each mesocosm were collected and evaluated for NH4+and NO3− at each sampling time, and the results were reported on dry weight basis. At three distinct stages of experiment, the nitrification potential of coprolites from both types of substrates was determined using the method107 as: Coprolites samples (2–4 g fresh weight) from both substrates and control were put into flasks with 25 ml (NH4+ & SO4–2) solution. Foam plugged flasks were shaken mechanically at 25 °C for 2 h. A mixture sample of 10 ml was taken for N2O analysis. Flasks were then exposed to aerobic conditions for two days followed by filtration and the amount of N2O was measured. The difference between the initial and final readings of N2O was recorded as the nitrification potential. Nitrification rates were expressed on a dry weight basis. During the experiment, denitrification rates were determined three time (2nd, 4th and 6th weeks) using surface coprolites taken from each mesocosm. All castings were removed from mesocosm surface two days before coprolite sampling, so all coprolite samples evaluated for denitrification in the experiment were ≤ 2 days old. The acetylene block method108 was used to determine denitrification rate as: Coprolite sample (2 g fresh weight) of A. caliginosa from each mesocosm surface were placed in10 ml test tubes fixed with headspace, and 1.0 ml of hydrogen and carbon was added to the headspace of each test tube to inhibit the reduction of N2O to N. N2O production was measured every 3 h for 14 h at 16 ± 2.5 °C. Modified automated method109, was used to determine the N2O.
Coprolite microbiome
For differential analysis of the micro flora, coprolites samples were sub-sampled. In addition, earthworms were put under starvation for two days in petri dishes with 1% sterile agar. This much time was sufficient to get the transit microbes come over the agar. Coprolite samples were dissolved into 10 ml of sterile 0.85% NaCl and stirred vigorously for 20 min using the method110 described as: Suspension was diluted by the serial dilution method using a dilution factor of 10–1–10–10 to make out the development of microbes on agar nutrient plates. Enumeration of microorganism was carried out by pour plate method on nutrient agar. Microbial samples were incubated for 24 h at 20 ± 2 °C. Using the purified streak-plate technique, each isolate with similar morphological features was eventually relocated to a new nutrient agar plate until a single colony was established. Pure microbial colonies were characterized and identified by perceiving morphological features and bacterial cell shapes through the gram staining technique111. Nitrogen transformation activity of pure isolates were tested by using Kjeldahl method112, on the basis of generation of initial turbidity in flasks with nitrogen free medium.
Chemical analysis
Coprolite samples were ignited in Muffle furnace at 500 °C for 90 min for determination of organic carbon using the method as described by Nelson and Sommer (1996). Phosphorus and potassium were quantified by the procedure of John (1970) through flame photometer-128 (Systronics) after digesting samples in a diacid suspension (HClO4: HNO3 in the ratio of 4:1). pH and electric conductivity (EC) were determined in double-distilled water blend each with a concentration ratio of 1:10 (w/v) plying digital meter (COM-100) and Eqip-tronics (EQ-614A) respectively. Nitrogen (N) was determined by the Micro-Kjeldhal method as described by Bremner and Mulvancy (1982) using digestion extract (H2SO4 + K2SO4: CuSO4: SeO2 in the ratio of 10:4:1). Phosphorus (P) content was determined by nitro-vanadomolybdate method, potassium (K) by using photometry and micronutrients (Zn and Fe) by atomic-absorption spectrometry (AAS) after digestion of both coprolite samples from GS and GM with HNO3:HClO4 by the method113. Diacid mixture digested samples were analyzed for transition metal elements using an atomic absorption spectrophotometer (Electronic Corporation of India).
Statistical analysis
Analysis of variance (ANOVA) was used to compare the results between the control and treatment groups followed by post hoc analysis. A significance level of P < 0.05 was used to determine significance in the treatment means using R-software. Regression analysis was carried out using an equation (y \(= b_{2} x^{2} - b_{1} x + a)\) and to workout maximum responses in different study parameters were determined from the formula \(\left( {x = - b_{1} /2b_{2} } \right)\). In the experiment, the mean differentiation of chemical parameters of nitrogen dynamics was done using student’s t-test. Parameters such as pH, moisture content (MC), total organic carbon (TOC), total phosphorus (TP), nitrates, total nitrogen (TN), potassium (K), iron (Fe), zinc (Zn), and the carbon and nitrogen ratio (CN ratio) were utilized to determine correlation matrices affecting coprolites’ quality and stability. The findings were plotted and tabulated using principal component analysis.
Conclusion
The study confirms that GM coprolites contains more nitrogen (NH4+-N and NO3–-N) than the GS, which is readily logical when the selective feeding habits of earthworms are considered. At low temperature, simultaneous heterotrophic nitrification and aerobic denitrification (SHNAD) was found to be stable processes and main N transformation mechanism in both substrates. A. caliginous promotes microorganism growth, which would otherwise be severely limited due to harsh winter and low ambient temperature. The study highlights the importance of the SHNAD as a pilot scale process showing positive interaction with A. caliginous contributing in physico-chemical parameters of its coprolites and thus has substantial potential for N removal from wastes at low temperatures. Interaction between psychrophillic earthworms and microbial genera need to be further investigated to provide insight evidences of co-occurrence pattern of both, which could help to minimize NH3 emission by effectively reducing N2O.
Data availability
The data sets generated are available as supplementary file.
References
Blume-Werry, G. et al. Invasive earthworms unlock arctic plant nitrogen limitation. Nat. Commun. 11, 1–10 (2020).
Marhan, S. & Scheu, S. Mixing of different mineral soil layers by endogeic earthworms affects carbon and nitrogen mineralization. Biol. Fertil. Soils 42, 308 (2006).
Sanchez-Hernandez, J. C. Vermiremediation of Pharmaceutical-Contaminated Soils and Organic Amendments (Springer, Berlin, 2020).
Gómez-Brandón, M., Aira, M., Lores, M. & Domínguez, J. Changes in microbial community structure and function during vermicomposting of pig slurry. Bioresour. Technol. 102, 4171–4178. https://doi.org/10.1016/j.biortech.2010.12.057 (2011).
Aira, M. & Domínguez, J. Earthworm effects without earthworms: Inoculation of raw organic matter with worm-worked substrates alters microbial community functioning. PLoS ONE 6, e16354. https://doi.org/10.1371/journal.pone.0016354 (2011).
Blair, J., Parmelee, R. W., Allen, M. F., McCartney, D. & Stinner, B. R. Changes in soil N pools in response to earthworm population manipulations in agroecosystem with different N sources. Soil Biol. Biochem. 29, 361–367. https://doi.org/10.1016/S0038-0717(96)00098-3 (1997).
Abail, Z. & Whalen, J. K. Earthworm contributions to soil nitrogen supply in corn-soybean agroecosystems in Quebec. Canada. Pedosphere 31, 405–412. https://doi.org/10.1016/S1002-0160(20)60086-8 (2021).
Frelich, L. E. et al. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol. Invasions 8, 1235–1245. https://doi.org/10.1007/s10530-006-9019-3 (2006).
Ding, W. et al. Effect thresholds for the earthworm Eisenia fetida: Toxicity comparison between conventional and biodegradable microplastics. Sci. Total Environ. 781, 146884 (2021).
Treder, K., Jastrzębska, M., Kostrzewska, M. K. & Makowski, P. Do long-term continuous cropping and pesticides affect earthworm communities?. Agronomy 10, 586 (2020).
Fonte, S. J., Kong, A. Y. Y., van Kessel, C., Hendrix, P. F. & Six, J. Influence of earthworm activity on aggregate-associated carbon and nitrogen dynamics differs with agroecosystem management. Soil Biol. Biochem. 39, 1014–1022. https://doi.org/10.1016/j.soilbio.2006.11.011 (2007).
Scheu, S., Schlitt, N., Tiunov, A. V., Newington, J. E. & Jones, H. T. Effects of the presence and community composition of earthworms on microbial community functioning. Oecologia 133, 254–260. https://doi.org/10.1007/s00442-002-1023-4 (2002).
Sheikh, T. et al. Unveiling the efficiency of psychrophillic aporrectodea caliginosa in deciphering the nutrients from dalweed and cow manure with bio-optimization of coprolites. Sustainability 13, 5338 (2021).
Lavelle, P. & Spain, A. V. Soil Ecology (Springer, Dordrecht, 2001).
Aubert, L., Konradova, D., Barris, S. & Quinet, M. Different drought resistance mechanisms between two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. https://doi.org/10.1111/ppl.13248 (2020).
Sistla, S. A., Asao, S. & Schimel, J. P. Detecting microbial N-limitation in tussock tundra soil: Implications for Arctic soil organic carbon cycling. Soil Biol. Biochem. 55, 78–84. https://doi.org/10.1016/j.soilbio.2012.06.010 (2012).
Chkrebtii, O. A., Cameron, E. K., Campbell, D. A. & Bayne, E. M. Transdimensional approximate Bayesian computation for inference on invasive species models with latent variables of unknown dimension. Comput. Stat. Data Anal. 86, 97–110. https://doi.org/10.1016/j.csda.2015.01.002 (2015).
Szlavecz, K. et al. Invasive earthworm species and nitrogen cycling in remnant forest patches. Appl. Soil. Ecol. 32, 54–62. https://doi.org/10.1016/j.apsoil.2005.01.006 (2006).
Liu, M., Cao, J. & Wang, C. Bioremediation by earthworms on soil microbial diversity and partial nitrification processes in oxytetracycline-contaminated soil. Ecotoxicol. Environ. Saf. 189, 109996 (2020).
Lubbers, I. M. et al. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Change 3, 187–194. https://doi.org/10.1038/nclimate1692 (2013).
Wang, Z., Chen, Z., Niu, Y., Ren, P. & Hao, M. Feasibility of vermicomposting for spent drilling fluid from a nature-gas industry employing earthworms Eisenia fetida. Ecotoxicol. Environ. Saf. 214, 111994 (2021).
Elyamine, A. M. & Hu, C. Earthworms and rice straw enhanced soil bacterial diversity and promoted the degradation of phenanthrene. Environ. Sci. Eur. 32, 124. https://doi.org/10.1186/s12302-020-00400-y (2020).
Sun, M. et al. Ecological role of earthworm intestinal bacteria in terrestrial environments: A review. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140008 (2020).
Turp, G. A., Turp, S. M., Ozdemir, S. & Yetilmezsoy, K. Vermicomposting of biomass ash with bio-waste for solubilizing nutrients and its effect on nitrogen fixation in common beans. Environ. Technol. Innov. https://doi.org/10.1016/j.eti.2021.101691 (2021).
Lv, B., Zhang, D., Chen, Q. & Cui, Y. Effects of earthworms on nitrogen transformation and the correspond genes (amoA and nirS) in vermicomposting of sewage sludge and rice straw. Bioresour. Technol. 287, 121428. https://doi.org/10.1016/j.biortech.2019.121428 (2019).
Sharma, K. & Garg, V. K. Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresour. Technol. 250, 708–715. https://doi.org/10.1016/j.biortech.2017.11.101 (2018).
Castillo Diaz, J. M., Martin-Laurent, F., Beguet, J., Nogales, R. & Romero, E. Fate and effect of imidacloprid on vermicompost-amended soils under dissimilar conditions: Risk for soil functions, structure, and bacterial abundance. Sci. Total Environ. 579, 1111–1119. https://doi.org/10.1016/j.scitotenv.2016.11.082 (2017).
Samal, K., Raj Mohan, A., Chaudhary, N. & Moulick, S. Application of vermitechnology in waste management: A review on mechanism and performance. J. Environ. Chem. Eng. 7, 103392. https://doi.org/10.1016/j.jece.2019.103392 (2019).
Katakula, A. A. N., Handura, B., Gawanab, W., Itanna, F. & Mupambwa, H. A. Optimized vermicomposting of a goat manure-vegetable food waste mixture for enhanced nutrient release. Sci. Afr. 12, e00727 (2021).
Cáceres, R., Malińska, K. & Marfà, O. Nitrification within composting: A review. Waste Manag. 72, 119–137. https://doi.org/10.1016/j.wasman.2017.10.049 (2018).
Lv, B., Cui, Y., Wei, H., Chen, Q. & Zhang, D. Elucidating the role of earthworms in N2O emission and production pathway during vermicomposting of sewage sludge and rice straw. J. Hazard. Mater. 400, 123215 (2020).
Zhang, L. et al. The non-negligibility of greenhouse gas emission from a combined pre-composting and vermicomposting system with maize stover and cow dung. Environ. Sci. Pollut. Res. 28, 19412–19423 (2021).
Chen, C., Whalen, J. K. & Guo, X. Earthworms reduce soil nitrous oxide emissions during drying and rewetting cycles. Soil Biol. Biochem. 68, 117–124. https://doi.org/10.1016/j.soilbio.2013.09.020 (2014).
Wang, X. et al. Treating low carbon/nitrogen (C/N) wastewater in simultaneous nitrification-endogenous denitrification and phosphorous removal (SNDPR) systems by strengthening anaerobic intracellular carbon storage. Water Res. 77, 191–200. https://doi.org/10.1016/j.watres.2015.03.019 (2015).
Yang, Z., Sun, H. & Wu, W. Intensified simultaneous nitrification and denitrification performance in integrated packed bed bioreactors using PHBV with different dosing methods. Environ. Sci. Pollut. Res. 27, 21560–21569. https://doi.org/10.1007/s11356-020-08290-6 (2020).
Pan, Z. et al. Effects of COD/TN ratio on nitrogen removal efficiency, microbial community for high saline wastewater treatment based on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 301, 122726. https://doi.org/10.1016/j.biortech.2019.122726 (2020).
Xia, L., Li, X., Fan, W. & Wang, J. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp .ND7 isolated from municipal activated sludge. Bioresour. Technol. 301, 122749. https://doi.org/10.1016/j.biortech.2020.122749 (2020).
Dad, J. M. & Khan, A. B. Threatened medicinal plants of Gurez Valley, Kashmir Himalayas. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 7, 20–26. https://doi.org/10.1080/21513732.2011.602646 (2011).
Cameira, M. D. & Mota, M. Nitrogen related diffuse pollution from horticulture production—mitigation practices and assessment strategies. Horticulturae https://doi.org/10.3390/horticulturae3010025 (2017).
Yuvaraj, A., Thangaraj, R., Ravindran, B., Chang, S. W. & Karmegam, N. Centrality of cattle solid wastes in vermicomposting technology—A cleaner resource recovery and biowaste recycling option for agricultural and environmental sustainability. Environ. Pollut. 268, 115688. https://doi.org/10.1016/j.envpol.2020.115688 (2021).
Kuzyakov, Y., Friedel, J. K. & Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498. https://doi.org/10.1016/S0038-0717(00)00084-5 (2000).
Bertrand, M. et al. Earthworm services for cropping systems. A review. Agron. Sustain. Dev. 35, 553–567 (2015).
Makoto, K., Bryanin, S. V. & Takagi, K. The effect of snow reduction and Eisenia japonica earthworm traits on soil nitrogen dynamics in spring in a cool-temperate forest. Appl. Soil. Ecol. 144, 1–7. https://doi.org/10.1016/j.apsoil.2019.06.019 (2019).
Huang, K. et al. Optimal growth condition of earthworms and their vermicompost features during recycling of five different fresh fruit and vegetable wastes. Environ. Sci. Pollut. Res. Int. 23, 13569–13575. https://doi.org/10.1007/s11356-016-6848-1 (2016).
Makoto, K., Minamiya, Y. & Kaneko, N. Differences in soil type drive the intraspecific variation in the responses of an earthworm species and consequently, tree growth to warming. Plant Soil 404, 209–218. https://doi.org/10.1007/s11104-016-2827-z (2016).
Grenon, F., Bradley, R. L. & Titus, B. D. Temperature sensitivity of mineral N transformation rates, and heterotrophic nitrification: possible factors controlling the post-disturbance mineral N flush in forest floors. Soil Biol. Biochem. 36, 1465–1474. https://doi.org/10.1016/j.soilbio.2004.04.021 (2004).
Zhang, H., Li, J., Zhang, Y. & Huang, K. Quality of vermicompost and microbial community diversity affected by the contrasting temperature during vermicomposting of dewatered sludge. Int. J. Env. Res. Public Health 17, 1748 (2020).
Velasco-Velasco, J., Parkinson, R. & Kuri, V. Ammonia emissions during vermicomposting of sheep manure. Bioresour. Technol. 102, 10959–10964 (2011).
Dan, X. et al. Effects of changing temperature on gross N transformation rates in acidic subtropical forest soils. Forests 10, 894 (2019).
Gusain, R. & Suthar, S. Vermicomposting of invasive weed Ageratum conyzoids: Assessment of nutrient mineralization, enzymatic activities, and microbial properties. Bioresour. Technol. 312, 123537 (2020).
Klaasen, H. L., Koopman, J. P., Poelma, F. G. & Beynen, A. C. Intestinal, segmented, filamentous bacteria. FEMS Microbiol. Rev. 8, 165–180. https://doi.org/10.1111/j.1574-6968.1992.tb04986.x (1992).
Fischer, K., Hahn, D., Daniel, O., Zeyer, J. & Amann, R. I. In situ analysis of the bacterial community in the gut of the earthworm Lumbricus terrestris L. by whole-cell hybridization. Can. J. Microbiol. 41, 666–673. https://doi.org/10.1139/m95-092 (1995).
Karsten, G. R. & Drake, H. L. Comparative assessment of the aerobic and anaerobic microfloras of earthworm guts and forest soils. Appl. Environ. Microbiol. 61, 1039–1044. https://doi.org/10.1128/AEM.61.3.1039-1044.1995 (1995).
Hobson, A. M., Frederickson, J. & Dise, N. B. CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manag. 25, 345–352. https://doi.org/10.1016/j.wasman.2005.02.015 (2005).
Singh, A. et al. Earthworms and vermicompost: An eco-friendly approach for repaying nature’s debt. Environ. Geochem. Health 42, 1617–1642 (2020).
Zedelius, J. et al. Alkane degradation under anoxic conditions by a nitrate-reducing bacterium with possible involvement of the electron acceptor in substrate activation. Environ Microbiol. Rep. 3, 125–135. https://doi.org/10.1111/j.1758-2229.2010.00198.x (2011).
Liu, R., Suter, H. C., He, J.-Z., Hayden, H. & Chen, D. Influence of temperature and moisture on the relative contributions of heterotrophic and autotrophic nitrification to gross nitrification in an acid cropping soil. J. Soils Sed. https://doi.org/10.1007/s11368-015-1170-y (2015).
Zhang, Y. et al. Composition of soil recalcitrant C regulates nitrification rates in acidic soils. Geoderma 337, 965–972. https://doi.org/10.1016/j.geoderma.2018.11.014 (2019).
Zhang, J., Sun, W., Zhong, W. & Cai, Z. The substrate is an important factor in controlling the significance of heterotrophic nitrification in acidic forest soils. Soil Biol. Biochem. 76, 143–148. https://doi.org/10.1016/j.soilbio.2014.05.001 (2014).
Abail, Z., Sampedro, L. & Whalen, J. K. Short-term carbon mineralization from endogeic earthworm casts as influenced by properties of the ingested soil material. Appl. Soil. Ecol. 116, 79–86 (2017).
Coq, S., Barthès, B. G., Oliver, R., Rabary, B. & Blanchart, E. Earthworm activity affects soil aggregation and organic matter dynamics according to the quality and localization of crop residues—an experimental study (Madagascar). Soil Biol. Biochem. 39, 2119–2128 (2007).
Medina-Sauza, R. M. et al. Earthworms building up soil microbiota, a review. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2019.00081 (2019).
Aira, M., Monroy, F. & Domínguez, J. Ageing effects on nitrogen dynamics and enzyme activities in casts of Aporrectodea caliginosa (Lumbricidae). Pedobiologia 49, 467–473 (2005).
Clause, J., Barot, S., Richard, B., Decaëns, T. & Forey, E. The interactions between soil type and earthworm species determine the properties of earthworm casts. Appl. Soil. Ecol. 83, 149–158 (2014).
McDaniel, J. P., Stromberger, M. E., Barbarick, K. A. & Cranshaw, W. Survival of Aporrectodea caliginosa and its effects on nutrient availability in biosolids amended soil. Appl. Soil. Ecol. 71, 1–6 (2013).
Ravishankara, A., Daniel, J. S. & Portmann, R. W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 (2009).
Lubbers, I., Brussaard, L., Otten, W. & Van Groenigen, J. Earthworm-induced N mineralization in fertilized grassland increases both N2O emission and crop-N uptake. Eur. J. Soil Sci. 62, 152–161 (2011).
Peter, S. D. J., George, G. B., Siu, M. T., Marcus, A. H. & Harold, L. D. Emission of nitrous oxide and dinitrogen by diverse earthworm families from Brazil and resolution of associated denitrifying and nitrate-dissimilating taxa. FEMS Microbiol. Ecol. 83, 375–391. https://doi.org/10.1111/j.1574-6941.2012.01476.x (2013).
Firestone, M. K. & Davidson, E. A. Microbiological basis of NO and N2O production and consumption in soil. Exch. Trace Gases terr. Ecosyst. Atmos. 47, 7–21 (1989).
Zhu, X. et al. Exploring the relationships between soil fauna, different tillage regimes and CO2 and N2O emissions from black soil in China. Soil Biol. Biochem. 103, 106–116 (2016).
Yu, D.-S. et al. Simultaneous nitrogen and phosphorus removal characteristics of an anaerobic/aerobic operated spndpr system treating low C/N urban sewage. Huan Jing ke Xue Huanjing Kexue 39, 5065–5073 (2018).
Wang, F., Zhao, Y., Xie, S. & Li, J. Implication of nitrifying and denitrifying bacteria for nitrogen removal in a shallow lake. Clean: Soil, Air, Water 45, 1500319 (2017).
Wang, J. et al. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag. 34, 1546–1552. https://doi.org/10.1016/j.wasman.2014.04.010 (2014).
Nigussie, A., Kuyper, T. W., Bruun, S. & de Neergaard, A. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J. Clean. Prod. 139, 429–439. https://doi.org/10.1016/j.jclepro.2016.08.058 (2016).
Yang, F., Li, G., Zang, B. & Zhang, Z. The maturity and CH4, N2O, NH3 emissions from vermicomposting with agricultural waste. Compost Sci. Util. 25, 262–271. https://doi.org/10.1080/1065657X.2017.1329037 (2017).
Ma, L. et al. Soil properties alter plant and microbial communities to modulate denitrification rates in subtropical riparian wetlands. Land Degrad. Dev. 31, 1792–1802 (2020).
Xu, X. et al. Effective nitrogen removal in a granule-based partial-denitrification/anammox reactor treating low C/N sewage. Bioresour. Technol. 297, 122467 (2020).
Ma, X., Xing, M., Wang, Y., Xu, Z. & Yang, J. Microbial enzyme and biomass responses: Deciphering the effects of earthworms and seasonal variation on treating excess sludge. J. Environ. Manag. 170, 207–214. https://doi.org/10.1016/j.jenvman.2016.01.022 (2016).
Kremen, A., Bear, J., Shavit, U. & Shaviv, A. Model demonstrating the potential for coupled nitrification denitrification in soil aggregates. Environ. Sci. Technol. 39, 4180–4188. https://doi.org/10.1021/es048304z (2005).
Zhang, Y., Song, C., Zhou, Z., Cao, X. & Zhou, Y. Coupling between nitrification and denitrification as well as its effect on phosphorus release in sediments of Chinese Shallow Lakes. Water 11, 1809 (2019).
Das, D. & Deka, H. Vermicomposting of harvested waste biomass of potato crop employing Eisenia fetida: Changes in nutrient profile and assessment of the maturity of the end products. Environ. Sci. Pollut. Res. 28, 35717–35727 (2021).
Fernández-Gómez, M. J., Romero, E. & Nogales, R. Feasibility of vermicomposting for vegetable greenhouse waste recycling. Bioresour. Technol. 101, 9654–9660. https://doi.org/10.1016/j.biortech.2010.07.109 (2010).
Biruntha, M. et al. Vermiconversion of biowastes with low-to-high C/N ratio into value added vermicompost. Bioresour. Technol. 297, 122398 (2020).
Devi, C. & Khwairakpam, M. Feasibility of vermicomposting for the management of terrestrial weed Ageratum conyzoides using earthworm species Eisenia fetida. Environ. Technol. Innov. 18, 100696. https://doi.org/10.1016/j.eti.2020.100696 (2020).
Garg, P., Gupta, A. & Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparative study. Bioresour. Technol. 97, 391–395. https://doi.org/10.1016/j.biortech.2005.03.009 (2006).
Huang, K., Li, F., Wei, Y., Fu, X. & Chen, X. Effects of earthworms on physicochemical properties and microbial profiles during vermicomposting of fresh fruit and vegetable wastes. Bioresour. Technol. 170, 45–52. https://doi.org/10.1016/j.biortech.2014.07.058 (2014).
Gusain, R. & Suthar, S. Vermicomposting of duckweed (Spirodela polyrhiza) by employing Eisenia fetida: Changes in nutrient contents, microbial enzyme activities and earthworm biodynamics. Bioresour. Technol. 311, 123585 (2020).
Karmegam, N. et al. Precomposting and green manure amendment for effective vermitransformation of hazardous coir industrial waste into enriched vermicompost. Bioresour. Technol. 319, 124136. https://doi.org/10.1016/j.biortech.2020.124136 (2021).
Bhattacharya, S. S. & Chattopadhyay, G. N. Transformation of nitrogen during vermicomposting of fly ash. Waste Manag. Res. 22, 488–491. https://doi.org/10.1177/0734242X04048625 (2004).
Hussain, N., Abbasi, T. & Abbasi, S. Transformation of the pernicious and toxic weed parthenium into an organic fertilizer by vermicomposting. Int. J. Environ. Stud. 73, 731–745 (2016).
Rai, R. & Suthar, S. Composting of toxic weed Parthenium hysterophorus: Nutrient changes, the fate of faecal coliforms, and biopesticide property assessment. Bioresour. Technol. 311, 123523 (2020).
Whalen, J. K., Parmelee, R. W. & Subler, S. Quantification of nitrogen excretion rates for three lumbricid earthworms using 15N. Biol. Fertil. Soils 32, 347–352. https://doi.org/10.1007/s003740000259 (2000).
Esmaeili, A., Khoram, M. R., Gholami, M. & Eslami, H. Pistachio waste management using combined composting-vermicomposting technique: Physico-chemical changes and worm growth analysis. J. Clean. Prod. 242, 118523 (2020).
Karmegam, N., Vijayan, P., Prakash, M. & Paul, J. A. J. Vermicomposting of paper industry sludge with cowdung and green manure plants using Eisenia fetida: A viable option for cleaner and enriched vermicompost production. J. Clean. Prod. 228, 718–728 (2019).
Paul, J. A., Karmegam, N. & Daniel, T. Municipal solid waste (MSW) vermicomposting with an epigeic earthworm Perionyx ceylanensis Mich. Bioresour. Technol. 102, 6769–6773. https://doi.org/10.1016/j.biortech.2011.03.089 (2011).
Huang, K., Xia, H., Cui, G. & Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 578, 337–345. https://doi.org/10.1016/j.scitotenv.2016.10.172 (2017).
Mokgophi, M. M., Manyevere, A., Ayisi, K. K. & Munjonji, L. Characterisation of chamaecytisus tagasaste, moringa oleifera and vachellia karroo vermicomposts and their potential to improve soil fertility. Sustainability 12, 9305 (2020).
Pathma, J. & Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 1, 26–26. https://doi.org/10.1186/2193-1801-1-26 (2012).
Villar, I., Alves, D., Pérez-Díaz, D. & Mato, S. Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Waste Manag. 48, 409–417 (2016).
Wang, Y. et al. Speciation of heavy metals and bacteria in cow dung after vermicomposting by the earthworm, Eisenia fetida. Bioresour. Technol. 245, 411–418 (2017).
Svensson, B. H., Boström, U. & Klemedtson, L. Potential for higher rates of denitrification in earthworm casts than in the surrounding soil. Biol. Fertil. Soils 2, 147–149. https://doi.org/10.1007/BF00257593 (1986).
Syers, J. K. & Springett, J. A. Earthworms and Soil Fertility. In Biological Processes and Soil Fertility (eds Tinsley, J. & Darbyshire, J. F.) 93–104 (Springer Netherlands, Dordrecht, 1984).
Mohanty, S. R. et al. nitrification rates are affected by biogenic nitrate and volatile organic compounds in agricultural soils. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.00772 (2019).
Cui, G. et al. Changes of quinolone resistance genes and their relations with microbial profiles during vermicomposting of municipal excess sludge. Sci. Total Environ. 644, 494–502 (2018).
Paliwal, R. & Julka, J. Checklist of earthworms of western Himalaya, India. Zoos’ Print J. 20, 1972–1976 (2005).
Gal, C., Frenzel, W. & Möller, J. Re-examination of the cadmium reduction method and optimisation of conditions for the determination of nitrate by flow injection analysis. Microchim. Acta 146, 155–164. https://doi.org/10.1007/s00604-004-0193-7 (2004).
Schmidt, L. W. in Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties (ed A.L. Page) 1027–1042 (1982).
Yoshinari, T., Hynes, R. & Knowles, R. Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol. Biochem. 9, 177–183. https://doi.org/10.1016/0038-0717(77)90072-4 (1977).
Parkin, T. B. Automated analysis of nitrous oxide. Soil Sci. Soc. Am. J. 49, 273 (1985).
Hussain, M. et al. Bacteria in combination with fertilizers improve growth, productivity and net returns of wheat (Triticum aestivum L.). Pak. J. Agric. Sci. https://doi.org/10.21162/PAKJAS/16.4901 (2016).
Gislin, D., Sudarsanam, D., Antony Raj, G. & Baskar, K. Antibacterial activity of soil bacteria isolated from Kochi, India and their molecular identification. J Genet. Eng. Biotechnol. 16, 287–294. https://doi.org/10.1016/j.jgeb.2018.05.010 (2018).
Rashid, K. M. H., Mohiuddin, M. & Rahman, M. Enumeration, isolation and identification of nitrogen-fixing bacterial strains at seedling stage in rhizosphere of rice grown in non-calcareous grey flood plain soil of Bangladesh. J. Fac. Environ. Sci. Technol. 13, 97 (2008).
Williams, S. & Association of Official Analytical, C. Official methods of analysis of the Association of official analytical chemists. (Association of official analytical chemists, 1984).
Acknowledgements
This project was supported by Researchers Supporting Project number (RSP-2022R7) King Saud University, Riyadh, Saudi Arabia.
Funding
This project was supported by Researchers Supporting Project number (RSP-2022R7) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
T.S.: Conceptualization, methodology and writing of original draft. Z.B.: Supervision, writing- reviewing and editing. A.M.Y.: Designed the figure reading, writing review and editing. B.H.: Proof reading. S.I.: Analysis and Review of literature. F.W.: Statistics. S.F.: Revisions and editing. S.A.: Investigation and critical editing. M.J.A.: Reviewing of whole manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheikh, T., Baba, Z., Yatoo, A.M. et al. Deciphering waste bound nitrogen by employing psychrophillic Aporrectodea caliginosa and priming of coprolites by associated heterotrophic nitrifiers under high altitude Himalayas. Sci Rep 12, 9556 (2022). https://doi.org/10.1038/s41598-022-12972-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12972-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.