Abstract
The solubility of empagliflozin in supercritical carbon dioxide was measured at temperatures (308 to 338 K) and pressures (12 to 27 MPa), for the first time. The measured solubility in terms of mole faction ranged from 5.14 × 10–6 to 25.9 × 10–6. The cross over region was observed at 16.5 MPa. A new solubility model was derived to correlate the solubility data using solid–liquid equilibrium criteria combined with Wilson activity coefficient model at infinite dilution for the activity coefficient. The proposed model correlated the data with average absolute relative deviation (AARD) and Akaike’s information criterion (AICc), 7.22% and − 637.24, respectively. Further, the measured data was also correlated with 11 existing (three, five and six parameters empirical and semi-empirical) models and also with Redlich-Kwong equation of state (RKEoS) along with Kwak-Mansoori mixing rules (KMmr) model. Among density-based models, Bian et al., model was the best and corresponding AARD% was calculated 5.1. The RKEoS + KMmr was observed to correlate the data with 8.07% (correspond AICc is − 635.79). Finally, total, sublimation and solvation enthalpies of empagliflozin were calculated.
Similar content being viewed by others
Introduction
Supercritical carbon dioxide (ScCO2) is a fluid above its critical point. It has physical properties (density, diffusivity, viscosity and surface tension) intermediate to that of gas and liquid1,2. ScCO2 has been used as a solvent in various process applications, because it has gas like diffusivity and liquid like density with low viscosity and surface tension1,3,4,5. The major applications are in drug particle micronization, food processing, textile dyeing, ceramic coating, extraction and many more4,6,7,8,9,10,11,12. Although, several supercritical fluids are utilized as solvent in process industry, ScCO2 is the most desirable solvent8,13,14,15,16,17. In general, phase equilibrium information is necessary to implement supercritical fluid technology (SFT)6,7,9. The solubility is the basic information for the design and development of SFT. In literature, solubility of many drug solids in ScCO2 is readily available18,19,20,21,22,23,24,25,26,27,28,29,30, however, the solubility of empagliflozin has not been reported, therefore in this work for the first time, its solubility in ScCO2 has been measured. This data may be used in the particle micronization process using ScCO2. The molecular formula of empagliflozin is C23H27 ClO7 and its molecular weight is 450.91. The chemical structure is shown in Fig. 1.
Empagliflozin is an inhibitor of sodium-glucose co-transporter-2 (SGLT2), the transporters primarily responsible for the re-absorption of glucose in the kidney. Further, it is useful in reducing the risk of cardiovascular death in adults with type 2 diabetes mellitus and cardiovascular disease31. Sufficient drug dosage is very essential for those treatments and this is achieved through a proper particle size. Therefore, the present study is quite useful in particle micronization using ScCO2. Solubility measurement at each desired condition is very cumbersome and hence, there is a great need to develop a model that correlates/predicts the solubility32. Recent developments such as machine learning methods may be considered with the improvement of artificial intelligence prediction methods for the data correlation33,34,35. However, in general, the solubility models are classified into five types; however, only three are user friendly, and they are equation of state, density-based and mathematical models36. Directly or indirectly all of them are derived based on thermodynamic frame work. The derived models make use of the basic concepts related to phase equilibrium criteria (solid–gas or solid–liquid), solvent–solute association theory, dilute solution theory, solution theory and Wilson model or any other model37. In fact, most of the literature models correlate the solubility of the solid solutes in ScCO2 quite well. A solid–gas equilibrium models need the critical properties and vapour pressure of the solute, while these properties are rarely available in literature, due to this, the group contribution methods are commonly used38. On the other hand, the solid–liquid equilibrium (SLE) criterion requires an appropriate model for activity coefficient calculation. A recent study reveals that SLE model in combination with Van Laar activity coefficient model can be a simple approach in model development, but this method resulted in an implicit expression in terms of mole fraction38,39. Therefore, there is a need to develop an explicit solubility model and hence, this task is taken up in this work.
The main motives of this study were in two levels. In the first level, empagliflozin solubility in ScCO2 was determined and in the second level, a new explicit solubility model was developed based on solid–liquid equilibrium criterion in combination with Wilson activity coefficient model for the activity coefficient calculation.
Experimental
Materials
Gaseous CO2 (purity > 99.9%) was obtained from Fadak company, Kashan (Iran), empagliflozin (CAS Number: 864070-44-0, purity > 99%) was purchased from Amin Pharma company, and dimethyl sulfoxide (DMSO, CAS No. 67-68-5, purity > 99%) was provided from Sigma Aldrich company. Table 1 indicates all the information about the chemicals utilized in this work.
Experiment details
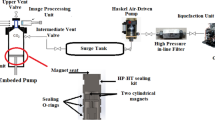
The detailed discussion of the solubility apparatus and equilibrium cell has been presented in our earlier studies (Fig. 2)19,25,40,41. However, a brief description about the apparatus is presented in this section. This method may be classified as an isobaric-isothermal method42. Each measurement was carried out with high precision and temperatures and pressures were controlled within ± 0.1 K and ± 0.1 MPa, respectively. For all measurement, 1 g of empagliflozin drug was used. As mentioned in our previous works, the equilibrium was observed within 60 min. After equilibrium, 600 µL saturated ScCO2 sample was collected via 2-status 6-way port valve in a DMSO preloaded vial. After discharging 600 µL saturated ScCO2, the port valve was washed with 1 ml DMSO. Thus, the total saturation solution was 5 ml. Each measurement was repeated thrice and their average values were reported. Mole fraction is obtained as follows:
where \({n}_{\text{solute}}\) is the moles number of the drug, and \({n}_{{\text{CO}}_{2}}\) is the moles number of CO2 in the sampling loop.
Experimental setup for solubility measurement, E1—CO2 cylinder; E-2—Filter; E-3—Refrigerator unit; E-4—Air compressor; E-5—High pressure pump; E-6—Equilibrium cell; E-7—Magnetic stirrer; E-8—Needle valve; E-9—Back-pressure valve; E-10—Six-port, two position valve; E-11—Oven; E-12—Syringe; E13—Collection vial; E-14—Control panel.
Further, the above quantities are given as:
where \({C}_{\text{s}}\) is the drug concentration in saturated sample vial in g/L. The volume of the sampling loop and vial collection are V1(L) = 600 \(\times \) 10–6 m3 and Vs(L) = 5 \(\times \) 10–3 m3, respectively. The \(M_{s}\) and \(M_{{\text{CO}_{2} }}\) are the molecular weight of drug and CO2, respectively. Solubility is also described as
The relation between S and \(y_{2}\) is
A UV–Visible spectrophotometer (Model UNICO-4802) and DMSO solvent were used for the measurement of empagliflozin solubility. The samples were analyzed at 276 nm.
Existing and new models and their correlations
In this section, the details of various solubility models are presented along with a new explicit solubility model.
Existing models
Alwi–Garlapati model (three parameters model)43
It is one of the latest models for the solubility correlation. It is mathematically explained as
where \(A_{1} - \;C_{1}\) are model constants.
Bartle et al., model (three parameters model)44
It is an empirical model and mathematically stated as:
where \(A_{2} - C_{2}\) are model constants. From parameter \(B_{2}\), one can estimate sublimation enthalpy using the relation, \(\Delta_{sub} H = - B_{2} R\), in which R is universal gas constant.
Bian et al., model (five parameters model)45
It is an empirical model and mathematically formulated as:
where \(A_{3} - E_{3}\) are model constants.
Chrastil model (three parameters model)46
It is a semi-empirical model and mathematically stated as:
where \(\kappa ,A_{4} \;{\text{and}}\;B_{4}\) are model constants.
In terms of mole fraction, it is written as47:
Garlapati–Madras model (five parameters model)48
It is a mathematical model and mathematically formulated as
where \(A_{5} - E_{5}\) are model constants.
Kumar–Johnstone model (three parameters model)49
It is a semi empirical model and mathematically described as:
where \(A_{6} - C_{6}\) are model constants.
Mahesh–Garlapati model (three parameters model)39
It is one of the latest models. It is based on degree of freedom and mathematically stated as:
where \(A_{7} - C_{7}\) are model constants.
Mendez–Teja model (three parameters model)50
It is a semi-empirical model and mathematically explained as:
where \(A_{8} - C_{8}\) are model constants.
Sodefian et al., model (six parameters model)40
It is a mathematical model and stated as:
where \(A_{9} - F_{9}\) are model constants.
Reformulated Chrastil model (three parameters model)47,51
It is a semi-empirical model and mathematically explained as:
where \(\kappa^{\prime},A_{10} \;{\text{and}}\;B_{10}\) are model constants.
Tippana–Garlapati model (six parameters model)52
It is a degree of freedom model and mathematically stated as:
where \(A_{11} - F_{11}\) are model constants.
New model
According to solid–liquid phase equilibrium criteria, the fugacity of the solute in the solid phase and liquid phase is equal at equilibrium. The liquid phase is considered as an expanded liquid phase of ScCO2. At equilibrium, the solubility may be expressed as53,54,55,56,57
where \(\gamma_{2}^{\infty }\) is drug activity coefficient at infinitesimal dilution in ScCO2 and \(f_{2}^{S}\) and \(f_{2}^{L}\) are fugacities of drug in the solid and ScCO2 phases, respectively. The \({f_{2}^{S} }/{f_{2}^{L} }\) ratio may be expressed as follows:
where,\(\Delta C_{p}\) is heat capacity difference of the drug in solid phase and that of SCCO2 phase. The terms that include △Cp is much smaller than the term that has \(\Delta H_{2}^{m}\)58, thus leaving △Cp term yields a much simpler expression for fugacity ratio as:
Combining Eq. (19) with Eq. (17) give the expression for the solubility model (Eq. (20)).
In order to use Eq. (20), the appropriate model for \(\gamma_{2}^{\infty }\) is essential.
In this work, the required activity coefficient is obtained from Wilson activity coefficient model56 at infinite dilution and it is given by the Eq. (21).
where \(\lambda_{12} = \left( {{{V_{2} }/ {V_{1} }}} \right)\exp \left( { - {{a_{12} } /{RT}}} \right)\) and \(\lambda_{21} = \left( {{{V_{1} } / {V_{2} }}} \right)\exp \left( { - {{a_{21} } /{RT}}} \right)\), \(V_{1}\) and \(V_{2}\) are molar volumes of solvent and solute, respectively.
When \(\rho_{1} = {1 / {V_{1} }}\), the final expression for the infinite dilution activity coefficient is obtained as:
The quantities \(a_{12}\) and \(a_{21}\) are assumed to be functions of reduced solvent density57, and molar volume of the solute is assumed as a constant value. In this work, \(a_{12}\) and \(a_{21}\) are assumed to have the following form:
Combining Eqs. (22), (23) and (24) with Eq. (20), give the following new explicit solubility model:
Equation (25) has four temperature independent adjustable variables namely \(A\),\(B\),\(C\) and \(D\).
Equation of state (EoS) model
The solubility of drug i (solute) in ScCO2 (solvent) is expressed as59,60,61:
where \(P_{i}^{s}\) is the sublimation pressure of the pure solid at system temperature T, P is the system pressure,\(V_{s}\) is the molar volume of the pure solid, R is the universal gas constant. The fugacity coefficient of the pure solute at saturation (\(\hat{\varphi }_{i}^{S}\)) is usually taken to be unity. In this work, the fugacity coefficient of the solute in the supercritical phase \(\hat{\varphi }_{i}^{{ScCO_{2} }}\) is calculated using EoS along with KMmr57. The expression used for calculation of \(\hat{\varphi }_{i}^{{ScCO_{2} }}\) is obtained from the following basic thermodynamic relation60:
The expression for \(\hat{\varphi }_{i}^{{ScCO_{2} }}\) is
\({\text{where}}\) \(\alpha = \sum\limits_{i}^{n} {\sum\limits_{j}^{n} {x_{i} x_{j} a_{{ij}}^{{2/3}} } } b_{{ij}}^{{1/3}} \)
and the associated mixing rules are:
The main reason for considering RKEoS is that it has only two adjustable constants \(k_{ij}\) and \(l_{ij}\).
All the models (density-based, new and RKEoS models) are correlated with the following objective function58:
The regression ability of a model is indicated in terms of an average absolute relative deviation percentage (AARD %).
For the regression, fminsearch (MATLAB 2019a®) algorithm has been used.
Results and discussion
Table 1 shows some physicochemical properties of the used materials. Empagliflozin solubility in ScCO2 is reported at various temperatures (T = 308 to 338 K) and pressures (P = 12 to 27 MPa). Table 2 indicates the solubility data and ScCO2 density. The reported ScCO2 density is obtained from the NIST data base. Figure 3 shows the effect of pressure on various isotherms. The cross over region is observed at 16.5 MPa. From Fig. 3, below the cross over region, solubility decreases with increase in temperature, and on the other hand, above the cross over region, the solubility increases with increase in temperature. The EoS model requires critical properties which are computed with standard group contribution methods based on the chemical structure62,63,64,65. The summary of the critical properties computed are shown in Table 3. Figure 4 presents the self-consistency of the measured data with MT model.
The density-based models considered in this work have different number of adjustable parameters. These parameters range from three to six numbers. The regression results of all the models are indicated in Tables 4 and 5. The correlating ability of the models is depicted in Figs. 5, 6, 7, 8, 9, 10, 11. From the results, it is clear that all the models are able to correlate the data reasonably well and maximum AARD% is observed to be 10.4%. It is believed that, more parameter models are able to correlate the data more accurately. Sodefian et al., model is able to correlate the data with AARD = 5.84% and Akaike’s information criterion (AIC = − 637.59) (more relevant information is presented in the following section). Among density models, Bian et al., model (five parameters model) is able to correlate the data well and corresponding AARD% is 5.1%. Interestingly, Chrastil (three parameters model) and Reformulated Chrastil models (three parameters model) are also able to correlate the data quite well. Further, Chrastil and Reformulated Chrastil models are able to provide the total enthalpy. Whereas, Bartle et al., model parameters are able to provide sublimation enthalpy of the empagliflozin drug. From the magnitude difference between the total and sublimation enthalpies, a solvation enthalpy is calculated. These results are reported in Table 6.
A new explicit solubility model based on solid–liquid equilibrium criteria combined with Wilson activity coefficient model corresponding to infinitesimal dilution is derived. The new model has four parameters \(A\), \(B\), \(C\) and \(D\). While regression, new model parameters are treated as temperature independent and solid molar volume is kept constant. The new model requires melting point, melting enthalpy and molar volume of empagliflozin drug, and these values are obtained from literature and group contribution methods. From literature31, the melting point of empagliflozin drug (426.1 K), molar volume (3.2699 × 10–4 m3/mol) and melting enthalpy (60.238 kJ/mol) are calculated based on literature, Immirzi and Perini63 and Jain et al., methods66, respectively. The new model makes use of objective function given in Eq. (33). Similarly, RKEoS along KMmr correlations are established with the help of critical properties given in Table 3 (temperature independent correlations). The optimization results of the new solubility and RKEoS models are indicated in Table 5.
In order to examine the ability of models for correlating the solubility data, AIC is applied67,68,69,70. When the data number is less than < 40, the corrected AIC (AICc) is used.
where AIC, N, \(Q\) and SSE are \(N\;\ln \left( {{{SSE} / N}} \right) + 2Q\), the number of observations, the number of adjustable parameters of the model and the error sum of squares, respectively. According to AICc criterion, the best model has the least AICc value. Table 7 shows AICc values for various models considered in this study. In terms of AICc, all the models are able to correlate the data closely. However, Reformulated Chrastil model has AICc value (− 637.02), hence it is treated as the best model and at the same time, Tippana–Garlapati model has the highest AICc value (− 621.69), therefore, it is considered as a poorly correlating model. Three parameters models namely Chrastil, Alwi–Garlapati and Mendez–Teja models have AICc values − 636.95, − 635.3 and − 635.4, respectively. The new model which has four parameters, indicating comparable performance with the best model (AICc value of − 637.24).
Conclusion
Solubilities of empagliflozin in ScCO2 at temperatures (T = 308–338 K) and pressures (P = 12–27 MPa) were reported for the first time. The measured solubility in terms of mole faction ranged from 5.14 × 10–6 to 25.9 × 10–6. The data was successfully correlated with several models, Bian et al., model (AARD = 5.1%) was observed to be the best model in correlating the solubility data. All the models are able correlate the data reasonable. However, the correlating ability in ascending order of various models in terms of the lowest AICc values is as follows: Bian et al., Reformulated Chrastil, Chrastil, new solid–liquid equilibrium, Mendez–Teja, RKEoS + KMmr, Alwi–Garlapati, Sodefian et al., Mahesh–Garlapati, Bartle et al., Tippana–Garlapati models. The new model proposed in this work may be useful for correlating solids solubility in any SCF.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to confidential cases are available from the corresponding author on reasonable request.
Abbreviations
- A-D:
-
New model constants
- \(A_{1} - \;C_{1}\) :
-
Alwi–Garlapati model constants
- \(A_{2} - C_{2}\) :
-
Bartle model constants
- \(A_{3} - E_{3}\) :
-
Bian model constants
- \(A_{4} \;{\text{and}}\;B_{4}\) :
-
Chrastil model constants
- \(A_{5} - E_{5}\) :
-
Garlapati–Madras model constants
- \(A_{6} - C_{6}\) :
-
Kumar–Johnstone model constants
- \(A_{7} - C_{7}\) :
-
Mahesh–Garlapati model constants
- \(A_{8} - C_{8}\) :
-
Mendez–Teja model constants
- \(A_{9} - F_{9}\) :
-
Sodefian model constants
- \(A_{10} \;{\text{and}}\;B_{10}\) :
-
Reformulated Chrastil model constants
- \(A_{11} - F_{11}\) :
-
Tippana–Garlapati model constants
- AARD:
-
Absolute average relative deviation
- Adj.R2 :
-
Adjusted R2
- AIC:
-
Akaike’s information criterion
- aij :
-
EoS energy parameter
- bij :
-
EoS volume correction
- C:
-
Solubility in Chrastil model
- Cp :
-
Heat capacity
- EoS:
-
Equation of state
- Hsol :
-
Solvation enthalpy
- Hsub :
-
Sublimation enthalpy
- HTotal :
-
Total enthalpy
- H2 m :
-
Melting enthalpy of the solute
- Mscf:
-
Molecular weight of supercritical fluid
- N:
-
Number of data points
- P:
-
Total pressure
- Psub :
-
Sublimation pressure
- RK:
-
Redlich–Kwong
- Pr :
-
Reduced pressure
- Pc :
-
Critical pressure
- Q:
-
Number of parameters of a model
- R:
-
Universal gas constant
- R2 :
-
Square of correlation coefficient
- RMSE:
-
Root mean square deviation
- SSE:
-
Error sum of squares
- T:
-
Temperature
- Tc :
-
Critical temperature
- Tm :
-
Melting temperature
- Tr:
-
Reduced temperature
- y2 :
-
Solubility in molefraction
- \(\Delta\) :
-
Difference
- \(\hat{\varphi }_{i}^{S}\) :
-
Fugacity coefficient of the pure substance at saturation
- \(\hat{\varphi }_{i}^{{ScCO_{2} }}\) :
-
Solute fugacity in supercritical carbon dioxide (ScCO2)
- \(\varpi\) :
-
Acentric factor
- \(\rho\) :
-
Density
- \(\rho_{r}\) :
-
Reduced density
- \(\kappa_{ij}\) :
-
EoS mixing rule parameter
- \(l_{ij}\) :
-
EoS mixing rule parameter
- \(\lambda_{12} ,\lambda_{21}\) :
-
Wilson model parameters
- \(\gamma_{2}^{\infty }\) :
-
Infinite dilution activity coefficient
- exp:
-
Experimental
- cal:
-
Calculated
- 1:
-
Solvent (CO2)
- 2:
-
Solute (drug)
- c:
-
Critical
- m:
-
Melting
- r:
-
Reduced
References
Akgerman, A. & Madras, G. Supercritical Fluids: Fundamentals and Applications (Kluwer Academic, 1994).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): Experimental correlations and thermodynamic modeling. J. Mol. Liq. 297, 111740 (2020).
Hitchen, S. & Dean, J. Properties of supercritical fluids. In Applications of Supercritical Fluids in Industrial Analysis 1–11 (Springer, 1993).
Subramaniam, B., Rajewski, R. A. & Snavely, K. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 86(8), 885–890 (1997).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Hazaveie, S. M. Experimental data and thermodynamic modeling of solubility of Sorafenib tosylate, as an anti-cancer drug, in supercritical carbon dioxide: Evaluation of Wong-Sandler mixing rule. J. Chem. Thermodyn. 142, 105998 (2020).
Bahrami, M. & Ranjbarian, S. Production of micro-and nano-composite particles by supercritical carbon dioxide. J. Supercrit. Fluids 40(2), 263–283 (2007).
Elvassore, N. & Kikic, I. Pharmaceutical processing with supercritical fluids. In High Pressure Process Technology: Fundamentals and Applications (ed. Bertucco, A. G. V. G.) 612–625 (Elsevier Science, 2001).
Gupta, R. B. & Chattopadhyay, P Method of forming nanoparticles and microparticles of controllable size using supercritical fluids and ultrasound. US patent No. 20020000681 (2002).
Reverchon, E., Adami, R., Caputo, G. & De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 47(1), 70–84 (2008).
Sodeifian, G., Sajadian, S. A., Ardestani, N. S. & Razmimanesh, F. Production of loratadine drug nanoparticles using ultrasonic-assisted rapid expansion of supercritical solution into aqueous solution (US-RESSAS). J. Supercrit. Fluids 147, 241–253 (2019).
Razmimanesh, F., Sodeifian, G. & Sajadian, S. A. An investigation into Sunitinib malate nanoparticle production by US-RESOLV method: Effect of type of polymer on dissolution rate and particle size distribution. J. Supercrit. Fluids 170, 105163 (2021).
Sodeifian, G., Sajadian, S. A. & Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 140, 72–84 (2018).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Abadian, M. Solubility of dasatinib monohydrate (anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling. J. Mol. Liq. 346, 117899 (2022).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Tamura, K. Solubility of quetiapine hemifumarate (antipsychotic drug) in supercritical carbon dioxide: Experimental, modeling and hansen solubility parameter application. Fluid Phase Equilib. 537, 113003 (2021).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483, 122–143 (2019).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Ghanaat-Ghamsari, M. Measurement and modeling of clemastine fumarate (antihistamine drug) solubility in supercritical carbon dioxide. Sci. Rep. 11(1), 1–16 (2021).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. CO2 utilization for determining solubility of teriflunomide (immunomodulatory agent) in supercritical carbon dioxide: Experimental investigation and thermodynamic modeling. J. CO2 Util. 58, 101931 (2022).
Reverchon, E., Russo, P. & Stassi, A. Solubilities of solid octacosane and triacontane in supercritical carbon dioxide. J. Chem. Eng. Data 38(3), 458–460 (1993).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Measurement, correlation and thermodynamic modeling of the solubility of Ketotifen fumarate (KTF) in supercritical carbon dioxide: Evaluation of PCP-SAFT equation of state. J. Fluid Phase Equilib. 458, 102–114 (2018).
Sodeifian, G., Detakhsheshpour, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 154, 104606 (2019).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. Solubility of amlodipine besylate (calcium channel blocker drug) in supercritical carbon dioxide: Measurement and correlations. J. Chem. Eng. Data 66(2), 1119–1131 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. The solubility of Sulfabenzamide (an antibacterial drug) in supercritical carbon dioxide: Evaluation of a new thermodynamic model. J. Mol. Liq. 335, 116446 (2021).
Sodeifian, G., Hazaveie, S. M., Sajadian, S. A. & SaadatiArdestani, N. Determination of the solubility of the repaglinide drug in supercritical carbon dioxide: experimental data and thermodynamic modeling. J. Chem. Eng. Data 64(12), 5338–5348 (2019).
Sodeifian, G., Hsieh, C.-M., Derakhsheshpour, R., Chen, Y.-M. & Razmimanesh, F. Measurement and modeling of metoclopramide hydrochloride (anti-emetic drug) solubility in supercritical carbon dioxide. Arab. J. Chem. 15, 103876 (2022).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. Measuring and modeling the solubility of an antihypertensive drug (losartan potassium, Cozaar) in supercritical carbon dioxide. J. Mol. Liq. 331, 115745 (2021).
Sodeifian, G., Razmimanesh, F., Ardestani, N. S. & Sajadian, S. A. Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq. 299, 112179 (2020).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (Loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. J. Fluid Phase Equilib. 472, 147–159 (2018).
Sodeifian, G. & Sajadian, S. A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 133, 239–252 (2018).
Sodeifian, G., Sajadian, S. A. & Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid Phase Equilib. 450, 149–159 (2017).
Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Hazaveie, S. M. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: Measurements and empirical correlations. Sci. Rep. 11(1), 1–13 (2021).
Niguram, P., Polaka, S. N., Rathod, R., Kalia, K. & Kate, A. S. Update on compatibility assessment of empagliflozin with the selected pharmaceutical excipients employed in solid dosage forms by thermal, spectroscopic and chromatographic techniques. Drug Dev. Ind. Pharm. 46(2), 209–218 (2020).
Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Ardestani, N. S. A comprehensive comparison among four different approaches for predicting the solubility of pharmaceutical solid compounds in supercritical carbon dioxide. J. Korean J. Chem. Eng. 35(10), 2097–2116 (2018).
Bian, X.-Q., Zhang, L., Du, Z.-M., Chen, J. & Zhang, J.-Y. Prediction of sulfur solubility in supercritical sour gases using grey wolf optimizer-based support vector machine. J. Mol. Liq. 261, 431–438 (2018).
Nguyen, H. C. et al. Computational prediction of drug solubility in supercritical carbon dioxide: Thermodynamic and artificial intelligence modeling. J. Mol. Liq. 354, 118888 (2022).
Sadeghi, A. et al. Machine learning simulation of pharmaceutical solubility in supercritical carbon dioxide: Prediction and experimental validation for busulfan drug. Arab. J. Chem. 15(1), 103502 (2022).
Sodeifian, G., Garlapati, C., Hazaveie, S. M. & Sodeifian, F. Solubility of 2, 4, 7-Triamino-6-phenylpteridine (triamterene, diuretic drug) in supercritical carbon dioxide: Experimental data and modeling. J. Chem. Eng. Data 65(9), 4406–4416 (2020).
Rathnam, V. M., Lamba, N. & Madras, G. Evaluation of new density based model to correlate the solubilities of ricinoleic acid, methyl ricinoleate and methyl 10-undecenoate in supercritical carbon dioxide. J. Supercrit. Fluids 130, 357–363 (2017).
Alwi, R. S., Garlapati, C. & Tamura, K. Solubility of anthraquinone derivatives in supercritical carbon dioxide: New correlations. Molecules 26(2), 460 (2021).
Mahesh, G. & Garlapati, C. Modelling of solubility of some parabens in supercritical carbon dioxide and new correlations. Arab. J. Sci. Eng. 1–13 (2021).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 146, 89–99 (2019).
Sodeifian, G., SaadatiArdestani, N., Sajadian, S. A., Golmohammadi, M. R. & Fazlali, A. Prediction of solubility of sodium valproate in supercritical carbon dioxide: Experimental study and thermodynamic modeling. J. Chem. Eng. Data 65(4), 1747–1760 (2020).
Peper, S., Fonseca, J. M. & Dohrn, R. High-pressure fluid-phase equilibria: Trends, recent developments, and systems investigated (2009–2012). Fluid Phase Equilib. 484, 126–224 (2019).
Alwi, R. S. & Garlapati, C. A new semi empirical model for the solubility of dyestuffs in supercritical carbon dioxide. Chem. Pap. 75(6), 2585–2595 (2021).
Bartle, K. D., Clifford, A., Jafar, S. & Shilstone, G. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20(4), 713–756 (1991).
Bian, X.-Q., Zhang, Q., Du, Z.-M., Chen, J. & Jaubert, J.-N. A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid Phase Equilib. 411, 74–80 (2016).
Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86(15), 3016–3021 (1982).
Sridar, R., Bhowal, A. & Garlapati, C. A new model for the solubility of dye compounds in supercritical carbon dioxide. Thermochim. Acta 561, 91–97 (2013).
Garlapati, C. & Madras, G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta 500(1), 123–127 (2010).
Kumar, S. K. & Johnston, K. P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1(1), 15–22 (1988).
Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 158, 501–510 (1999).
Garlapati, C. & Madras, G. Solubilities of palmitic and stearic fatty acids in supercritical carbon dioxide. J. Chem. Thermodyn. 42(2), 193–197 (2010).
Reddy, T. A. & Garlapati, C. Dimensionless empirical model to correlate pharmaceutical compound solubility in supercritical carbon dioxide. Chem. Eng. Technol. 42(12), 2621–2630 (2019).
Huang, C.-Y., Lee, L.-S. & Su, C.-S. Correlation of solid solubilities of pharmaceutical compounds in supercritical carbon dioxide with solution model approach. J. Taiwan Inst. Chem. Eng. 44(3), 349–358 (2013).
Iwai, Y., Koga, Y., Fukuda, T. & Arai, Y. Correlation of solubilities of high-boiling components in supercritical carbon dioxide using a solution model. J. Chem. Eng. Jpn. 25(6), 757–760 (1992).
Kramer, A. & Thodos, G. Solubility of 1-hexadecanol and palmitic acid in supercritical carbon dioxide. J. Chem. Eng. Data 33(3), 230–234 (1988).
Narayan, R. C., Dev, J. V. & Madras, G. Experimental determination and theoretical correlation for the solubilities of dicarboxylic acid esters in supercritical carbon dioxide. J. Supercrit. Fluids 101, 87–94 (2015).
Nasri, L., Bensetiti, Z. & Bensaad, S. Modeling of the solubility in supercritical carbon dioxide of some solid solute isomers using the expanded liquid theory. J. Sci. Technol. 3(2) (2018).
De Zordi, N., Kikic, I., Moneghini, M. & Solinas, D. Solubility of pharmaceutical compounds in supercritical carbon dioxide. J. Supercrit. Fluids 66, 16–22 (2012).
Garlapati, C. & Madras, G. Temperature independent mixing rules to correlate the solubilities of antibiotics and anti-inflammatory drugs in ScCO2. Thermochim. Acta 496(1–2), 54–58 (2009).
Kwak, T. & Mansoori, G. Van der Waals mixing rules for cubic equations of state. Applications for supercritical fluid extraction modelling. Chem. Eng. Sci. 41(5), 1303–1309 (1986).
Valderrama, J. O. & Alvarez, V. H. Temperature independent mixing rules to correlate the solubility of solids in supercritical carbon dioxide. J. Supercrit. Fluids 32(1–3), 37–46 (2004).
Fedors, R. F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 14(2), 147–154 (1974).
Immirzi, A. & Perini, B. Prediction of density in organic crystals. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 33(1), 216–218 (1977).
Lyman, W. J., Reehl, W. F. & Rosenblatt, D. H. Hand Book of Chemical Property Estimation Methods (McGraw-Hill, 1982).
Reid, R. C., Prausnitz, J. M. & Poling, B. E. The Properties of Gases and Liquids. (1987).
Jain, A., Yang, G. & Yalkowsky, S. H. Estimation of melting points of organic compounds. Ind. Eng. Chem. Res. 43(23), 7618–7621 (2004).
Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory (eds Petrov, B. N. & Csaki, F.) 267–281 (AkademiaaiKiado, 1973).
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33(2), 261–304 (2004).
Deepitha, J., Pitchaiah, K., Brahmmananda Rao, C., Madras, G. & Sivaraman, N. Solubilities of dialkylhydrogen phosphonates in supercritical carbon dioxide and their correlation using semi-empirical equations. Sep. Sci. Technol. 54(10), 1650–1660 (2019).
Kletting, P. & Glatting, G. Model selection for time-activity curves: The corrected Akaike information criterion and the F-test. Z. Med. Phys. 19(3), 200–206 (2009).
https://webbook.nist.gov/chemistry/01 (Institute of Standards and Technology U.S. Department of Commerce, 2018).
Acknowledgements
Corresponding authors would like to thank the research deputy of university of Kashan (Grant # Pajoohaneh-1400/26) for the financial support of this project.
Author information
Authors and Affiliations
Contributions
G.S. conceptualization, methodology, validation, investigation, supervision, project administration, writing—review and editing; C.G. methodology, investigation, software, writing—original draft; F.R. investigation, validation, resources; H.N. measurement.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sodeifian, G., Garlapati, C., Razmimanesh, F. et al. Experimental solubility and thermodynamic modeling of empagliflozin in supercritical carbon dioxide. Sci Rep 12, 9008 (2022). https://doi.org/10.1038/s41598-022-12769-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12769-2
This article is cited by
-
Studies on solubility measurement of codeine phosphate (pain reliever drug) in supercritical carbon dioxide and modeling
Scientific Reports (2023)
-
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2
Scientific Reports (2023)
-
Solubility measurement and modeling of hydroxychloroquine sulfate (antimalarial medication) in supercritical carbon dioxide
Scientific Reports (2023)
-
Utilization of CO2 in supercritical conditions for the synthesis of cyclic poly (N-isopropylacrylamide) via emulsion and homogeneous reactions
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.