Abstract
High serum glucose to potassium ratio (GPR) at admission is implicated for a poor outcome in acute brain injury, acute intracranial hemorrhage, and aneurysmal subarachnoid hemorrhage. However, the relationship between GPR and the outcome of ischemic stroke (IS) remains unknown. In all, 784 IS patients from a large emergency Norwegian cohort were included for secondary analysis. The exposure and outcome were GPR at baseline and all-cause mortality within 30 days after the first admission. Multivariable logistic regression analysis was performed to estimate the risk of 30-day mortality based on GPR levels. In addition, we examined whether there was a nonlinear relationship between admission GPR and 30-day mortality using two-piecewise linear regression with a smoothing function and threshold level analysis. The results of multivariable regression analysis showed that GPR at baseline was positively associated with the 30-day mortality (OR 2.01, 95% CI 1.12, 3.61) after adjusting for potential confounders (age, gender, department, serum sodium, serum albumin, serum-magnesium, hypertension, heart failure, chronic renal failure, and pneumonia). When GPR was translated to a categorical variable, the ORs and 95% CIs in the tertiles 2 to 3 versus the tertile 1 were 1.24 (0.60, 2.56) and 2.15 (1.09, 4.24), respectively (P for trend = 0.0188). Moreover, the results of the two-piecewise linear regression and curve fitting revealed a linear relationship between GPR and 30-day mortality. In IS patients, GPR is positively correlated with 30-day mortality, and the relationship between them is linear. The GPR at admission may be a promising predictor for the short-term outcome in IS patients.
Similar content being viewed by others
Introduction
Ischemic stroke (IS) is a commonly acute and severe disease with high mortality and disability rates, imposing an increasingly heavy socioeconomic burden globally1. Currently, intense efforts are being made to find novel risk predictors, which are simple and easily accessible, to better guide clinical decision-making for patients with IS. Circulating biomarkers in blood samples were clinically very common; thus, the relationship between circulating biomarkers and prognosis has gained increasing attention for stroke in recent years2,3.
Serum glucose and potassium are two important blood indicators that are commonly used clinically. As the main energy source of cells in the human body, glucose is a critical factor for maintaining cellular metabolism4. Potassium ion, the most abundant cation in the cells of a human body, plays a crucial role in physiological processes including neural conduction, cardiac pulsation, muscle contraction, and maintenance of normal renal function5. In addition, both serum glucose and potassium disturbances have been revealed to be correlated with the risk of stroke6,7. Previous studies have demonstrated that there were complex interactions among potassium and glucose in the human body8,9. Given the potential combined effects of glucose and serum potassium, the serum glucose to potassium ratio (GPR) has been used in a few studies and has been shown to be an early prognostic factors for central nerve injures including aneurysmal subarachnoid hemorrhage (aSAH)10, acute intracerebral hemorrhage11, severe traumatic brain injury12, and neuropsychiatric syndrome after carbon monoxide poisoning13.
However, the relationship between GPR and the clinical outcome of IS remains unknown. Therefore, we aimed to explore the association between GPR at admission and short-term mortality in IS patients based on a retrospective cohort study.
Methods
Data source
Original data were published by Tazmini et al.14 on the “DRYAD” website (www.datadryad.org). And Tazmini et al.15 authorized the ownership of their raw data to the “DRYAD” database. Thus, this secondary research based on the raw data for a different research hypothesis was permitted.
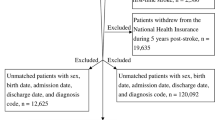
The original research was a single-center retrospective cohort study that included 31,966 unique patients (62,991 registered admission information) who visited the emergency department of the Diakonhjemmet Hospital in Oslo (Norway) from 2010 to 2015. According to the ICD-10 standard of classification, 974 visits (admission information) were diagnosed as IS (ICD-10, I63). The raw data included information on multiple hospitalizations for the same patient, but only the first visit of each patient was considered in this study. Thus, we excluded the second or subsequent admissions (n = 88). Only 886 unique IS patients were then considered during analysis. Subsequently, 102 patients were excluded for missing data concerning serum glucose or potassium levels (n = 6), incorrectly recorded days of death (n = 5), and presence of diabetes mellitus or serum glucose level > 200 mg/dL (n = 91) at admission. Finally, 784 unique participants were included in the study (Fig. 1).
Exposure
All the laboratory indicators were obtained from the first-time laboratory results at admission. Serum calcium (mmol/L), serum-albumin (g/L), serum-sodium (mmol/L), serum-potassium (mmol/L), serum-glucose (mmol/L), serum-phosphate (mmol/L), and serum-magnesium (mmol/L) were recorded in the original data. The serum glucose to potassium ratio was calculated as serum glucose concentration (mmol/L) divided by serum potassium concentration (mmol/L)12.
Co-morbidities and other variables
Secondary diagnostic information was used to identify co-morbidities including diabetes (ICD-10: E10–E14), hypertension (ICD-10: I10), hyperlipemia (ICD-8: E78), atrial fibrillation/atrial flutter (ICD-10: I48), heart failure (ICD-10: I50), acute renal failure(ICD-10: N17), chronic renal failure (ICD-10: N18), chronic obstructive pulmonary disease (ICD-10: J42–44), coronary heart disease (ICD-10: I25), chronic obstructive pulmonary disease (ICD-10: J42–44), cancer (ICD-10: C0–C9, Z51.0-3), malnutrition (ICD-10: E40–E46), and pneumonia (ICD-10: J98, J69, J11–18).
All IS patients were categorized into five subtypes according to the TOAST stroke subtype classification system: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, other determined etiology, and undetermined16. The information of patients admitted to the medical or surgical department was identified as a binary variable.
Outcome
The primary outcome was all-cause mortality within 30 days after first admission.
Missing data
All missing data of covariates are stated in Table 1. Considering that missing data may reduce statistical power or even lead to bias, covariates with too much missing data (e.g., concerning serum phosphate and serum magnesium levels) were handled as categorical variables. And dummy variables were used to identify the missing values of the covariate17.
In addition, to further assess whether missing data being handled as dummy variables can introduce bias into the results, we used multiple imputations based on five replications and chained equation approach in the R MI procedure to handle all the missing data for sensitivity analysis18. The process of multiple imputation and the results of multivariate regression analysis based on five multiple imputation data are shown in Supplementary Fig. 1 and Supplementary Table 1 respectively.
Statistical analysis
All analyses were performed with EmpowerStats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the statistical software package R (http://www.R-project.org, The R Foundation). P < 0.05 was considered statistically significant.
Continuous and categorical variables are expressed as Mean ± SDs and percentages, respectively. To examine the differences among subgroups of variables, we used one-way ANOVA test for continuous variables with normal distribution, Kruskal–Wallis H test for continuous variables with skewed distribution, and chi-square test (or Fisher’s exact test) for categorical variables.
Multiple logistic regression analysis was used to explore the association between GPR and outcome (30-day mortality); OR and 95% CI were used for risk evaluation. To evaluate whether there is a potential non-linear relationship between GPR and 30-day mortality, curve fitting and two-piecewise linear regression analysis were performed.
We built three models to regulate the potential confounding factors, they are : (1) Crude model, i.e., unadjusted; (2) Model I, adjusted for age and gender; (3) Model II, adjusted for age, gender, department, serum sodium, serum albumin, serum-magnesium tertiles, hypertension, heart failure, chronic renal failure, and pneumonia. Covariates were selected based on their relationship to 30-day mortality or their ability to change the effect value by more than 10%19, gender was also included as a basic covariate.
Sensitivity analysis
GPR tertiles were also used to test the stability of multiple regression analysis results, and the linear tests were performed by assigning medians to each GPR tertile as a continuous variable in the models20.
An E-value was used to explore the potential of unmeasured confounding between GPR and 30-day mortality. The E-value was defined as the required magnitude for an unmeasured confounder to overturn the observed association between GPR and 30-day mortality21.
Ethics approval and consent to participate
Ethics of the previous study was approved by the Norway regional committee (Regional Committee for Medical and Health Research Ethics South East) and informed consent was exempt for anonymous data. Thus, our secondary analysis based on this original study did not require separate ethical approval. And our study was carried out following all the relevant guidelines and regulations.
Results
Baseline characteristics of participants
The participants’ average age of participants was 77.64 ± 12.32 (range 34–100) years and 54.68% were female. The baseline characteristics and co-morbidities of participants are demonstrated in Table 1 by GPR tertiles. Serum-phosphate tertiles, atrial fibrillation/atrial flutter, cancer, pneumonia, and 30-day mortality of the GPR tertiles groups were statistically different (all P < 0.05). Large-artery atherosclerosis, cardioembolism, small-vessel occlusion, other determined etiology, and undetermined.
Univariate analysis in relation to 30-day mortality
The 30-day mortality was chosen as a dependent variable, and univariate analysis were performed to determine which covariables were related to 30-day mortality. The results indicated that age (OR 1.10, 95% CI 1.057–1.13, P < 0.0001), department (Surgical vs Medical: OR 19.11, 95% CI 1.71–213.26, P = 0.0165), GPR (OR 2.18, 95% CI 1.31–3.63, P = 0.0028), serum-glucose (OR 1.33, 95% CI 1.14–1.55, P = 0.0003), serum-albumin (OR 0.82, 95% CI 0.78–0.87, P < 0.0001), hypertension (yes vs no: OR 0.50, 95% CI 0.26–0.96, P = 0.0378), heart failure (yes vs no: OR 5.44, 95% CI 2.22–13.28, P = 0.0002), chronic renal failure (yes vs no: OR 4.51, 95% CI 2.06–9.89, P = 0.0002), pneumonia (yes vs no: OR 9.17 , 95% CI 4.52–18.63, P < 0.0001) were associated with 30-day mortality (Table 2).
Multivariate logistic regression analysis of GPR and 30-day mortality
In multivariate regression analysis, we built three models adjusting for different covariates to verify the stability of the results. The results of the crude model without adjusting for confounder factors showed that GPR and 30-day mortality were positively correlated (OR 2.18, 95% CI 1.31–3.63).Model I which was adjusted for age and gender also indicated the same association (OR 2.09, 95% CI 1.24–3.54). Model II which was further adjusted for age, gender, department, serum sodium, serum albumin, serum-magnesium tertiles, hypertension, heart failure, chronic renal failure, and pneumonia also revealed that GPR was independently associated with 30-day mortality (OR 2.01, 95% CI 1.12–3.61) (Table 3).
Curve fitting and two-piecewise linear regression model of GPR and 30-day mortality
Curve fitting analysis, was adjusted according to three models (crude model, model I, model II) all indicated that a curve that that continues to rise, and the inflection point was approximately 1.6 (Fig. 2). According to the inflection point (GPR = 1.6), two-piecewise linear regression analysis models for different confounding factors (crude model, model I, model II) were used to explore the potential non-liner relationship. The result of model II showed two different effective sizes in two-piecewise linear regression equations, but the P-value for likelihood ratio test was 0.55 which was not statistically significant. The other two models showed similar results and the P-values for the likelihood ratio test were all > 0.05 (Table 4). Thus, the relationship of GPR with 30-day mortality was linear.
Multivariate adjusted smooth curve-fitting for association between GPR and 30-day mortality. GPR glucose to potassium ratio. (a) Crude model: not adjusted. (b) Mode I: adjusted for age and gender. (c) Mode II: adjusted for age, gender, department, serum sodium, serum albumin, serum-magnesium tertiles, hypertension, heart failure, chronic renal failure, and pneumonia. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. The potential demarcation points are 1.6 according to the smoothing spline plots.
Sensitivity analysis
GPR (tertiles) were also plugged into the multiple regression equation for sensitivity analysis. The results of GPR as categorical variable (tertile) were consistent with the results of GPR as a continuous variable, the top tertile had 115% increment of diabetes risk when compared with the bottom tertile in the full model (model II), and found that the trend across the tertiles was significant (P for trend = 0.0188). Other two models (crude model, model I) showed similar results (Table 3).
An E-value was calculated to assess the sensitivity to unmeasured confounding. The primary findings were stable unless an unmeasured confounder existed and high positively related to GPR (OR ≥ 3.43) and 30 day-mortality (OR ≥ 3.43).
Discussion
To the best of our knowledge, this is the first study to explore the relationship between GPR and the clinical IS outcome. Our study showed a significantly positive correlation between GPR levels and 30-day mortality. Further, the stability of the association was verified by adjusting for potential confounding factors (mode I, OR 2.09, 95% CI 1.24–3.54; model II, OR 2.01, 95% CI 1.24–3.54). In sensitivity analysis, we handled GPR as a categorical variable (tertiles) and the results showed an increasing trend of OR values from tertile 1 to tertile 3 in the three models (P values for trend all < 0.05). Moreover, the curve fittings of GPR levels and 30-day mortality showed a gradual upward curve in smoothing plots for the three different models (Supplementary Fig. 1). According to the inflection point in the curve fitting plot, two-piecewise linear regression analyses with three different adjustment methods were performed and all the results showed a linear relationship between GPR and 30-day mortality.
GPR is a novel parameter that can be measured quickly in clinics. Fujiki et al. first reported the potential association between baseline GPR and H–K grade and Glasgow score at discharge in a retrospective cohort study including 565 aSAH patients10. In another study, they investigated cerebral vasospasm after aSAH, and reported that elevated GPR levels were related to cerebral vasospasm grades and ischemic events induced by cerebral vasospasm22. In addition, the roles of GPR levels in other acute neurological injury related disease including acute intracerebral hemorrhage. Neuropsychiatric syndrome after carbon monoxide poisoning, and severe traumatic brain injury, have been proven11,12,13. In these studies, the baseline GPR levels were all observed in worse clinical outcome group than the normal group. Our results added evidence with regard to association between GPR levels and short-term outcome (30-day mortality) in cerebral ischemic injury. The results of the aforesaid studies suggested that GPR levels were closely related to pathological neurological disorders.
Hyperglycemia is very common in the acute phase of IS, even among non-diabetic IS patients23. The phenomenon of post-stroke hyperglycemia was believed to be a type of stress hyperglycemia induced by high cortisol and catecholamine levels after ischemic injury24. In addition, stress hyperglycemia had been suggested to be associated with stroke severity. Patients with stress hyperglycemia often had more serious strokes than those with type 2 diabetes mellitus (T2DM)25. Guo et.al have reported that IS patients with stress hyperglycemia had a higher risk of 90-day stroke recurrence than those with T2DM26. However, inconsistent results were shown in studies to explore the association between hyperglycemia and clinical outcomes of IS patients25,27,28,29. A study by Zonneveld et.al showed that stress hyperglycemia was associated with post-stroke infections and poor functional outcome27. Further, among both IS patients treated with intravenous thrombolysis28 and those treated via mechanical thrombectomy29, stress hyperglycemia was proven to be associated with a poor outcome. However, Tziomalos et al. believed that stress hyperglycemia was correlated with stroke severity rather than directly being related to an adverse outcome25. Besides, a recent clinical trial showed that glucose-lowering therapy did not help in improving the prognosis30. In addition to the heterogeneity of study design, and the potential non-linear relationship between admission serum glucose and outcome reported previously31, the complicated and multifaceted path mechanism underlying stress hyperglycemia may lead to the discordance of results. Likewise, as another important clinical blood biomarker, serum potassium levels play a crucial role in maintaining basic cellular functions. Normally, potassium ion is mostly stored in the cells and transported outside the membrane by sodium/potassium ATPase when necessary. Some population-based evidences have demonstrated that a potassium-rich diet could lower the risk of stroke32,33. Nevertheless, existing studies investigating the association between serum potassium and stroke outcome have shown contrasting results34,35,36. In our univariate analysis, admission serum potassium was not associated with 30-day mortality. This may be because of the intermediate factors, such as serum glucose, which impacted the relationship between serum potassium and short-term outcome. Accordingly, current research on the specific relationship between serum potassium level and the outcome of IS patients is still limited.
Despite these observations investigating the baseline GPR levels and IS outcome, the mechanisms underlying these findings still remain unknown. In severe stress injury conditions, sympathetic activation would result in an increased secretion of stress hormones including catecholamines, growth hormone, cortisol, and cytokines, and then induce a hyperglycemic response and insulin resistance24. In acute IS patients, the regulation of sodium/potassium ATPase by high catecholamine levels and secretion of insulin all would lead to potassium influx37. Thus, post-stroke hyperglycemia and hypokalemia may reflect the stress-related activation and a disorder of the hypothalamic–pituitary–adrenal (HPA) axis. HPA axis dysregulation believed to play a key role in the process of successive energy pump failure and various signaling cascades of IS38. In addition, a high cortisol level would activate the renin–angiotensin–aldosterone system (RAAS) to induce low serum potassium39. Brown et.al also thought that lower serum potassium level may represent increased activity of RAAS40. Current evidences show that RAAS plays a pivotal role in the progression of IS41, and angiotensin II receptors blockers could help to stroke prevention42. Based on the abovementioned discussion and considering the combined effects of serum potassium and serum glucose levels, the GPR index may be a good indicator for reflecting the status of HPA axis and RAAS dysregulation after IS. Moreover, studies of other stress damage types including acute myocardial infarction43, blunt abdominal trauma44, pulmonary embolism45, and even intermediate syndrome induced by anticholinesterase-containing chemicals poisoning46 all showed a stable correlation between increased GPR and poor outcomes or more severe symptoms. These studies also suggest that GPR may be a potential marker of stress injury for reflecting the condition of the whole body in severe disease.
The pathogenesis of IS is complex, and there was limited knowledge concerning it until now. Therefore, most treatment strategies currently followed that were developed by targeting known key pathogenetic links are often inadequately effective. The role of stress responses38 and stress-related markers3 has been proven to play a pivotal role in IS progression and has gained increasing attention. Our results suggested that comprehensive treatments including appropriate potassium supplementation, hypoglycemic treatment, and stress response blocker (β-blocker, RAAS inhibitor) may improve the short-term prognosis with high GPR levels. However, because of the nature of this retrospective study, the causality of high GPR levels and short-term outcome in IS patients could not be established. Future studies were needed to explore the causal relationship and verify the effectiveness of these treatments. In addition, because the raw data of our study were from a large emergency cohort, GPR may have a broad application prospect for IS patients admitted in emergency departments, especially in primary or smaller emergency departments as a brief blood biomarker, information on which could quickly be obtained at admission.
Conclusion
Among IS patients, GPR is positively correlated with 30-day mortality, and the relationship between them is linear. Thus, GPR at admission may be a promising predictor of the short-term outcome of IS patients.
Strengths and limitations
There are several advantages to our study. First, the results of univariate analysis, regression coefficient change, and previous literature were used to select covariates. Second, curve fitting and two-piecewise linear regression analysis were performed to explore the potential non-liner relationship, which had been shown in a previous study. Third, one crude model and three models which had been adjusted for potential confounding variables were used to test the stability of the results. Fourth, to avoid the contingency of analysis, GPR was considered as a continuous variable and categorical variable in the multiple regression equation, and sensitivity analysis and trend test were performed.
However, this study also has some limitations. First, the presence of unmeasured confounders could not be excluded. Since the secondary analysis originated from a retrospective cohort, variables that were not collected could not be adjusted. E-value was used to explore the potential for unmeasured confounding between GPR and 30-day mortality and the result showed that an unmeasured confounder was unlikely to explain the entirety of the mortality effect. Second, ICD-10 codes for renal failure may not be clear enough to identify renal function status, and hospitalization information after emergency admission including intensive care unit duration and length of hospital stay were not included in the analysis; future studies collecting renal function indicators (serum creatinine and baseline eGFR), intensive care unit duration, and length of hospital stay are required to more accurately explore the association and mechanisms. Second, there was no record concerning the levels of serum hormones such as catecholamines, glucagon, and corticosteroids in the original data; thus, we could not clarify the reason for high GPR in patients with severe IS. Third, lacking treatment information before the first blood tests (dextrose, potassium, or insulin) and after admission may lead to bias. However, given that the treatment would tend to a bias toward the null, we believed that the unmeasured confounding of medication treatment may underestimate the observed effect. Fourth, though first-time laboratory results at admission, which are more likely to reflect the initial state of the patient at the onset, were used, it would be better to examine the dynamic changes in GPR in future studies to understand the potential mechanism of the associations. Because of the retrospective study design, we could not confirm the time of blood collection, which will influence the GPR level. Thus, further prospective studies with predesigned identical examination time are required. Finally, the participants of this study are Norwegian populations, and the findings do not necessarily apply to other populations.
Data availability
The data are available from the ‘DataDryad’ database (www.datadryad.org).
Abbreviations
- GPR:
-
Serum glucose to potassium ratio
- IS:
-
Ischemic stroke
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- AF:
-
Atrial fibrillation/atrial flutter
- COPD:
-
Chronic obstructive pulmonary disease
- CHD:
-
Coronary heart disease
References
Ding, Q. et al. Global, Regional, and National Burden of Ischemic Stroke, 1990–2019. Neurology 98, e279–e290 (2022).
Makris, K., Haliassos, A., Chondrogianni, M. & Tsivgoulis, G. Blood biomarkers in ischemic stroke: Potential role and challenges in clinical practice and research. Crit. Rev. Clin. Lab. Sci. 55, 294–328 (2018).
Montellano, F. A. et al. Role of blood-based biomarkers in ischemic stroke prognosis: A systematic review. Stroke 52, 543–551 (2021).
Remesar, X. & Alemany, M. Dietary energy partition: The central role of glucose. Int. J. Mol. Sci. 21, 7729 (2020).
Kovesdy, C. P. et al. Potassium homeostasis in health and disease: A Scientific Workshop Cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am. J. Kidney Dis. 70, 844–858 (2017).
Shi, H. et al. Fasting blood glucose and risk of Stroke: A dose-response meta-analysis. Clin. Nutr. 40, 3296–3304 (2021).
Johnson, L. S., Mattsson, N., Sajadieh, A., Wollmer, P. & Soderholm, M. Serum potassium is positively associated with stroke and mortality in the large, population-based malmo preventive project cohort. Stroke 48, 2973–2978 (2017).
Rowe, J. W., Tobin, J. D., Rosa, R. M. & Andres, R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism 29, 498–502 (1980).
McTaggart, J. S., Clark, R. H. & Ashcroft, F. M. The role of the KATP channel in glucose homeostasis in health and disease: More than meets the islet. J. Physiol. 588, 3201–3209 (2010).
Fujiki, Y. et al. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 129, 870–875 (2018).
Wu, X. Y. et al. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J. Int. Med. Res. 49, 675896679 (2021).
Zhou, J., Yang, C. S., Shen, L. J., Lv, Q. W. & Xu, Q. C. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clin. Chim. Acta 506, 166–171 (2020).
Demirtas, E., Korkmaz, I., Tekin, Y. K., Demirtas, E. & Caltekin, I. Assessment of serum glucose/potassium ratio as a predictor for delayed neuropsychiatric syndrome of carbon monoxide poisoning. Hum. Exp. Toxicol. 40, 207–213 (2021).
Tazmini, K. et al. Data from: Electrolyte imbalances in an unselected population in an emergency department: A retrospective cohort study. Dryad. https://doi.org/10.5061/dryad.f3h26j3 (2019).
Tazmini, K., Nymo, S. H., Louch, W. E., Ranhoff, A. H. & Oie, E. Electrolyte imbalances in an unselected population in an emergency department: A retrospective cohort study. PLoS ONE 14, e215673 (2019).
Adams, H. J. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24, 35–41 (1993).
Vetter, C. et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315, 1726–1734 (2016).
Bernhardt, P. W. Model validation and influence diagnostics for regression models with missing covariates. Stat. Med. 37, 1325–1342 (2018).
Jaddoe, V. W. et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ 348, g14 (2014).
Park, S. Y. et al. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann. Intern. Med. 167, 228–235 (2017).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321, 602–603 (2019).
Matano, F. et al. Serum glucose and potassium ratio as risk factors for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 28, 1951–1957 (2019).
Luitse, M. J., Biessels, G. J., Rutten, G. E. & Kappelle, L. J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 11, 261–271 (2012).
Dungan, K. M., Braithwaite, S. S. & Preiser, J. C. Stress hyperglycaemia. Lancet 373, 1798–1807 (2009).
Tziomalos, K. et al. Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metabolism 67, 99–105 (2017).
Guo, Y. et al. Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging (Albany). 13, 9108–9118 (2021).
Zonneveld, T. P. et al. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology 88, 1415–1421 (2017).
Merlino, G. et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J. Thromb. Thrombol. 51, 789–797 (2021).
Rinkel, L. A. et al. High admission glucose is associated with poor outcome after endovascular treatment for ischemic stroke. Stroke 51, 3215–3223 (2020).
Johnston, K. C. et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The SHINE randomized clinical trial. JAMA 322, 326–335 (2019).
Ntaios, G., Egli, M., Faouzi, M. & Michel, P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 41, 2366–2370 (2010).
Aburto, N. J. et al. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 346, f1378 (2013).
Vinceti, M. et al. Meta-analysis of potassium intake and the risk of stroke. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.116.004210 (2016).
Mattsson, N. et al. Prognostic impact of mild hypokalemia in terms of death and stroke in the general population—A prospective population study. Am. J. Med. 131, 318–319 (2018).
Gariballa, S. E., Robinson, T. G. & Fotherby, M. D. Hypokalemia and potassium excretion in stroke patients. J. Am. Geriatr. Soc. 45, 1454–1458 (1997).
Fofi, L. et al. An observational study on electrolyte disorders in the acute phase of ischemic stroke and their prognostic value. J. Clin. Neurosci. 19, 513–516 (2012).
Thier, S. O. Potassium physiology. Am. J. Med. 80, 3–7 (1986).
Gulyaeva, N. V., Onufriev, M. V. & Moiseeva, Y. V. Ischemic stroke, glucocorticoids, and remote hippocampal damage: A translational outlook and implications for modeling. Front. Neurosci. 15, 781964 (2021).
Back, C. et al. RAAS and stress markers in acute ischemic stroke: Preliminary findings. Acta Neurol. Scand. 131, 132–139 (2015).
Paulson, O. B., Barry, D. I., Strandgaard, S. & Lassen, N. A. Does angiotensin-II protect against strokes? Lancet 2, 927–928 (1986).
Sokol, S. I. et al. Modulation of the renin-angiotensin-aldosterone system for the secondary prevention of stroke. Neurology 63, 208–213 (2004).
Jiang, F. et al. Angiotensin-converting enzyme 2 and angiotensin 1–7: Novel therapeutic targets. Nat. Rev. Cardiol. 11, 413–426 (2014).
Plakht, Y., Gilutz, H. & Shiyovich, A. The association of concomitant serum potassium and glucose levels and in-hospital mortality in patients with acute myocardial infarction (AMI). Soroka acute myocardial infarction II (SAMI-II) project. Int. J. Cardiol. 287, 39–45 (2019).
Katipoglu, B. & Demirtas, E. Assessment of serum glucose potassium ratio as a predictor for morbidity and mortality of blunt abdominal trauma. Ulus Travma Acil Cerrahi Derg. 28, 134–139 (2022).
Boyuk, F. The predictor potential role of the glucose to potassium ratio in the diagnostic differentiation of massive and non-massive pulmonary embolism. Clin. Appl. Thromb. Hemost. 28, 1309709646 (2022).
Sharif, A. F. & Fayed, M. M. Assessment of the serum glucose/potassium GLU/K ratio as a predictor of intermediate syndrome following acute anticholinesterase exposure. Neurotoxicology 89, 161–173 (2022).
Acknowledgements
The authors specially thank the other authors of the original study. They completed the whole study and shared their data selflessly and kindly. They are (the rankings and institutions of these researchers are ranked according to the original reference 15): Professor Kiarash Tazmini (Department of Endocrinology, Morbid Obesity and Preventive Medicine, Faculty of Medicine, Oslo University Hospital, Oslo, Norway), Professor Ståle H. Nymo (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway), Professor William E. Louch (Institute of Experimental Medical Research, Oslo University Hospital, Ullevål and University of Oslo, Oslo, Norway; Center for Heart Failure Research, University of Oslo, Oslo, Norway), Professor Anette H. Ranhoff (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Science, University of Bergen, Bergen, Norway), and Professor (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Science, University of Bergen, Bergen, Norway). Besides, the authors are grateful to Professor Xinglin Chen and Professor Changzhong Chen (Statistical consultant, X&Y Solution, lnc. Boston MA) for their assistance in statistics. Finally, the first author YzL would like to especially thank his daughter (Mrs. Yanli Lu) and other family memberships for their encouragement and kind support.
Funding
This study was supported by National Natural Science Foundation of China (No. 81960330) (http://www.nsfc.gov.cn) and Jiangxi Provincial Department of Science and Technology (Grant Number: 20202BABL206053, 20192BAB205045, 20161BBI90018).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology: Y.L. and X.M. Software: Y.L. Visualization: X.M. Writing—Original draft preparation: Y.L. Writing—Reviewing and Editing: Y.W., Y.M., and X.Z. All the authors listed have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Y., Ma, X., Zhou, X. et al. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci Rep 12, 8233 (2022). https://doi.org/10.1038/s41598-022-12393-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12393-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.