Abstract
The safety and efficacy of drug-eluting beads transarterial chemoembolization (DEB-TACE) for unresectable renal cell carcinoma (RCC) still unknown. We aimed to assess the feasibility, safety and clinical efficacy of DEB-TACE with doxorubicin-loaded CalliSpheres beads (CB) in patients with unresectable RCC. Between 2016 and 2020, thirty-five patients with unresectable RCC underwent DEB-TACE with doxorubicin-loaded CB. The objective response rate (ORR) was the primary endpoint, and overall survival (OS) and progression-free survival (PFS) were the secondary endpoints. Fifteen-seven times of DEB-TACE were performed in 35 patients using doxorubicin-loaded (median 60 mg) CB. Fifteen patients underwent an additional session of DEB-TACE, with intervals of 1 to 1.5 months. Twenty-one patients underwent transarterial infusion with cisplatin or oxaliplatin before DEB-TACE. The median follow-up time was 9.0 months (Range 1.8–43.6 months). ORR and DCR were 47.1% and 94.1%, 29.0% and 87.1%, 23.1% and 84.6% respectively at 1-, 3-, and 6- months after DEB-TACE. The median PFS was 21.4 months, and the 3-, 6- and 12- month PFS rates were 84.7%, 73.7% and 62.3%, respectively. The median OS was 24.6 months, and the 3-, 6- and 12- month OS rates were 93.9%, 87.6% and 65.2%, respectively. There were no treatment-related deaths or severe adverse events of grade 3 or more. In conclusion, DEB-TACE with doxorubicin-loaded CB is a safe, feasible and effective palliative treatment option for patients with unresectable RCC.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) is a common cause of cancer-related death and surgical resection is the preferred treatment of localized disease of RCC. Transarterial chemoembolization (TACE) is a palliative treatment option for unresectable RCC, which may increase the clinical efficacy and decrease adverse events than systemic chemotherapy by increasing local drug concentration1. Emulsions of chemotherapeutic drugs and lipiodol were usually used in traditional TACE; however, drugs can neither release slowly nor reside long enough time2 As a novel drug delivery and embolization system, drug-eluting beads TACE (DEB-TACE) is able to release drugs slowly into the malignant tissue after embolizing the tumor-feeding arteries3,4,5. CalliSpheres beads (CB) has been used recently for patients with hepatocellular carcinoma6,7, uterus8 or lung9. In animal study, CB can effectively and safely embolize porcine renal artery10. However, the safety and efficacy of DEB-TACE have not been assessed in patients with unresectable RCC. This preliminary study aims to assess the feasibility, safety and clinical efficacy of DEB-TACE with doxorubicin-loaded CB in patients with unresectable RCC.
Patients and methods
Study design
The observational study was approved by the Institutional Review Board of Zhengzhou university committee on human investigation. Written informed consent was obtained from all patients. All methods were performed in accordance with the relevant guidelines and regulations. This study was conducted in 35 patients with unresectable RCC who underwent DEB-TACE using doxorubicin-loaded CB from July 2016 to May 2020. Indications for DEB-TACE: age < 85 years; pathological confirm of RCC (Fig. 1A); recurrence or progression after operation or standard treatments; refused or ineligible to receive standard treatments due to severe visceral dysfunction; no life-threatening diseases. Exclusion criteria: with other carcinoma but receive no treatment; white blood cell count < 3.0 × 109/L; platelets count < 40.0 × 109/L; active and severe infection; breastfeeding woman; pregnant woman.
A 55-year female with left RCC treated by CB. (A) Pathological diagnosis of clear-cell type RCC in left kidney. (B) CT examination before operation revealed RCC of left kidney. (C) The left renal tumor was found to shrink after 3 months' follow-up. (D) A RCC was shown in left kidney by renal artery angiography. (E) The tumor-feeding artery was superselectively incubated via microcatheter. (F) The blood supply artery was embolized by the drug-loaded microspheres.
Data collection
We retrospectively collected baseline data such as demographic data, clinical data, illness history, complications, tumor size, tumor markers, white blood cell count, computed tomography (CT) imaging ( Fig. 1B,C; Fig. 4A–C) or MRI (Fig. 2), and so on (Fig. 4D).
A 37-year female with bilateral RCCs followed up by MRI. (A) T2W-TSE series in coronal view showed bilateral RCCs before treatment. (B) Tumors shrunk significantly 1 month after DEB-TACE. (C) The right RCC almost disappeared after 3 months. T2-SPIR series in cross-sectional view showed the change of bilateral RCCs before (D), 1 month (E) and 3 months later (F).
DEB-TACE procedures
All procedure was performed under fluoroscopic monitoring and local anesthesia via injection of 5 ml of lidocaine (Figs. 1D–F; 3; Fig. 4E,F). Right femoral artery was accessed and a 5F-pigtail catheter (Terumo, Japan) was introduced to the level of kidney, then abdominal aortic angiography was performed to show the bilateral kidneys. A Cobra catheter was used to identify the tumor-feeding arteries of RCC. A microcatheter (Asahi, Japan) was advanced selectively into feeding arteries. Cisplatin or oxaliplatin was infused if patient received no platinum-based chemotherapy previously. doxorubicin (20–60 mg) was loaded with 100–300 μm or 300–500 μm of CB (Jiangsu Hengrui Medicine Co. Ltd., Nanjing, China) for about 30 min, with shaking every 5 min. Then CB was slowly injected into tumor-feeding arteries after mixture with iodixanol. Polyvinyl alcohol of 350–560 μm (Merit, American) was used if embolization was insufficient by CB.
DEB-TACE for the female patients with bilateral RCCs. (A) A moderated RCC was shown in left kidney by renal artery angiography. (B) The tumor-feeding artery was superselectively incubated via microcatheter. (C) The blood supply artery was embolized by the drug-loaded microspheres. (D) A small RCC was shown in right kidney by renal artery angiography. (E) The tumor-feeding artery was superselectively incubated via microcatheter. (F) The blood supply artery was embolized by the drug-loaded microspheres.
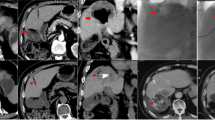
A 72-year male with right RCC treated by CB. (A) CT preoperative examination revealed a large tumor of the right kidney with tumor invasion into inferior vena cava. (B) The right renal tumor maintained stable after one month's follow-up. (C) The tumor shrunk at 3 months after DEB-TACE. (D) The right kidney showed a lower GFR (43.7 ml/min) than the left kidney (60.1 ml/min). (E) The right renal artery was the blood supply artery of the tumor. (F) The right renal artery was incubated and embolized.
Endpoint
ORR, the sum of CR and PR, the primary endpoint, was assessed by abdominal CT. The disease control rate (DCR), the sum of CR, PR and SD, is also the primary endpoint. The secondary endpoints were progression-free survival (PFS) and overall survival (OS).
Safety assessment
According to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0)11, adverse events and serious adverse events were recorded.
Follow-up
Some patients were hospitalized to received second session of DEB-TACE, the remained patients were followed up by outpatient visit. Patients underwent abdominal CT every 1 to 2 months after procedure. All patients received telephone follow up with the last date on 26 July 2020.
Results
Patient characteristics
This study enrolled 19 men and 16 women (mean age 67.5 ± 10.8 years, range 37–84 years). Patient characteristics on admission are listed in Table 1. Twenty-seven patients were diagnosed with clear-cell type RCC and four patients were Bellini duct carcinoma. Local or distant metastases were present in 13 and 6 patients, respectively. Three patients showed recurrence after surgery, and 5 patients received radiotherapy or chemotherapy before DEB-TACE.
DEB-TACE treatments
Fifteen-seven times of DEB-TACE were performed in 35 patients using doxorubicin-loaded DEB-TACE, with a median dose of 60 mg (IQR 40, 60). CB of 100–300 μm was used in 17 patients, and CB of 300–500 μm was used in the remained 18 patients. Polyvinyl alcohol of 350–560 μm was used in 25 patients after DEB-TACE. Twenty-one patients also received transarterial infusion with cisplatin (n = 7) or oxaliplatin (n = 14). Fifteen patients underwent an additional session of DEB-TACE, with an interval of 1 to 1.5 months. One patient underwent bronchial transarterial chemoembolization, and one received placement of esophageal stent due to severe esophageal stenosis. Two patients underwent 125I seeds implantation and 4 patients underwent thermal ablation for RCC after DEB-TACE. The median inpatient duration was 14.0 days (IQR 9.0, 17.5) and the mean cost of hospitalizations was (5.7 ± 2.3) × 104¥ (Table 2).
Endpoint
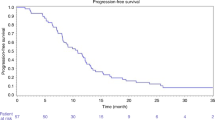
The median follow-up time was 9.0 months (Range 1.8–43.6 months). ORR and DCR were 47.1% and 94.1%, 29.0% and 87.1%, 23.1% and 84.6% respectively at 1, 3, and 6 months after DEB-TACE (Table 3). The median PFS was 21.4 months, and the 3-, 6- and 12 month PFS rates were 84.7%, 73.7% and 62.3%, respectively. The median OS was 24.6 months, and the 3-, 6 and 12 month OS rates were 93.9%, 87.6% and 65.2%, respectively (Fig. 5).
Safety
No serious adverse event was observed, including perioperative deaths or treatment-related adverse events of grade 3 or more. Abdominal pain and abdominal distension were found in 12 and 4 patients, respectively. Five patients (14.3%) showed nausea or vomiting and were controlled within 2–3 days. One patient showed hematuria of grade 1 after DEB-TACE and was successfully treated by hemostatics. Three patients showed moderate fever for 2–3 days and physical cooling was used (Table 4).
Discussion
DEB-TACE is as a new embolization option for unresectable RCC, which can embolize the tumor-feeding arteries and block blood supply of tumor tissue4,5. DEB-TACE can also slowly release and increase local concentration of antitumor drug, and thus increasing retention time and efficacy of tumor necrosis12,13,14. Currently, DEB-TACE has been widely used in the treatment of unresectable carcinoma of substantial organs (e.g. liver6,7, uterus8 or lung9) rather than cavity organs such as bladder and digestive tract3,15. Our results indicated that DEB-TACE using doxorubicin-loaded CB is feasible, safe and showed good short-term efficacy without serious adverse events. doxorubicin-loaded DEB-TACE appears to be a well-tolerated treatment option for unresectable RCC.
Doxorubicin-loaded DEB-TACE had been used for unresectable hepatocellular carcinoma, and showed significantly elevated ORR or DCR5,6. TACE using superabsorbent polymer microspheres is able to decrease tumor size of refractory lung cancer16,17,18. However, very few studies have reported the safety and efficacy of doxorubicin-loaded DEB-TACE in patients with RCC. In our study, the ORR and DCR were 47.1% and 94.1%, 29.0% and 87.1%, 23.1% and 84.6% respectively at 1, 3, and 6 months after doxorubicin-loaded DEB-TACE. Our data indicated that doxorubicin-loaded DEB-TACE showed a good disease control rate during a short-term follow up.
When compared with the conventional TACE, DEB-TACE using CB showed survival benefit for the treatment of hepatocellular carcinoma19. However, some investigator reported no survival benefit20. In our study, the median PFS and OS were 21.4 and 24.6 months after DEB-TACE, respectively. The 3-, 6- and 12- month PFS rates were 84.7%, 73.7% and 62.3%, and the 3-, 6 and 12- month OS rates were 93.9%, 87.6% and 65.2%, respectively.
Patients with more previous treatments or larger tumor size may show a poorer therapeutic response to DEB-TACE21, and combined therapeutic options should be used to improve prognosis, such as thermal ablation, 125I seeds implantation and targeted therapy, and so on21,22,23. In this study, 4 patients underwent thermal ablation and 2 patients received 125I seeds implantation. Additionally, other complications, such as thrombosis in inferior venae cava and esophageal stenosis, should be managed. In this study, one patient received esophageal stent insertion and 2 patients underwent inferior venae cava filter placement.
In line with previous studies, we found that DEB-TACE showed no serious adverse events. Only one patient showed hematuria and was successfully treated by hemostatics. Three patients showed moderate fever for 2–3 days and physical cooling was used. doxorubicin loaded DEB-TACE appears to be a safe treatment for unresectable RCC.
There are some shortcomings in our study. This is a retrospective observational study conducted in a single center, with a relatively small sample size. Cox regression analysis should be used to look for prognostic factors of patients with RCC, however, the sample of this preliminary study was too small to perform cox regression analysis. Fifteen patients rather than all patients received one more session, which may underestimate the efficacy of DEB-TACE. More studies with large sample size are needed to further study its safety, efficacy and prognostic factors.
In conclusion, DEB-TACE with doxorubicin-loaded CB is a safe, feasible and effective palliative treatment option for patients with unresectable RCC.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computerized tomography
- IQR:
-
Mean ± interquartile range
- DEB-TACE:
-
Drug-eluting beads transarterial chemoembolization
- RCC:
-
Renal cell carcinoma
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- CB:
-
CalliSpheres beads
References
Kwan, K. C. Oral bioavailability and first-pass effects. Drug Metab. Dispos. 25(12), 1329–1336 (1997).
de Baere, T. et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc. Intervent. Radiol. 39(3), 334–343 (2016).
Bi, Y. et al. Transarterial chemoembolization with doxorubicin-loaded beads for inoperable or recurrent colorectal cancer. Abdom. Radiol. (NY) 46(6), 2833–2838 (2021).
Melchiorre, F. et al. DEB-TACE: a standard review. Fut. Oncol. 14(28), 2969–2984 (2018).
Wu, B., Zhou, J., Ling, G., Zhu, D. & Long, Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J. Surg. Oncol. 16(1), 69 (2018).
Xiang, H. et al. CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma. Technol. Cancer Res. Treat. 18, 1533033819830751 (2019).
Zhou, G. H. et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres beads in Chinese hepatocellular carcinoma patients. BMC Cancer 18(1), 644 (2018).
Bi, Y. et al. Clinical outcomes of uterine arterial chemoembolization with drug-eluting beads or advanced-stage or recurrent cervical cancer. Abdom. Radiol. (NY) 46(12), 5715–5722 (2021).
Bi, Y., Shi, X., Yi, M., Han, X. & Ren, J. Pirarubicin-loaded CalliSpheres drug-eluting beads for the treatment of patients with stage III-IV lung cancer. Acta Radiol. 63, 311–318 (2021).
Ren, B., Wang, W., Shen, J., Ni, C. & Zhu, X. In vivo evaluation of callispheres microspheres in the porcine renal artery embolization model. Am. J. Transl. Res. 11, 4166–4179 (2019).
NCI. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Choi, J. W. et al. Comparison of drug release and pharmacokinetics after transarterial chemoembolization using diverse lipiodol emulsions and drug-eluting beads. PLoS ONE 9(12), e115898 (2014).
Mikhail, A. S. et al. Mapping drug dose distribution on CT images following transarterial chemoembolization with radiopaque drug-eluting beads in a rabbit tumor model. Radiology 289(2), 396–404 (2018).
Kunisada, K., Ishikawa, H., Takafuji, J. & Komuta, K. A case of a 91-year-old patient with advanced squamous cell lung cancer complicated with renal dysfunction successfully treated with trans-arterial chemo-embolization. Gan To Kagaku Ryoho 40(7), 917–919 (2013).
Bi, Y. et al. Clinical outcomes of doxorubicin-eluting CalliSpheres beads-transarterial chemoembolization for unresectable or recurrent esophageal carcinoma. BMC Gastroenterol. 21(1), 231 (2021).
Kennoki, N., Hori, S., Yuki, T., Sueyoshi, S. & Hori, A. Transcatheter arterial chemoembolization with super absorbent polymer microspheres for a large lung cystic adenocarcinoma in the left pulmonary cavity. Gan To Kagaku Ryoho 42(11), 1407–1410 (2015).
Kennoki, N., Hori, S., Yuki, T., Sueyoshi, S. & Hori, A. Trans-arterial chemoembolization therapy for refractory advanced non-small cell lung cancer with spherical embolic material–a single case report. Gan To Kagaku Ryoho 42(12), 1827–1829 (2015).
Seki, A. et al. Transcatheter arterial embolization with spherical embolic agent for pulmonary metastases from renal cell carcinoma. Cardiovasc. Intervent. Radiol. 36(6), 1527–1535 (2013).
Liu, Y. S. et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 18(1), 124 (2018).
Cheung, A. H. et al. Nine-year experience of doxorubicin-eluting beads chemoembolization for hepatocellular carcinoma. Hepatobil. Pancreat. Dis. Int. 15(5), 493–498 (2016).
Bie, Z. et al. The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer. Thorac. Cancer 10(9), 1770–1778 (2019).
Chen, Y. et al. Bronchial artery chemoembolization combined with radioactive iodine-125 seed implantation in the treatment of advanced nonsmall cell lung cancer. J. Cancer Res. Ther. 13(4), 636–641 (2017).
Yang, X. G. et al. Efficacy for artery chemoembolization combined with radiofrequency ablation in the treatment of advanced non-small cell lung cancer. Zhonghua Yi Xue Za Zhi 96(7), 539–543 (2016).
Author information
Authors and Affiliations
Contributions
X.W. Han and J.Z. Ren: guarantor of integrity of the entire study. X.W. Han: study concepts. X.W. Han and J.Z. Ren: study design. X.N. Shi and M. F. Yi: literature research. Y.H. Bi and X.N. Shi: clinical studies. X.N. Shi and M. F. Yi: data acquisition. Y.H. Bi and X.N. Shi: data analysis and statistical analysis. Y.H. Bi and X.N. Shi: manuscript preparation. X.W. Han and J.Z. Ren: manuscript editing and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, Y., Shi, X., Ren, J. et al. Transarterial chemoembolization of unresectable renal cell carcinoma with doxorubicin-loaded CalliSpheres drug-eluting beads. Sci Rep 12, 8136 (2022). https://doi.org/10.1038/s41598-022-12334-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12334-x
This article is cited by
-
Efficacy and safety analysis of TACE + sunitinib vs. sunitinib in the treatment of unresectable advanced renal cell carcinoma: a retrospective study
BMC Cancer (2023)
-
Vision transformer and explainable transfer learning models for auto detection of kidney cyst, stone and tumor from CT-radiography
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.