Abstract
Background
Chemo-embolisation with drug-eluting beads loaded with irinotecan (DEBIRI) increased survival as compared with intravenous irinotecan in chemorefractory patients with liver-dominant metastases from colorectal cancer (LMCRC). First-line DEBIRI with systemic chemotherapy may increase survival and secondary resection.
Methods
In the FFCD-1201 single-arm Phase 2 study, patients with untreated, non-resectable LMCRC received DEBIRI plus mFOLFOX6. Four courses of DEBIRI were performed alternating right and left lobe or two sessions with both lobes treated during the same session.
Results
Fifty-seven patients were enrolled. Grade 3–5 toxicities were more frequent when both lobes were treated during the same session (90.5% versus 52.8%). Nine-month PFS rate was 53.6% (95% CI, 41.8–65.1%). The objective response rate (RECIST 1.1) was 73.2%, and the secondary R0 surgery was 33%. With a median follow-up of 38.3 months, median OS was 37.4 months (95% CI, 25.7–45.8), and median PFS 10.8 months (95% CI, 8.2–12.3).
Conclusions
Front-line DEBIRI + mFOLFOX6 should not be recommended as the hypothesised 9-month PFS was not met. However, high response rate, deep responses, and prolonged OS encourage further evaluation in strategies integrating biologic agent, in particular in patients with secondary surgery as the main goal.
Clinical trial registration
NCT01839877.
Similar content being viewed by others
Background
Over 80% of patients with liver metastases from colorectal cancer (LMCRC) present with unresectable disease. The intensification of first-line chemotherapy in unresectable patients allows a significant rate of secondary resection and led to an increased survival.1,2,3 Intrahepatic arterial delivery of chemotherapy has been proposed in patients with liver-only disease to treat tumour cells with high local concentrations of anticancer agents and decreased systemic toxicity.
Transarterial chemo-embolisation (TACE) is a standard treatment for hepatocellular carcinoma and has for many years been used to treat LMCRC, albeit with a lack of prospective and comparative studies. Most reports are of small retrospective studies with various chemotherapy regimens and embolisation procedures in heterogeneous patient populations.4 Intra-arterial hepatic administration of drug-eluting beads (DEB) loaded with chemotherapy drugs have been developed to standardise the embolisation procedure compared to conventional TACE with use of calibrated non-resorbable beads, which can load and continuously release cytotoxic drugs into the target tissues.
In a Phase 3 trial on 74 patients,5 DEB loaded with irinotecan (DEBIRI) plus intravenous 5-fluoro-uracil (5FU) was compared to intravenous 5FU plus irinotecan (Folfiri) in patients with unresectable LMCRC after failure of at least two lines of treatment. Overall survival (OS), progression-free survival (PFS), and response rate significantly improved in the DEBIRI arm. However, heavily pretreated patients with liver-limited disease (LLD) are not frequent and the rate of secondary resectability is very low in this subgroup of patients. Use of DEBIRI before surgery also increases the histological response, as suggested by the PARAGON II study,5 and thus may potentially reduce the risk of recurrence.6
We hypothesise that upfront use of DEBIRI combined with systemic chemotherapy in liver-dominant metastatic colorectal cancer (mCRC) patients could improve treatment efficacy, limit systemic toxicity, and thus improve survival and secondary resectability. Our prospective, multi-centre, single-arm, Phase 2 study evaluated the feasibility, safety, and efficacy of a conventional systemic chemotherapy regimen 5FU plus oxaliplatin (FOLFOX) combined with intra-arterial hepatic DEBIRI as first-line treatment of mCRC patients with liver-dominant mCRC.
Methods
Study design
This multi-centre, single-arm, Phase 2 study was approved by the French ethics committee “CCP Ile de France 8” (No. 1212113). All patients provided informed consent before study enrolment. The main eligibility criterion was previously untreated mCRC with unresectable liver metastases, as defined at a local multidisciplinary team meeting. Main exclusion criteria included liver involvement >60% or impaired hepatic function and extrahepatic metastases on computed tomographic (CT) scan, except lung nodules if <4 and <1 cm each. Inclusion/exclusion criteria are detailed in Supplementary Information 1. Concomitant administration of any targeted therapies was not permitted, considering that toxicity of the combination of DEBIRI plus anti-vascular endothelial growth factor (anti-VEGF) or anti-epidermal growth factor receptor (anti-EGFR) is unknown (no Phase 1 study available), and the biliary or vascular cumulative toxicity that have been reported in other trials with hepatic arterial chemotherapy.7
Procedures
Patients received induction chemotherapy with FOLFOX: oxaliplatin 85 mg/m2 as a 2-h infusion at day 1, leucovorin 400 mg/m2 as a 120-min infusion at day 1 followed by 5FU 400 mg/m2 bolus at day 1 and 2400 mg/m2 46-h continuous 5FU infusion, 1 cycle every 2 weeks. Patients received treatment with DC Bead LUMI™ 100–300 (Biocompatibles UK limited) loaded with irinotecan 50 mg/ml, 1 vial per lobe and per treatment (meaning 1 vial in case of unilobar administration, and 2 vials in case of bilobar administration). Each treatment session was performed 48–72 h after a chemotherapy cycle. Treatment administration was performed using a unilateral femoral approach. Depending of patient case and according to the choice of the investigators, the treatment could be administered in a bilobar approach or a sequential unilobar approach: in patients treated with a bilobar approach, both lobes were treated at each session after the second and fourth chemotherapy cycles; in patients treated with a sequential unilobar approach, only one lobe was treated per session, each lobe being treated alternately, after the second, third, fourth, and fifth cycles of chemotherapy. After a preplanned safety analysis performed after 27 patients were treated, the safety board recommendation was to treat patients with the sequential unilobar approach because of better tolerability. The procedure and periprocedural medication are described in Supplementary Information 2 and 3.
Prophylaxis with granulocyte-colony stimulating factor (G-CSF) could be used as primary prophylaxis, at the investigator’s discretion. Patients continued treatment until unacceptable toxicity, disease progression, consent withdrawal, or investigator choice.
Endpoints/statistical analysis
The primary endpoint was the rate of PFS at 9 months, according to the local investigator evaluation. A one-step Fleming plan was used, with an α risk of 5% and unilateral power (1 − β) of 90%, testing the following assumptions: H0: 55% of patients alive without progression at 9 months is uninteresting; H1: 75% is expected. Taking into account a rate of 20% of patients lost to follow-up (without evaluation during the first 9 months of treatment), 58 patients had to be included.
Secondary endpoints included safety (according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.0), objective response rate (ORR) according to response evaluation criteria in solid tumours (RECIST) 1.1 criteria, PFS, OS, secondary resectability rate, depth of response and early tumour shrinkage at 8 weeks; definitions are provided in Supplementary Information 4.
All adverse events were graded according to the NCI-CTCAE version 4.0. All patients with LMCRC who received at least one dose of treatment were included in the analysis of safety population. An interim safety analysis was planned after treatment of 27 patients, and an independent safety committee board was implemented.
The primary endpoint was analysed in a modified intention-to-treat (mITT) population defined as all patients who fulfilled eligibility criteria and has received at least one session of chemo-embolisation, at least one dose of chemotherapy, and who had at least one radiological evaluation during the 9 months of follow-up. Secondary endpoint was analysed in the ITT population.
Exploratory analyses to determine prognostic factors of PFS and OS were performed using univariate and multivariate Cox proportional hazards models. Hazard ratios and 95% confidence interval (CI) were estimated. Variables with p < 0.20 in univariate analyses were used in multivariate analyses.
All data were reported using the usual descriptive statistics: qualitative variables are described with percentages and quantitative variables with mean, standard deviation, median, interquartile interval (Q1–Q3), and range (minimum–maximum). Analyses were done using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
From May 2013 to December 2016, 58 patients were included and treated in 10 participating centres in France. Diagnosis of LMCRC was reconsidered and requalified as LM from pancreatic adenocarcinoma after inclusion in one patient, who was excluded from the final analysis. One patient was not analysed in the mITT population because he had no evaluation after the treatment during the study period (9 months). Patient characteristics are described in Table 1. Briefly, all patients had synchronous metastatic disease with primary tumour removed in 10 patients, including one with preoperative radio-chemotherapy for rectal cancer. Patients had bilobar metastatic disease in 88% of cases and a mean 9 LM (range 1–20). Lung nodules were described in 12% of patients on CT scan in respect of inclusion criteria.
Treatment compliance
All patients received at least one session of DEBIRI. The full DEBIRI treatment plan (2 or 4 sessions) was performed in 49% of patients. Bilobar administration was performed in 36.8% of patients, and a sequential unilobar administration was performed in 63.2%. Six patients planned for 2 bilobar sessions received only one bilobar administration instead of 2 due to grade 3–4 toxicity after the first session. The median number of FOLFOX cycles was 8 (range 2–28). The median dose intensities (all cycles of FOLFOX) were 92.4% for oxaliplatin, 80% for 5FU bolus, and 97.8% for continuous 5FU infusion. Twenty-eight patients (49%) received G-CSF.
Efficacy
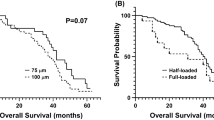
The 6- and 9-month PFS rates according to investigators were 82.4% [95% CI 69.8–90.1] and 53.6% [95% CI 41.8–65.1], respectively, in mITT population. After a median follow-up of 38.3 months [95% CI 32.2–41.9], 4 patients were alive without progression in ITT population. Median PFS was 10.8 months [95% CI 8.2–12.3] (Fig. 1) and median OS was 37.4 months [95% CI, 25.7–45.8] (Fig. 2). Median-specific liver PFS was 10.9 months [95% CI, 8.2–12.4]. Post-progression treatments were available in 49 patients. After progression, patients received a median of two treatments (Supplementary Table S1).
A blind central review assessment for the primary objective was made by a single independent radiologist. Results were concordant with investigator assessment, with a median PFS of 10.4 months [95% CI 7.2–13.6].
According to investigator evaluation, an ORR was observed in 41 patients (73.2%) including 4 complete responses. Disease control rate (DCR) was 92.9%. The median depth of response was −43.6% (Q1: −61.7; Q3: −31) (Fig. 3). The rate of early tumour shrinkage was 52.1% [95% CI 37.2–66.7].
Secondary resection or ablative treatment
Nineteen patients (33%) underwent R0 resection of LM; for this subgroup, median PFS was 13 months [95% CI 8.8; 16.6] and median OS was not reached. OS rate at 2 and 3 years were 94.74% [95% CI 68.12; 99.24] and 75.49% [95% CI 45.80; 90.37], respectively. Of the 17 patients with recurrence after curative intent surgery, 11 were eligible for a second curative intent surgery/ablation. Altogether, 8 of these 19 patients were alive with no evidence of disease after a median follow-up of 3.7 years (range 2.5–4.7).
A post hoc independent evaluation of resectability by three experienced hepatic surgeons was performed based on a CT scan at baseline. According to this centralised review, 53/56 patients (94.6%) were retrospectively confirmed as non-resectable by at least 2 surgeons (Fig. 4). Two patients were considered resectable at baseline (3.5%) by the 3 surgeons and 1 by 2 of the 3 surgeons.
Prognostic factors
The effect on survival of clinical and laboratory findings and unilobar versus bilobar administration was assessed. BRAF mutation was the only factor associated with worse PFS and OS in both univariate and multivariate analysis. Primary tumour location and administration modality were not prognostic (Supplementary Tables S2 and S3).
Safety
One toxic death possibly related to DEBIRI was reported (peritonitis). The main grade 3–4 toxicities were neutropenia (24.6%), diarrhoea (12.3%), abdominal pain (14%), and pancreatitis/cholecystitis (8.8%/5.3). Importantly, almost all G3/4/5 toxicities were more frequent with the bilobar approach than the unilobar approach (87.5% versus 47.2%; Table 2). Adverse events are detailed in Supplementary Table S4. Two patients stopped chemotherapy due to major toxicity after 2 and 6 cycles of FOLFOX, respectively. A delay in administration of chemotherapy due to toxicity was reported in 16 patients.
Periprocedural adverse events (occurring during the first 24 h after DEBIRI) of any grade was observed in 75.4% of patients. Post-embolisation syndrome was the most frequent adverse event, with significant abdominal pain (visual analogue scale >3) occurring in 75.4% of patients, nausea/vomiting in 22.8% of patients, and fever in 5.3% of patients. Cardiovascular side effects were not rare, with acute hypertension in 19.3% of patients, thoracic pain in 3 patients, among which 1 was identified as coronary spasm with transitory elevation of troponin, and 2 tachycardia (Supplementary Table S5, online only).
Discussion
We demonstrated that combination of DEBIRI plus FOLFOX is feasible in LLD mCRC, leading to a high ORR of 73.2% and allowing secondary resection in one-third of patients. Moreover, prolonged OS was observed, with a median of 37.4 months. However, our study did not meet the prespecified primary endpoint, as the 9-month PFS rate was under 75% expected (53.6%). A posteriori, 75% appears challenging considering recent trials with comparable populations in LMCRC.8 Nevertheless, a long OS may have been favoured by the depth of response and early tumour shrinkage,9 and the subsequent treatment lines considering the spare of targeted agent, and limited irinotecan systemic release10 with Folfox + DEBIRI. Finally, while the toxicity profile was manageable, DEBIRI + FOLFOX may lead to serious and specific side effects when two lobes are treated during the same session, but the safety profile was better when DEBIRI was administered in the hepatic lobes one by one.
Doublet chemotherapies with a targeted agent led to an ORR between 33% and 53%, median PFS from 6.8 to 9.2 months, and median OS from 15.1 to 25.8 months.1,2,3,11 ORR reached 55–62% in recent trials in RAS wild-type patients only.12,13 Compared to doublet chemotherapies, FOLFOXIRI +/− bevacizumab significantly increased ORR and median PFS in patients with non-resectable LMCRC,1,2,3,14 ranging from 43% to 80% and 9.8 to 12.3 months, respectively. The ORR and PFS reported in our study therefore seem in line with those observed in patients treated with intensive systemic regimens such as FOLFOXIRI +/− bevacizumab. Median OS was 37.4 months compared to 25–29 months with the use of triplet +/− bevacizumab.1,2,3,14 However, we have to point that the trials studying triplet included unselected non-resectable mCRC patients, while our study included only liver-dominant patients. Nevertheless, our survival results seem promising in mCRC patients with liver-dominant disease, even though the primary 9-month PFS endpoint was not reached.
Reported secondary resection rates are around 15% with doublet CT + anti-VEGF or anti-EGFR15,16 and 23–60% in patients with exclusive LMCRC.11,14,15,16,17 However, in previous studies of LMCRC, secondary resectability may have been overestimated owing to a significant proportion of patients may actually have been resectable upfront. Indeed, in most of these studies, non-resectability at baseline was defined by the number of metastases >4 and/or size >5 cm and/or by the presence of bilobar metastases or no extrahepatic metastases. However, it is now accepted that these criteria no longer apply.18,19,20,21 The modern definition of resectability includes the potential for complete resection with tumour-free margins; preservation of viable vascular inflow, outflow, and biliary drainage; and a future minimal remnant liver volume of 30%.6,22 Interestingly, in the CELIM study and the FIRE-3 study, retrospective assessment of baseline resectability found that 30% and 22% of patients were technically resectable initially.15,23 Our non-resectability assessment before inclusion did not use these size/number criteria and was made by the local multidisciplinary team meeting and 95% of the patients were retrospectively confirmed as non-resectable at baseline by an independent committee of three experienced liver surgeons. Therefore, the resectability rate of 33% is solid and compared favourably with the other studies.
Other intensified hepatic intra-arterial strategies have been or are currently being investigated. Radio-embolisation (selective internal radiation therapy (SIRT)) combined with FOLFOX failed to increase ORR, OS, PFS, or the resection rate, which remained <15% in the FOXFIRE GLOBAL large Phase 3 trial that included patient with liver-dominant disease.8 In this study, inclusion criteria and design were very close from our study, and this trial provides recent data in a very similar population and with an alternative transarterial approach and the same systemic regimen (despite that biologic agent could be added after the induction treatment in FOXFIRE GLOBAL trial according to the investigator choice). In this trial, FOLFOX + SIRT led to a median PFS of 11 months and a response rate of 72.4%, similar to those observed with FOLFOX + DEBIRI. The resection rate remained <15% and median OS was only 22.6 months, contrasting with the 33% and 38.3 months reported in our study, which may be explained in part by the depth of response and the early tumour shrinkage observed with DEBIRI, rather than by the liver-specific PFS, which was lower with DEBIRI than with SIRT.
Hepatic arterial infusion of floxuridine chemotherapy showed a response rate of 92% and conversion to resection in 47% of patients24 in a Phase 1 trial, but with some FUDR-related complications and biliary toxicity. Hepatic arterial infusion of oxaliplatin combined with systemic chemotherapy and targeted therapy is currently being tested in the PRODIGE 49 Phase 3 trial (NCT02885753). Compared to the latter, DEBIRI has the advantage of being an easy, accessible, and reproducible procedure, as TACE is used worldwide. When first-line DEBIRI in combination with FOLFOX +/− bevacizumab was assessed and compared with systemic chemotherapy alone, ORR increased with DEBIRI, as did resection rate (35% versus 6% [p = 0.05]).25 However, response was evaluated using the modified RECIST criteria, which are not adequate for DEBIRI in mCRC.26 When considering RECIST 1.1 criteria, this study found a response rate of 97% with DEBIRI + FOLFOX, but, more surprisingly, also 95% in patients treated with FOLFOX alone, a value far from those generally reported with this regimen.
We observed a high rate of grade 3–5 adverse events with one toxic death possibly due to DEBIRI. Most frequent toxicities were gastrointestinal and the consequence of extrahepatic perfusion. Interestingly, these specific toxicities, as well as non-specific toxicities, were notably more frequent after DEBIRI was used in a bilobar approach, whereas efficacy parameters were not affected by the bilobar or unilobar approach. This observation during a planned interim safety analysis led to the recommendation to treat patients preferentially with a unilobar approach after inclusion of 27 patients. The reported rate of post-embolisation syndrome in the literature is quite difficult to analyse due to the large heterogeneity in monitoring and reporting of this side effect, ranging from 6% to 100%.27 The post-embolisation syndrome rate still seemed high in our trial compared to previous studies. The timing of the procedure with respect to chemotherapy may be involved, since cumulative toxicities of chemotherapy followed by chemo-embolisation could have occurred. The use in front line could also be a reason to explain the higher rate of embolisation syndrome, compared to previous reports in late-line treatment in LMCRC. Indeed, it has been suggested that a majority of patients have a tumour load in late line remaining below their initial tumour load,28 and highest tumour load have been associated with more frequent post-embolisation syndromes.29
The addition of DEBIRI appears to have had limited impact on the administration of chemotherapy. Indeed, only two patients stopped chemotherapy due to unacceptable toxicity during the induction period. Nevertheless, the median number of cycles of FOLFOX was only eight, suggesting that a large part of patients had a stop-and-go strategy with early maintenance with 5FU or even a break from chemotherapy. Such strategy may have been favoured by the increased toxicity during the induction period.
The strength of our study is that it was conducted in patients carefully selected with non-resectable LMCRC, as confirmed by our panel expert, and was multicentric. Nevertheless, these results need confirmation in a randomised study compared with standard protocols, and in particular with other intensified regimens as triplet chemotherapy. As specified above, we decided to avoid targeted therapy, and FOLFOX + DEBIRI deserves to be tested in combination with targeted therapy. Another weakness is that we did not plan oxaliplatin continuation after the induction period, and some patients had a stop-and-go strategy with early maintenance with 5FU or even a break from chemotherapy, when others kept oxaliplatin until limiting toxicity. This heterogeneity may have impaired the evaluation of PFS.
In conclusion, although the primary endpoint was not met, front-line FOLFOX + DEBIRI without any targeted agent is feasible. Indeed, despite relevant toxicity, the unilobar approach showed a manageable tolerability profile and allows an excellent DCR in non-resectable LMCRC with deep responses, leading to resection in one-third of patients and prolonged survival. Induction treatment with FOLFOX + DEBIRI cannot be considered standard in unresectable patients as the present trial did not meet the prespecified primary endpoint. However, considering a promising response rate and OS, its optimal place in the therapeutic strategy must now be defined by further studies including biologic agents and a more selected patient population, possibly with secondary surgery as main goal, and should be used in a unilobar approach only.
References
Falcone, A., Ricci, S., Brunetti, I., Pfanner, E., Allegrini, G., Barbara, C. et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 25, 1670–1676 (2007).
Souglakos, J., Androulakis, N., Syrigos, K., Polyzos, A., Ziras, N., Athanasiadis, A. et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 94, 798–805 (2006).
Cremolini, C., Loupakis, F., Antoniotti, C., Lupi, C., Sensi, E., Lonardi, S. et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16, 1306–1315 (2015).
Riemsma, R. P., Bala, M. M., Wolff, R. & Kleijnen, J. Transarterial (chemo)embolisation versus no intervention or placebo intervention for liver metastases. Cochrane Database Syst. Rev. 4, CD009498 (2013).
Jones, R. P., Malik, H. Z., Fenwick, S. W., Terlizzo, M., O’Grady, E., Stremitzer, S. et al. PARAGON II - a single arm multicentre phase II study of neoadjuvant therapy using irinotecan bead in patients with resectable liver metastases from colorectal cancer. Eur. J. Surg. Oncol. 42, 1866–1872 (2016).
Adam, R., Gramont, A. D., Figueras, J., Guthrie, A., Kokudo, N., Kunstlinger, F. et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 17, 1225–1239 (2012).
Cercek, A., D’Angelica, M., Power, D., Capanu, M., Gewirtz, A., Patel, D. et al. Floxuridine hepatic arterial infusion associated biliary toxicity is increased by concurrent administration of systemic bevacizumab. Ann. Surg. Oncol. 21, 479–486 (2014).
Wasan, H. S., Gibbs, P., Sharma, N. K., Taieb, J., Heinemann, V., Ricke, J. et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 18, 1159–1171 (2017).
Aprile, G., Fontanella, C., Bonotto, M., Rihawi, K., Lutrino, S. E., Ferrari, L. et al. Timing and extent of response in colorectal cancer: critical review of current data and implication for future trials. Oncotarget 6, 28716–28730 (2015).
Martin, R. C. G., Scoggins, C. R., Tomalty, D., Schreeder, M., Metzger, T., Tatum, C. et al. Irinotecan drug-eluting beads in the treatment of chemo-naive unresectable colorectal liver metastasis with concomitant systemic fluorouracil and oxaliplatin: results of pharmacokinetics and phase I trial. J. Gastrointest. Surg. 16, 1531–1538 (2012).
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004). 2004.
Heinemann, V., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S.-E. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1065–1075 (2014).
Venook, A. P., Niedzwiecki, D., Lenz, H.-J., Innocenti, F., Fruth, B., Meyerhardt, J. A. et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317, 2392–2401 (2017).
Gruenberger, T., Bridgewater, J., Chau, I., García Alfonso, P., Rivoire, M., Mudan, S. et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann. Oncol. 26, 702–708 (2015).
Modest, D. P., Denecke, T., Pratschke, J., Ricard, I., Lang, H., Bemelmans, M. et al. Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer—central evaluation of FIRE-3. Eur. J. Cancer 88, 77–86 (2018).
Bokemeyer, C., Van Cutsem, E., Rougier, P., Ciardiello, F., Heeger, S., Schlichting, M. et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 48, 1466–1475 (2012).
Wong, R., Cunningham, D., Barbachano, Y., Saffery, C., Valle, J., Hickish, T. et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann. Oncol. 22, 2042–2048 (2011).
Chow, F. C.-L. & Chok, K. S.-H. Colorectal liver metastases: an update on multidisciplinary approach. World J. Hepatol. 11, 150–172 (2019).
Omichi, K., Shindoh, J., Cloyd, J. M., Mizuno, T., Chun, Y. S., Conrad, C. et al. Liver resection is justified for patients with bilateral multiple colorectal liver metastases: a propensity-score-matched analysis. Eur. J. Surg. Oncol. 44, 122–129 (2018).
Altendorf-Hofmann, A. & Scheele, J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg. Oncol. Clin. North Am. 12, 165–192 (2003).
Elias, D., Ouellet, J.-F., Bellon, N., Pignon, J.-P., Pocard, M. & Lasser, P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br. J. Surg. 90, 567–574 (2003).
Clavien, P.-A., Petrowsky, H., DeOliveira, M. L. & Graf, R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 356, 1545–1559 (2007).
Folprecht, G., Gruenberger, T., Bechstein, W. O., Raab, H.-R., Lordick, F., Hartmann, J. T. et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 11, 38–47 (2010).
Kemeny, N. E., Melendez, F. D. H., Capanu, M., Paty, P. B., Fong, Y., Schwartz, L. H. et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J. Clin. Oncol. 27, 3465–3471 (2009).
Martin, R. C. G., Scoggins, C. R., Schreeder, M., Rilling, W. S., Laing, C. J., Tatum, C. M. et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 121, 3649–3658 (2015).
Akinwande, O., Philips, P., Scoggins, C. R., Kelly, L., Tatum, C., Hahl, M. et al. Comparison of tumor response assessment methods in patients with metastatic colorectal cancer after locoregional therapy. J. Surg. Oncol. 113, 443–448 (2016).
Akinwande, O., Dendy, M., Ludwig, J. M. & Kim, H. S. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: a systematic review. Surg. Oncol. 26, 268–275 (2017).
Palmieri, L.-J., Fihri, A., Doat, S., Dubreuil, O., Manceau, G., Karoui, M. et al. Tumor-size responses to first-line is a predictor of overall survival in metastatic colorectal cancer. Eur. Radiol. 29, 3871–3880 (2019).
Martin, R. C. G., Howard, J., Tomalty, D., Robbins, K., Padr, R., Bosnjakovic, P. M. et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc. Intervent. Radiol. 33, 960–966 (2010).
Acknowledgements
This study was presented at the European Society for Medical Oncology (ESMO) Annual Congress in Poster Discussion session, 19–23 October 2018; (Munich, Germany): Pernot, S., Artru, P., Tougeron, D., Montérymard, C., Smith, D., De La Fouchardière, C. et al. 461PD Folfox and intra-arterial DEBIRI as front-line treatment in patients with non resectable colorectal cancer liver metastases (FFCD 1201 Phase 2 trial). Ann. Oncol. 29(suppl_8), viii150–viii204 (2018).
Author information
Authors and Affiliations
Consortia
Contributions
Study concepts: S.P., O.P., J.T. Study design: S.P., O.P., C.M., C.L., J.T. Data acquisition: S.P., O.P., P.A., D.S., J.-L.R., C.D.L.F., L.D., R.G., D.S., J.-L.J., D.T., J.T. Quality control of data and algorithms: C.M. Data analysis and interpretation: S.P., J.T., O.P., D.T. Statistical analysis: C.M., S.P., J.T. Manuscript preparation: S.P., C.M., J.T. Manuscript editing and review: all.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This multi-centre, single-arm, Phase 2 study was approved by the French ethics committee “CPP Ile de France 8” (committee’s reference No. 1212113). All patients provided informed consent before study enrolment. The study was performed in accordance with the declaration of Helsinki.
Consent for publish
This manuscript does not include any person’s individual data.
Data availability
Data can be found based on reasonable demand to the promoter “Fédération Française de Cancérologie digestive (FFCD)”.
Competing interests
S.P. has received honoraria as a speaker and/or advisor from Sanofi, Amgen, and Servier. O.P. has received honoraria as a speaker and/or advisor from Merit Medical and COGITh-SAS. J.T. has received honoraria as a speaker and/or advisor from Merck KGaA, Sanofi, Roche Genentech, MSD, Lilly, Celgene, Servier, Pierre Fabre, and Amgen. J.-L.R. has received honoraria for speaker/advisory role from Astra Zeneca, Bayer, BTG, Ipsen, and Merck. D.T. has received honoraria for speaker or/and advisory role from Merck KGaA, Sanofi, Roche Genentech, MSD, Novartis, BMS, Servier, Bayer, and Amgen; D.S. has received honoraria for speaker or/and advisory role from Amgen, Ipsen, and Servier. C.D.L.F. has received honoraria for speaker/advisory role from Servier, Roche Genentech, MSD, Lilly, Celgene, Pierre Fabre, and Amgen. R.G. has received honoraria for speaker/advisory role from Pierre Fabre, Astra Zeneca, Amgen, Servier, and Novartis. All the remaining authors have declared no conflict of interest.
Funding information
This work was supported, and product provided free of charge, by Biocompatibles UK Ltd.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pernot, S., Pellerin, O., Artru, P. et al. Intra-arterial hepatic beads loaded with irinotecan (DEBIRI) with mFOLFOX6 in unresectable liver metastases from colorectal cancer: a Phase 2 study. Br J Cancer 123, 518–524 (2020). https://doi.org/10.1038/s41416-020-0917-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0917-4
This article is cited by
-
Efficacy of transarterial chemoembolization with drug-eluting beads combined with systemic chemotherapy and targeted therapy in colorectal cancer liver metastasis
World Journal of Surgical Oncology (2023)
-
Preoperative transarterial chemoembolization with drug-eluting beads (DEB-TACE) in patients undergoing conversional hepatectomy: a propensity-score matching analysis
European Radiology (2022)
-
The CIREL Cohort: A Prospective Controlled Registry Studying the Real-Life Use of Irinotecan-Loaded Chemoembolisation in Colorectal Cancer Liver Metastases: Interim Analysis
CardioVascular and Interventional Radiology (2021)