Abstract
Cerebral small vessel disease (CSVD) plays an important role in cognitive impairment, stroke, disability, and death. Hypertension is the main risk factor for CSVD. The use of antihypertensive therapy has not resulted in the expected decrease in CSVD complications, which may be related to the underestimation of significance of daily blood pressure profile for blood–brain barrier (BBB) permeability. 53 patients with CSVD of varying severity (mean age 60.08 ± 6.8 years, 69.8% women, subjects with treated long-standing hypertension vs. normotensive subjects − 84.8% vs. 15.2%) and 17 healthy volunteers underwent ambulatory blood pressure monitoring (ABPM) and MRI, including T1-weighted dynamic contrast-enhanced magnetic resonance imaging for assessing BBB permeability. Most of ABPM parameters in CSVD patients did not differ from controls, but were associated with the severity of white matter hyperintensity (WMH) and the total CSVD score. BBB permeability in normal-appearing white matter (NAWM) and grey matter (GM) was significantly higher in CSVD patients, and the severity of BBB permeability remained similar in patients with different stages of WMH. Among BBB permeability parameters, the area under the curve, corresponding to an increase in the contrast transit time in NAWM, had the greatest number of correlations with deviations of ABPM parameters. BBB permeability in CSVD is a universal mechanism of NAWM and GM damage associated with a slight increase in ABPM parameters. It is obvious that the treatment of hypertension in patients with not severe WMH should be more aggressive and carried out under the control of ABPM.
Similar content being viewed by others
Introduction
Cerebral small vessel disease (CSVD), associated with age and vascular risk factors, is the main cause of vascular cognitive impairment, mixed neurodegenerative and vascular dementia, as well as a significant cause of stroke, disability and mortality in adult population1,2,3.
Hypertension is the main risk factor for age-related CSVD4,5,6. In most cases, the severity of hypertension correlates with MRI markers of CSVD, such as white matter hyperintensity (WMH) and lacunae4,7. The leading mechanism of brain damage in patients with chronic hypertension is hypoperfusion secondary to arteriolosclerosis, which is characterized by the loss of smooth muscle cells, accumulation of fibrotic hyaline deposits, thickening of the blood vessel walls, and luminal narrowing8,9.
Modern antihypertensive therapy, aimed primarily at preventing remodelling of small arteries and, accordingly, increasing blood flow, has led to a decrease in the incidence of stroke10,11, but not the prevalence of cognitive impairment12,13. This can partly be explained by a lack of or a faint effect of antihypertensive therapy on the increased blood–brain barrier (BBB) permeability14, which, along with ischemia, is another significant mechanism of brain damage in hypertension15,16,17. BBB damage in patients with hypertension is considered to be the main mechanism for the initiation and progression of CSVD and the development of forms mixed with neurodegeneration15,16,17. In these cases, hypertension is both a factor in BBB damage18 and a consequence of the damage to the cerebral autonomic centres caused by the high BBB permeability19.
For a long time, BBB damage with high permeability has been considered mainly as a failure of cerebral autoregulation due to high blood pressure in acute and chronic hypertension20,21. Further experiments on the spontaneously hypertensive rats and stroke‐prone spontaneously hypertensive rats have proven that the mechanism of BBB damage in CSVD is universal and can be observed in milder hypertension stages22. One of the possible explanations may be the effect of blood pressure variability on the high BBB permeability. This assumption is consistent with recent studies that have noted the importance of blood pressure variability in the development of CSVD, including in controlled hypertension according to outpatient measurements23,24.
In vivo study of the role of this mechanism in CSVD development has become possible with T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), enabling a quantitative assessment of BBB permeability25,26. Increased BBB permeability was found in normal-appearing white matter (NAWM) as compared to controls27,28; in WMH and adjacent NAWM, correlating with the severity of WMH, hypertension, age and leading to the delayed reduction in cognitive capacity25.
Thus, hypertension and increased BBB permeability are of great importance in the development of CSVD17,25,27,28, but antihypertensive therapy has insufficient effect on reduction of cognitive impairment12,13. Of particular interest is the clarification of the relationship between the features of hypertension, which is well controlled by outpatient measurements, and BBB permeability in patients with CSVD and cognitive impairment. The study aims to evaluate the relation between daily blood pressure profile and BBB permeability in patients with CSVD and cognitive impairment.
Materials and methods
Participants and ethics
The study included patients aged 46–70 years with cognitive and other neurological complaints, such as gait and balance problems, mood disorders or residual symptoms after stroke, brain changes on MRI corresponded to CSVD (WMH, lacunae, enlarged perivascular spaces, microbleeds and cerebral atrophy)29. Patients with low WMH burden (Fazekas scale score 1) were included in the study if they had hypertension stage 2 or 3 and/or ≥ 1 lacuna.
Exclusion criteria: (1) cognitive impairment due to probable Alzheimer's disease according to the U.S. National Institute on Aging criteria30,31; (2) patients with small subcortical infarcts/lacunes < 3 months after an acute cerebrovascular event; (3) CSVD due to other independent causes (genetic, inflammatory, thrombophilic, systemic, toxic, history of severe migraines); (4) a different cause of stroke and concomitant brain pathology other than CSVD; (5) > 50% atherosclerotic stenosis of the extra- or intracranial arteries; (6) serious medical condition—cardiac (ejection fraction < 50%), endocrine (diabetes mellitus type 1 or 2 with severe vascular complications, uncompensated thyroid disorder), renal (chronic kidney disease with glomerular filtration rate < 30 ml/min), etc.; (7) contraindications for MRI.
The control group consisted of volunteers with no clinical or MRI evidence of vascular and degenerative brain pathology, no hypertension in the medical history and during Ambulatory Blood Pressure Monitoring (ABPM), and matched for age and gender. Controls with hypertension according to ABPM were excluded from the study, in accordance with the European Society of Hypertension recommendations: awake blood pressure was ≥ 135/85 mmHg and/or asleep blood pressure was ≥ 120/70 mmHg, or if blood pressure increased by more than 24% over time during exertion32.
In total 53 patients (37 women, average age 60.1 ± 6.8 years) and 17 healthy volunteers (12 women, average age 56.7 ± 6.7 years) were enrolled in the study.
Traditional vascular risk factors, such as hypertension33, hypercholesterolemia, obesity, diabetes mellitus and smoking were assessed in the patients and controls.
The study was approved by the Local Ethics Committee of the Research Centre of Neurology № №2–4/16 dated 17.02.2016 and performed in accordance with the principles of the Declaration of Helsinki. All subjects signed an informed consent form for participation in the study.
Ambulatory blood pressure assessment
All participants underwent ABPM with an automated device (LLC DMS Advanced Technologies, Moscow) based on oscillometric method. Patients underwent ABPM during hospitalization with blood pressure measurement every 30 min during the day (8:00 am to 10:00 pm) and every 60 min during the night (10:00 pm to 8:00 am). The ABPM device inflatable cuff was placed on the non-dominant upper limb. In all cases, at least 70% of the measurements were suitable for analysis. We calculated mean 24-h systolic blood pressure (SBP) and diastolic blood pressure (DBP); mean, standard deviation and maximal values of awake and asleep SBP and DBP; and blood pressure load parameters as the percentage of readings in a given period (24-h, day, or night), which exceeded the normal levels for awake and asleep SBP and DBP32.
The grade of hypertension was determined from the medical history and was adjusted according to ABPM results. During hospitalization patients continued their antihypertensive therapy.
Neuroimaging
Imaging was carried out in a Siemens MAGNETOM Verio 3 T scanner (Siemens Medical Systems, Erlangen, Germany) with a standard 12-channel matrix head coil. To evaluate STRIVE criteria29, patients and the control group underwent axial spin echo T2-weighed imaging (TR 4000 ms; TE 118 ms; slice thickness 5.0 mm; duration: 2 min 02 s); sagittal 3D T2 FLAIR (TR 6000 ms; TE 395 ms; 1.0 mm3 cubic voxel; duration: 7 min 12 s); sagittal 3D T1-weighed imaging (TR 1900 ms; TE 2,5 ms; 1.0 mm3 cubic voxel; duration: 4 min 16 s); diffusion MRI (DWI) using axial spin-echo echo-planar imaging sequence with two b-values—0, 1000 s/mm2 (TR − 4000 ms, TE − 100 ms, slice thickness − 4 mm, duration: − 1 min 20 s); axial susceptibility weighted imaging sequence (SWI) with magnitude and phase images reconstruction (TR 28 ms; TE 20 ms; slice thickness 1.2 mm; duration: 8 min 12 s).
Two neuroradiologists evaluated MR images in a standardized manner, blinded to clinical information. No STRIVE criteria were found in volunteers from control group. There were no acute or recent small lacunar infarcts based on DWI analysis in patients with CSVD. MRI presence of lacunes, white matter hyperintensities, microbleeds, and perivascular spaces were summed in a score of 0–4 representing all CSVD features combined34,35.
The Fazekas Scale36 was used to quantify T2 FLAIR white matter hyperintensities (WMH) (score 0–3) as well as semi-automatic WMH segmentation using LST toolbox (http://www.applied-statistics.de/lst.htm) for SPM12 (http://www.fil.ion.ucl.ac.uk/spm) with further manual correction using ITK-SNAP viewer (http://itksnap.org). The obtained data were saved as a binary mask, which was taken into consideration when the NAWM mask was subsequently created to calculate BBB permeability.
DCE-MRI was performed for BBB leak assessment. After two T1-weighted volumetric interpolated breath-hold examination (T1-VIBE) acquisitions (flip angles 2 and 15) for pre-contrast T1-mapping, we injected gadodiamide (Omniscan; GE Healthcare) 0.2 mL/kg (i.e., 0.1 mmol/kg body weight) at a rate of 3 mL/s intravenously via injection pump and then performed continuous serial acquisitions of 100 volumes of T1-VIBE images for 15 min 33 s. The scanning parameters were: TR − 8.6 ms, TE − 4 ms, field of view − 250 mm, matrix − 256 × 230 px, flip angle − 15°, and slice thickness − 3.6 mm.
Image processing
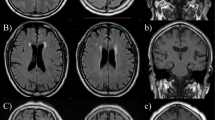
The entire DCE-MRI dataset underwent preliminary processing using the NordicNeuroLab software (NordicICE, Norway). This included automatic correction of motion artefacts, correction of pre- and post-contrast data in the dynamic series, concentration of contrast agent in the brain tissue calculation using relative signal change and T1 mapping. Individual vascular input functions were derived semi-automatically from the superior sagittal sinus37. The hematocrit, contrast agent dose and relaxivity of the contrast agent were set individually for each patient. The Patlak pharmacokinetic model was used to assess the low BBB permeability in CSVD resulting in Ktrans (volume transfer coefficient), Vp (fractional blood plasma volume) maps, and AUC (area under the curve—corresponding to increased contrast transit time in the brain) maps (Fig. 1A).
Once permeability parameter maps were obtained, further data processing was performed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm). This included the following steps: coregistration of each subject’s permeability parameter maps and the T1-weighted images; segmenting the T1-weighted images into grey matter and white matter, followed by the correction of obtained images using WMH masks based on a MatLab script (https://matlab.ru/), resulting in the binary images of the corrected grey and white matter. Permeability parameters were calculated in ITK-SNAP (http://itksnap.org) separately for grey matter (GM), NAWM and WMH by superimposing the relevant masks over the individual permeability maps (Fig. 1B). BBB permeability parameters have very low values (10−4), which do not visually differ, therefore, an analysis of the data of a patient with CSVD stage 2 according to Fazekas Scale is given as an example.
Statistical analysis
Statistical analysis was performed using IBM SPSS 23.0 (IBM SPSS Statistics, version 23.0, IBM Corp., Armonk, NY, USA) and R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) software. Data are presented as n (%) for categorical variables or as mean ± standard deviation (SD) or median [interquartile range (IQR)] for quantitative data. Differences between groups were determined using χ2, independent samples t-test or Mann–Whitney test, univariate analysis of variance or Kruskal–Wallis test with Bonferroni correction, where appropriate. In all cases, two-way statistical criteria were used. The null hypothesis was rejected if p < 0.05. Pearson's correlation coefficient and Spearman's correlation were used to assess the relationship between parameters.
Results
CSVD and the control groups were matched for age and gender, and consisted predominantly of women (Table 1). Vascular risk factors were comparable except for hypertension, which was the dropout criterion for the control group.
Most of the patients in the main group (84.8%) had hypertension of varying severity and were taking one or more antihypertensive drugs.
The main disease symptoms were cognitive impairment, gait disturbances unrelated to post-stroke hemiparesis, and MRI changes including WMH, lacunae, microbleeds and dilated perivascular spaces (Table 2).
Differences in mean asleep DBP, and asleep SBP and DBP load were found when ABPM results were compared between subjects with CSVD and those in the control group (Table 3).
ABPM results showed a good response to antihypertensive therapy in the main group. Most of ABPM parameters in the study (except for mean asleep DBP, asleep SBP load, and asleep DBP load) did not show intergroup differences corresponding to doctors’ and patients’ opinion that hypertension was well controlled as measured on the outpatient basis.
Although, there were found the relations of ABPM results with neuroimaging markers of CSVD (Table 4).
Increased SBP and DBP values had significant relations with WMH load, based on Fazekas Scale score, and correlations with WMH volume and total CSVD score.
Relationships between the standard deviation of blood pressure, which correspond to the indices of variability, and neuroimaging markers of CSVD were not found either by comparative or correlation analysis, therefore these data are not included in the table.
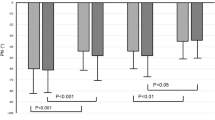
To clarify the link between daily blood pressure fluctuations and BBB permeability, the last one was assessed using DCE-MRI in patients and controls (Fig. 2).
According to DCE-MRI, all the study parameters of BBB permeability in NAWM and GM, except for Ktrans in NAWM, were significantly higher in patients with CSVD than in controls.
BBB permeability decreased as WMH Fazekas score increased, with significant differences in Vp and AUC in WMH (Table 5).
Differences in the BBB permeability parameters in GM and NAWM depending on the WMH Fazekas score were not found.
Statistically significant correlations were seen between AUC in GM and NAWM, Vp in NAWM and the parameters of 24-h and awake SBP and DBP (Table 6).
Increased BBB permeability, as assessed by AUC, was correlated with mean awake DBP, maximal awake SBP and DBP, awake SBP load (%) in GM, as well as with mean awake DBP and asleep SBP, maximal awake and asleep SBP and DBP, awake SBP load (%) in the NAWM.
AUC, which characterizes the contrast delay in the brain, had the highest sensitivity out of BBB permeability parameters.
No significant correlations were found between ABPM results and BBB permeability parameters in WMH, so these data are not provided.
Discussion
This study sought to clarify the relation between treated hypertension characteristics, assessed by ABPM, and BBB permeability, assessed by DCE-MRI, in patients with CSVD. Most of ABPM parameters in the study did not show intergroup differences, which confirms a good response to antihypertensive therapy in the main group. However, comparison of ABPM results with the severity of WMH based on Fazekas Score and its volume, as well as with total CSVD score, showed direct and significant relations. These data indicate the presence of certain mechanisms related to the abnormal ABPM parameters in patients compared with controls. Since a significant proportion of the studied patients had mild hypertension, as well as mild clinical and MRI signs of the disease, we could assume that BBB damage and its high permeability played a significant role in CSVD development. This hypothesis is based on the study results that indicate the special role of endothelial dysfunction with high BBB permeability as a mechanism of early CSVD25,38,39,40.
According to DCE-MRI, all the study parameters of BBB permeability in GM and NAWM, except for Ktrans in NAWM, were significantly higher in patients with CSVD compared to controls that is consistent with the previous studies26,27,28,41. There were no significant differences in BBB permeability in GM and NAWM between patients with WMH of varying severity. It should be noted that the results of previous studies regarding the significance of BBB permeability in the development of early or late CSVD are contradictory25,28,41. A reasonable explanation for our data may be that an increase in BBB permeability is possible with a relatively intact endothelium of small vessels in GM and NAWM in CSVD, corresponding the data about the universal mechanism of BBB damage in patients with CSVD and hypertension of varying severity22. On the other hand, the obtained data on a decrease in BBB permeability in the WMH may represent the conditions that characterize late-stage CSVD such as progressive endothelial death, impaired autoregulation due to small vessel remodelling, wall thickening and lumen narrowing, reduced microvascular perfusion9,28. This explanation is also supported by the fact that none of the abnormal ABPM parameters had relations with increased BBB permeability in the WMH. This result matches the different responses to elevated blood pressure in normotensive and hypertensive rats in following experiment. The hypertensive rats had higher permeability to sucrose which was absorbed more slowly by the brain, and the authors attributed this to changes in blood flow in hypertension18,42. AUC, which characterizes the contrast delay in the brain, had the highest sensitivity out of BBB permeability parameters. In our study, AUC in GM and NAWM had the most of relations with ABPM parameters. Although the latter in patients with CSVD did not differ from ones of the control group, we cannot exclude preceding rises in blood pressure that exceeds the upper threshold of cerebral autoregulation with an increase in BBB permeability21. It can be assumed that the use of antihypertensive therapy may change the upper threshold of cerebral autoregulation and the conditions for its disruption. This statement is indirectly supported by the fact that a greater reduction in blood pressure in elderly people with hypertension is associated with increased cerebral blood flow, corresponding to a shift in the autoregulation curve43. On the whole, these data support the necessity for more aggressive treatment of hypertension in patients with CSVD44,45. The risk of cardiovascular complications is decreased when blood pressure is reduced more aggressively, so the guidelines were rationally revised to the target SBP < 120 mmHg46. A recent randomized trial also supported this finding, as cerebral perfusion did not decrease in patients with severe CSVD when blood pressure was aggressively reduced, unlike in healthy controls47.
The obtained data on the universal nature of increased BBB permeability in the NAWM and GM in patients with CSVD indicate an ongoing pathological process in the small arteries, which leads to endothelial damage in relatively well-preserved small vessels. The connection between BBB permeability in NAWM and GM and elevated ABPM parameters indicated the importance of autoregulation dysfunction in promoting this mechanism. It is possible that the underestimation of the pathological mechanism of brain damage due to increased BBB permeability can partly explain the significance of hypertension in middle age for the development of cognitive impairment in the elderly48,49.
The lack of significant differences in ABPM results related to BBB permeability between patients and controls allow us to hypothesize the presence of additional factors of endothelial damage and increased BBB permeability alongside hypertension. These factors may be chronic inflammation50 or high salt sensitivity, which have been found to independently correlate with CSVD51.
The results of the study point out the necessity for more aggressive treatment of hypertension and repeat usage of ABPM as well as requirement for searching and affecting factors that potentiate the role of hypertension in CSVD development. It is obvious that further studies are needed on the effect of antihypertensive therapy on BBB permeability and its ability to protect the brain from damage in patients with CSVD.
Data availability
Raw data were generated at Research Center of Neurology. The data that support the findings of this study are available from the corresponding author upon reasonable request. Clinical, neurovisualization and statistical data will be available upon request from any qualified investigator.
Abbreviations
- CSVD:
-
Cerebral small vessel disease
- BBB:
-
Blood–brain barrier
- ABPM:
-
Ambulatory blood pressure monitoring
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- WMH:
-
White matter hyperintensity
- NAWM:
-
Normal-appearing white matter
- GM:
-
Grey matter
- MRI:
-
Magnetic resonance imaging
- DCE-MRI:
-
T1-weighted dynamic contrast-enhanced magnetic resonance imaging
- Ktrans:
-
Volume transfer coefficient
- Vp:
-
Fractional blood plasma volume
- AUC:
-
Area under the curve
References
Gorelick, P. B. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(9), 2672–2713. https://doi.org/10.1161/STR.0b013e3182299496 (2011).
Deramecourt, V. et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology 78, 1043–1050. https://doi.org/10.1212/WNL.0b013e31824e8e7f (2012).
Azarpazhooh, M. R. et al. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Demen. 14(2), 148–156. https://doi.org/10.1016/j.jalz.2017.07.755 (2018).
Dufouil, C. et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI cohort. Neurology 56, 921–926. https://doi.org/10.1212/wnl.56.7.921 (2001).
de Leeuw, F. E. et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125, 765–772. https://doi.org/10.1093/brain/awf077 (2002).
Wardlaw, J. M., Smith, C. & Dichgans, M. Mechanisms underlying sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497. https://doi.org/10.1016/S1474-4422(13)70060-7 (2013).
van Dijk, E. J. et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 44(5), 625–630. https://doi.org/10.1161/01.HYP.0000145857.98904.20 (2004).
Fisher, C. M. The arterial lesions underlying lacunes. Acta Neuropathol. 12(1), 1–15. https://doi.org/10.1007/BF00685305 (1969).
McAleese, K. E. et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 14(1), 129. https://doi.org/10.1186/s12916-016-0676-5 (2016).
Arima, H. & Chalmers, J. PROGRESS: prevention of recurrent stroke. J. Clin. Hypertens. 13(9), 693–702. https://doi.org/10.1111/j.1751-7176.2011.00530.x (2011).
Hankey, G. J. The global and regional burden of stroke. Lancet Glob. Health 1(5), e239–e240. https://doi.org/10.1016/S2214-109X(13)70095-0 (2013).
Weber, R. et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke 43(9), 2336–2342. https://doi.org/10.1161/STROKEAHA.111.648576 (2012).
Williamson, J. D. et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321(6), 553–561. https://doi.org/10.1001/jama.2018.21442 (2019).
Mamo, J. C. L. et al. Antihypertensive agents do not prevent blood-brain barrier dysfunction and cognitive deficits in dietary-induced obese mice. Int. J. Obes. 41(6), 926–934. https://doi.org/10.1038/ijo.2017.57 (2017).
Fan, Y. et al. Tight junction disruption of blood-brain barrier in white matter lesions in chronic hypertensive rats. Neuroreport 26(17), 1039–1043. https://doi.org/10.1097/WNR.0000000000000464 (2015).
Wardlaw, J. M., Valdés Hernández, M. C. & Muñoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4(6), 001140. https://doi.org/10.1161/JAHA.114.001140 (2016).
Iadecola, C. et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 68(6), e67–e94. https://doi.org/10.1161/HYP.0000000000000053 (2016).
Katsi, V. et al. Blood–brain barrier dysfunction: the undervalued frontier of hypertension. J. Hum. Hypertens. 34(10), 682–691. https://doi.org/10.1038/s41371-020-0352-2 (2020).
Biancardi, V. C., Son, S. J., Ahmadi, S., Filosa, J. A. & Stern, J. E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension 63(3), 572–579. https://doi.org/10.1161/HYPERTENSIONAHA.113.01743 (2014).
Hazama, A. F., Amano, S. & Ozaki, T. Pathological changes of cerebral vessel endothelial cells in spontaneously hypertensive rats, with special reference to the role of these cells in the development of hypertensive cerebrovascular lesions. Adv. Neurol. 20, 359–369 (1978).
Iadecola, C. & Davisson, R. L. Hypertension and cerebrovascular dysfunction. Cell Metab. 7(6), 476–484. https://doi.org/10.1016/j.cmet.2008.03.010 (2008).
Schreiber, S., Bueche, C. Z., Garz, C. & Braun, H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease?—New insights from a rat model. Exp. Transl. Stroke Med. 5(1), 4. https://doi.org/10.1186/2040-7378-5-4 (2013).
Ma, Y. et al. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke 51(1), 82–89. https://doi.org/10.1161/STROKEAHA.119.026739 (2020).
Tully, P. J. et al. Association between blood pressure variability and cerebral small-vessel disease: a systematic review and meta-analysis. J. Am. Heart Assoc. 9(1), e013841. https://doi.org/10.1161/JAHA.119.013841 (2020).
Wardlaw, J. M. et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 13(6), 634–643. https://doi.org/10.1016/j.jalz.2016.09.006 (2017).
Thrippleton, M. J. et al. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimers Dement. 15(6), 840–858. https://doi.org/10.1016/j.jalz.2019.01.013 (2019).
Topakian, R., Barrick, T. R., Howe, F. A. & Markus, H. S. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry 81(2), 192–197. https://doi.org/10.1136/jnnp.2009.172072 (2010).
Huisa, B. N. et al. Long-term blood-brain barrier permeability changes in binswanger disease. Stroke 46(9), 2413–2418. https://doi.org/10.1161/STROKEAHA.115.009589 (2015).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12(8), 822–838. https://doi.org/10.1016/S1474-4422(13)70124-8 (2013).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3), 270–279. https://doi.org/10.1016/j.jalz.2011.03.008 (2011).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.00 (2011).
O’Brien, E. et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 31(9), 1731–1768. https://doi.org/10.1097/HJH.0b013e328363e964 (2013).
Mancia, G. et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the European society of cardiology (ESC). J Hypertens. 31(7), 1281–1357. https://doi.org/10.1097/01.hjh.0000431740.32696.cc (2013).
Klarenbeek, P., van Oostenbrugge, R. J., Rouhl, R. P., Knottnerus, I. L. & Staals, J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke 44(11), 2995–2999. https://doi.org/10.1161/STROKEAHA.113.002545 (2013).
Staals, J. et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol. Aging 36(10), 2806–2811. https://doi.org/10.1016/j.neurobiolaging.2015.06.024 (2015).
Fazekas, F. et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 8(3), 421–426. https://doi.org/10.2214/ajr.149.2.351 (1987).
Li, Y. et al. Hemodynamic assessments of venous pulsatile tinnitus using 4D-flow MRI. Neurology. 91(6), e586–e593. https://doi.org/10.1212/WNL.0000000000005948 (2018).
Wardlaw, J. M., Sandercock, P. A., Dennis, M. S. & Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia?. Stroke 34(3), 806–812. https://doi.org/10.1161/01.STR.0000058480.77236.B3 (2003).
Rosenberg, G. A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 32(7), 1139–1151. https://doi.org/10.1038/jcbfm.2011.197 (2012).
Rajani, R. M. et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 10(448), eaam507. https://doi.org/10.1126/scitranslmed.aam9507 (2018).
Li, Y. et al. Higher blood–brain barrier permeability is associated with higher white matter hyperintensities burden. J. Neurol. 264(7), 1474–1481. https://doi.org/10.1007/s00415-017-8550-8 (2017).
Setiadi, A., May, C. N. & Yao, S. T. Ablation of astrocytes in the paraventricular nucleus disrupts the blood-brain barrier and increases blood pressure in rats. FASEB J. https://doi.org/10.1096/fasebj.31.1_supplement.1010.5 (2017).
Tryambake, D. et al. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension 61(6), 1309–1315. https://doi.org/10.1161/HYPERTENSIONAHA.112.200972 (2013).
SPRINT Research Group et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373(22), 2103–2116. https://doi.org/10.1056/NEJMoa1511939 (2015).
Mancia, G. et al. Cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the VALUE trial. Eur. Heart J. 37(12), 955–964. https://doi.org/10.1093/eurheartj/ehv633 (2016).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American heart Association. Task force on clinical practice guidelines. Hypertension 71(6), 1269–1324. https://doi.org/10.1161/HYP.0000000000000066 (2018).
Croall, I. D. et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: the preserve randomized clinical trial. JAMA Neurol. 75(6), 720–727. https://doi.org/10.1001/jamaneurol.2017.5153 (2018).
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C. & Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64(2), 277–281. https://doi.org/10.1212/01.WNL.0000149519.47454.F2 (2005).
Walker, K. A. et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 322(6), 535–545. https://doi.org/10.1001/jama.2019.10575 (2019).
Ihara, M. & Yamamoto, Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke 47(2), 554–560. https://doi.org/10.1161/STROKEAHA.115.009627 (2016).
Dobrynina, L. A. et al. The predictive value of salt sensitivity and osmotic fragility in the development of cerebral small vessel disease. Int. J. Mol. Sci. 21(6), 2036. https://doi.org/10.3390/ijms21062036 (2020).
Acknowledgements
MRI examinations were performed using the scanner at the Structural and Functional Brain Mapping Center for Collective Use at the Research Center of Neurology.
Author information
Authors and Affiliations
Contributions
L.A.D., K.V.S., E.I.K., M.R.Z., B.M.A., E.V.G., and M.V.K. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design—L.A.D. Acquisition of data—all authors. Data analysis and interpretation of data—L.A.D., E.I.K., K.V.S., M.R.Z., and B.M.A. Drafting of the manuscript—L.A.D., K.V.S., and E.I.K. Critical revision of the manuscript for important intellectual content—all authors. Statistical analysis—K.V.S., M.R.Z., and B.M.A. Study supervision: L.A.D., E.V.G., and M.V.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dobrynina, L.A., Shamtieva, K.V., Kremneva, E.I. et al. Daily blood pressure profile and blood–brain barrier permeability in patients with cerebral small vessel disease. Sci Rep 12, 7723 (2022). https://doi.org/10.1038/s41598-022-11172-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11172-1
This article is cited by
-
Cerebral Small Vessel Disease: a Review of the Pathophysiological Mechanisms
Translational Stroke Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.