Abstract
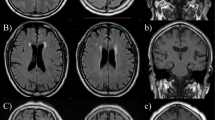
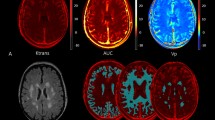
This study aimed to investigate the relationship between cerebral small vessel disease (CSVD) and orthostatic hypotension (OH) using self-measured blood pressure at home in community-dwelling older subjects. Between May 2016 and October 2018, 663 community-dwelling adults aged ≥60 years were enrolled in Shandong, China. CSVD, including white matter hyperintensities (WMHs), lacunes, enlarged Virchow–Robin spaces (EVRS) and microbleeds, was assessed using brain magnetic resonance imaging. After receiving appropriate training, the subjects participated in “home-measured (H)OH” by themselves for three consecutive days. Participants were classified into no-HOH, 1 HOH, and ≥2 HOH episode groups according to the presence of HOH episodes. The WMH volume, WMH-to-total intracranial volume (TIV) ratio, total numbers of lacunes and EVRS, and prevalence of Fazekas scale score ≥2, lacunes, and EVRS were elevated in the 1 and ≥2 HOH episode groups compared with the no-HOH episode group (P < 0.05). The prevalence and total number of microbleeds were significantly higher in the ≥2 HOH episodes group than in the no-HOH and 1 HOH episode groups (P < 0.05). HOH episodes were significantly associated with WMH volume, WMH-to-TIV ratio, and the total numbers of lacunes, EVRS, and microbleeds after adjustment for confounders (P < 0.05). The risks of Fazekas scale score ≥2, lacunes, EVRS, and microbleeds were 2.123-, 1.893-, 2.162-, and 1.656-fold higher in the 1 HOH episode group and 4.910-, 5.359-, 3.048-, and 2.418-fold higher in the ≥2 HOH episodes group, respectively, than those in the no-HOH group. The presence of HOH episodes was an independent risk factor for CSVD in the community-based older population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wardlaw JM, Smith C, Dichans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–97.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R.Standards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol.2013;12:822–38.
Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–56.
de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam scan study. J Neurol Neurosurg Psychiatry. 2011;70:9–14.
Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–6.
Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel disease: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88:669–74.
Hilal S, Baaij LGA, de Groot M, Niessen WJ, Ikram MK, Ikram MA, et al. Prevalence and clinical relevance of diffusion-weighted imaging lesions: the Rotterdam study. Neurology. 2019;93:e1058–67.
Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H.LADIS Study Group et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (Leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477.
Goldstein ED, Badi MK, Hasan TF, Lesser ER, Hodge DO, Lin MP, et al. Cerebral small vessel disease burden and all-cause mortality: Mayo clinic florida familial cerebrovascular diseases registry. J Stroke Cerebroavasc Dis. 2019;28:104285.
Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33:26–30.
Zhang H, Cui Y, Zhao Y, Dong Y, Wang J, Duan D, et al. Association of circadian rhythm of blood pressure and cerebral small vessel disease in community-based elderly population. J Gerontol A Biol Sci Med Sci. 2019;74:1322–30.
Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–53.
Matsubayashi K, Okumiya K, Wada T, Osaki Y, Fujisawa M, Doi Y, et al. Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke. 1997;28:2169–73.
Soennesyn H, Nilsen DW, Oppedal K, Greve OJ, Beyer MK, Aarsland D. Relationship between orthostatic hypotension and white matter hyperintensity load in older patients with mild dementia. PLoS ONE. 2012;7:e52196.
Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002;40:133–41.
Foster-Dingley JC, Moonen JEF, de Ruijter W, van der Mast RC, van der Grond J. Orthostatic hypotension in older persons is not associated with cognitive functioning, features of cerebral damage or cerebral blood flow. J Hypertens. 2018;36:1201–6.
Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurololgy. 1996;46:1470.
McNicholas T, Tobin K, Carey D, O’Callaghan S, Kenny RA. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4-year follow-up? Data from TILDA (The Irish Longitudinal Study on Ageing). J Am Heart Assoc. 2018;7:e008976.
Suemoto CK, Baena CP, Mill JG, Santos IS, Lotufo PA, Benseñor I. Orthostatic hypotension and cognitive function: cross-results from the ELSA-Brasil study. J Gerontol A Biol Sci Med Sci. 2019;74:358–65.
Peters R, Anstey KJ, Booth A, Beckett N, Warwick J, Antikainen R, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39:3135–43.
Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;66:848–60.
Finucane C, O’Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130:1780–9.
Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406–13.
Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32:1562–71.
Ko D, Preis SR, Lubitz SA, McManus DD, Vasan RS, hamburg NM, et al. Relation of orthostatic hypotension with new-onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2018;121:596–601.
Cremer A, Rousseau AL, Boulestreau R, Kuntz S, Tzourio C, Gosse P. Screening for orthostatic hypotension using home blood pressure measurements. J Hypertens. 2019;37:923–7.
Cohen A, Vidal JS, Roca F, Rananja H, hernandorena I, Coude du Foresto L, et al. Feasibility and determinants of orthostatic hypotension self-measurement at home in an elderly community-dwelling population. Am J Hypertens. 2019;32:824–32.
Belmin J, Abderrhamane M, Medjahed S, Sibony-Prat J, Bruhat A, Bojic N, et al. Variability of blood pressure response to orthostatism and reproducibility of the diagnosis of orthostatic hypotension in elderly subjects. J Gerontol A Biol Sci Med Sci. 2000;55:M667–71.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. List of authors/Task Force members:. 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284–309.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–594.
Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension—a report of the revision committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16:182–241.
Blacher J, Halimi JM, Hanon O, Mourad JJ, Pathak A, Schnebert B.French Society of Hypertension et al. Management of arterial hypetension in adults: 2013 guidelines of the French Society of Arterial Hypertension. Fundam Clin Pharm.2014;28:1–9.
Stergiou GS, Giovas PP, Gkinos CP, Patouras JD. Validation of the Microlife WatchBP Home device for self home blood pressure measurement according to the International Protocol. Blood Press Monit. 2007;12:185–8.
Ji T, Zhao Y, Wang J, Cui Y, Duan D, Chai Q, et al. Effect of low-dose statins and Apolipoprotein E genotype on cerebral small vessel disease in older hypertensive patients: a subgroup analysis of a randomized clinical trial. J Am Med Dir Assoc. 2018;19:995–1002.
Chen Y, Yu H, Zhu J, Zhang H, Zhao Y, Dong Y, et al. Low carotid endothelial shear stress associated with cerebral small vessel disease in an older population: a subgroup analysis of a population-based prospective cohort study. Atherosclerosis. 2019;288:42–50.
Robertson AD, Messner MA, Shirzadi Z, Kleiner-Fisman G, Lee J, Hopyan J, et al. Orthostatic hypotension, cerebral hypoperfusion, and visuospatial deficits in Lewy body disorders. Parkinsonism Relat Disord. 2016;22:80–86.
Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–82.
SPRINT MIND Investgators for the SPRINT Research Group, Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–34.
Zhang H, Cui Y, Zhao Y, Dong Y, Duan D, Wang J, et al. Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double-blind and placebo-controlled clinical trial. Hypertens Res. 2019;42:717–29.
Godin O, Tzourio C, Mailard P, Mazoyer B, Dufouil C. Antihypertnsive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–73.
White WB, Wakefield DB, Moscufo N, Guttmann CRG, Kaplan RF, Bohannon RW, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation. 2019;140:1626–35.
Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA. Neurol 2014;71:855–62.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Microbleeds Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Moran C, Phan TG, Srikanth VK. Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes. Int J Stroke. 2012;7:36–46.
Juraschek SP, Appel LJ, Miller ER III, Mukamal KJ, Lipsitz L. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease: results from the AASK trial. Hypertension. 2018;72:986–93.
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Auto Neurosci. 2011;161:46–48.
Wilson D, Werring DJ. Antithrombotic therapy in patients with cerebral microbleeds. Curr Opin Neurol. 2017;30:38–47.
Cheng Y, Liu J, Zhang S, Li J, Wei C, Wang D, et al. Prior antithrombotic therapy is associated with cerebral microbleeds in ischemic stroke patients with atrial fibrillation and/or rheumatic heart disease. Front Neurol. 2018;9:1184.
Badi MK, Vilanilam GK, Gupta V, Barrett KM, Lesser ER, Cochuyt JJ, et al. Pharmacotherapy for patients with atrial fibrillation and cerebral microbleeds. J Stroke Cerebrovasc Dis. 2019;28:2159–67.
Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005835.
Acknowledgements
The authors thank all participants and individuals who offered their assistance to this study.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81670432 and 81973139); Key Technology Research and Development Project of Shandong Province (grant numbers 2018GSF118044, 2017GSF218060, and 2019GSF108079); and The Innovation Project of Shandong Academy of Medical Sciences and Academic Promotion Program of Shandong First Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, Y., Zhang, H., Zhao, Y. et al. Home-measured orthostatic hypotension associated with cerebral small vessel disease in a community-based older population. Hypertens Res 43, 798–807 (2020). https://doi.org/10.1038/s41440-020-0429-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0429-x
Keywords
This article is cited by
-
Excessive salt intake accelerates the progression of cerebral small vessel disease in older adults
BMC Geriatrics (2023)
-
Efficacy and safety of Dengyinnaotong Capsule in patients with Cognitive impairment caused by cerebral Small Vessel Disease: study protocol of a multicenter, randomized, open-label, controlled trial (De-CSVD trial)
Trials (2022)
-
Hypertension management to prevent dementia
Hypertension Research (2022)