Abstract
This study was to investigate the association between serum interleukin 32 (IL-32) concentration and clinical parameters in patients with stable chronic obstructive pulmonary disease (COPD). One hundred and sixteen patients with stable COPD and 70 healthy subjects were included in the study. The serum concentration of IL-32 was detected by enzyme-linked immunosorbent assay. The correlation between serum IL-32 and clinical parameters of patients with COPD was analyzed by T-test, one-way analysis of variance, multiple linear regression and receiver operating characteristic curve. The serum concentration of IL-32 in patients with stable COPD was higher than that in healthy control group (p < 0.001) and increased serum IL-32 was positively correlated with GOLD grading (p = 0.026), mMRC score (p = 0.004) and clinical medical history (p = 0.005), but negatively related to FEV1/FVC (p = 0.001) and FEV1% predicted (p = 0.001). Patient's COPD grading (p = 0.001), clinical medical history (p < 0.001) and FEV1/FVC (p = 0.001) exerted a significant impact on serum IL-32. The sensitivity and specificity of serum IL-32 for discerning COPD patients from healthy individuals were 85.34% and 64.29%, and the area under the curve was 0.808 (p < 0.001). Increased IL-32 is involved in the chronic disease progression of COPD, suggesting that IL-32 may be a molecular biomarker that reflects the severity of COPD and contributes to the disease diagnosis.
Similar content being viewed by others
Chronic obstructive pulmonary disease (COPD), a common preventable and treatable disease, is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases1. COPD is one of the diseases with high morbidity and mortality worldwide. The prevalence of COPD in Chinese population has increased year by year due to environmental pollution and population aging. Despite some efforts by the government, the disease has become a huge burden for the patient population in the country. The World Health Organization (WHO) emphasizes that early diagnosis and proper treatment are key to controlling of COPD2. At present, the etiology and pathogenesis of COPD are still not fully elucidated. The imbalance of various inflammatory mediators, cytokines and proteases during the development of COPD play an important role in the persistence of small airway inflammation and alveolar tissue destruction, which leads to the activation of inflammatory cell deposits and autoimmune reactions, and small airway remodeling3,4,5,6. Scholars generally believe that airway inflammation and immune mechanisms, protease-antiprotease imbalance, oxidative stress, autonomic dysfunction, malnutrition and climate change are involved in occurrence and development of COPD1,4,7.
Interleukin-32 (IL-32) is an inflammatory cytokine that can be produced by activated T cells, natural killer cells, monocytes, and epithelial cells8,9. IL-32 gene is located on human chromosome 16p13.3 and contains eight exons, consisting of 705 pairs of bases10. Previous study shows that IL-32 is highly expressed in the lung tissue of patients with COPD, and alveolar wall and bronchial epithelial cells are the main expression sites11, it has been confirmed to participate in the inflammatory process of COPD as a pro-inflammatory factor12. In this study, we examined the concentration of serum IL-32 in patients with stable COPD and explored the correlation between serum IL-32 and clinical parameters of stable COPD.
Results
Serum IL-32 concentration in patients with stable COPD is higher than that in healthy individuals
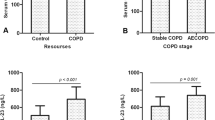
The homogeneity test of variance showed that the two population variances on the data of IL-32 concentration was equal (F = 4.005 and p = 0.052). The results of T test showed that serum concentration of IL-32 in patients with stable COPD (169.9 ± 51.9 pg/mL) was higher than that in healthy individuals (105.1 ± 43.3 pg/mL) (T = − 7.476, Df = 144, p < 0.001) (Fig. 1A; Table 1).
Relationship between clinical parameters and serum concentration of IL-32 in stable COPD patients. (A) Patients with stable COPD had a higher serum concentration of IL-32 compared with control group (p < 0.001). (B) Serum IL-32 concentration of patients with GOLD-3 and 4 was increased compared with that of GOLD-1 and 2 (p = 0.026). (C) Serum IL-32 concentration in patients with mMRC score of 4 was increased than that in patients with mMRC score of 3 and 2 (p = 0.004). (D) Serum IL-32 concentration in the patients with long clinical history was increased compared to those with short history (p = 0.005). COPD, chronic obstructive pulmonary disease; IL-32, interleukin-32; M ± SD, mean ± standard deviation; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified british medical research council.
Serum IL-32 concentration in patients with stable COPD positively correlates with GOLD grade, mMRC score and clinical medical history
The results of the T test showed that serum IL-32 concentration in patients with stable COPD was not associated with gender, smoking and BMI (p > 0.05) (Table 1). The data variance test for the correlation between serum IL-32 and GOLD grade, mMRC score and clinical medical history of patients showed that the overall variance of each group was equal (F = 3.295 and p = 0.073 for GOLD grade; F = 2.169 and p = 0.120 for mMRC score; F = 0.922 and p = 0.401 for clinical medical history) (Table 1). The statistical results showed that serum IL-32 concentration in stable COPD patients with GOLD-3 to 4 (186.5 ± 50.4 pg/mL), mMRC score of 4 (189.8 ± 48.2 pg/mL) and longer clinical medical history (187.6 ± 45.8 pg/mL for ≧ 20 years; 169.9 ± 51.1 pg/mL for history ≧ 10 < 20 years) was higher than that in patients with GOLD-1 to 2 (149.6 ± 52.3 pg/mL) (p = 0.026; Fig. 1B), mMRC score of 2 (154.1 ± 55.3 pg/mL) and 3 (155.3 ± 52.8 pg/mL) (p = 0.004; Fig. 1C) and shorter medical history (147.7 ± 48.9 pg/mL for history < 10 years) (p = 0.005; Fig. 1D).
Serum IL-32 concentration in patients with stable COPD negatively correlates with pulmonary function
The serum concentration of IL-32 was negatively correlated with FEV1/FVC (correlation coefficient = − 0.356, p = 0.001; F = 11.207, p = 0.001; T = − 3.348, p = 0.001; the regression equation = ^Y = 0.593–0.096X) (Fig. 2A–C; Table 2) and FEV1% predicted (Correlation coefficient = − 0.300, p = 0.001; F = 11.295, p = 0.001; T = − 3.361, p = 0.001; the regression equation = ^Y = 0.634–0.062X) (Fig. 2D–F; Table 2) in stable COPD patients.
Relationship between serum IL-32 concentration and pulmonary function in stable COPD patients. (A) The histogram of normal curve showed that the normalized residuals were normal distributions (p > 0.05). (B) The dependent variable was approximately linear with the standardized predictive value, indicating that the IL-32 and FEV1/FVC had a negative linear correlation (p > 0.05). (C) The vast majority of normalized residuals did not exceed 3, suggesting no specific value was found. (D) The histogram of normal curve showed that the normalized residuals were normal distributions (p > 0.05). (E) The dependent variable was approximately linear with the standardized predictive value, indicating that the IL-32 and FEV1% predicted had a negative linear correlation (p > 0.05). (F) The vast majority of normalized residuals did not exceed 3, suggesting no specific value was found. COPD, chronic obstructive pulmonary disease; IL-32, interleukin-32; M ± SD, mean ± standard deviation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Serum concentration of IL-32 is affected by COPD grading, clinical medical history and FEV1/FVC
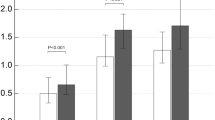
The stepwise regression of multiple linear showed that patient's COPD grading (p = 0.001; 95% CI = 6.63 to 26.61), clinical medical history (p = 0.023; 95% CI = 0.03 to 3.52) and FEV1/FVC (p = 0.001; 95% CI = − 175.16 to − 44.93) seemed to affect the serum IL-32 concentration (Fig. 3A–C; Table 3). The influence of FEV1/FVC (partial regression analysis = − 110.15) on IL-32 was greater than that of COPD grading (partial regression analysis = 16.62) and clinical medical history (partial regression analysis = 10.53).
Comparison of factors affecting serum IL-32 concentration in patients with stable COPD. (A) The patient's COPD grading showed a more effect on IL-32 concentration than the patient's clinical history (p < 0.05). (B) The effect of FEV1/FVC on IL-32 concentration was more direct than COPD grading (p < 0.05). (C) The effect of the patient’s FEV1/FVC on IL-32 expression was greater than the medical history (p < 0.05). GOLD, Global Initiative for Chronic Obstructive Lung Disease; IL-32, interleukin-32; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Diagnostic efficacy test of serum IL-32 in distinguishing patients with COPD from healthy individuals
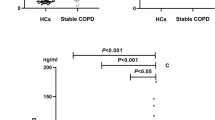
The ROC analysis suggested that the threshold value of serum IL-32 concentration for distinguishing patients with COPD from healthy individuals was 105 pg/mL (Fig. 4A; Table 4), the value showed a sensitivity of 85.34% and a specificity of 64.29% (Fig. 4B). Taking the value as a critical point, the area under the curve (AUC) was 0.808, the standard error was 0.0315, and the 95% confidence interval was 0.746–0.862 (Z = 9.77; p < 0.001) (Fig. 4C).
Diagnostic value and clinical significance of serum IL-32 concentration in stable COPD. (A) The serum IL-32 threshold value for discerning patients with COPD from healthy individuals was 105 pg/mL. (B) The sensitivity and specificity for distinguishing patients with COPD from healthy individuals were 85.34% and 64.29%. (C) The area under the curve (AUC) of serum IL-32 for discriminating COPD patients from healthy individuals was 0.808 and Z value was 9.77 (p < 0.001). GOLD Global Initiative for Chronic Obstructive Lung Disease, AUC area under the curve.
Discussion
Interleukin-32 (IL-32) is originally cloned from natural killer cells by interleukin-18 (IL-18) induction, it was later discovered that its biological role is similar to cytokines and plays a key role in chronic inflammation8,10,11,12 . Previous studies show that IL-32 can induce immune cells to produce a variety of other cytokines and chemokines, involving in the specific immune response and inflammatory response of COPD8,10,12,13. We investigated the concentration of serum IL-32 in stable COPD patients and found that the serum concentration of IL-32 in patients with stable COPD was higher than that in healthy individuals, indicating that there was a correlation between increased serum concentration of IL-32 and COPD. Previous studies show that IL-32 can activate the phosphorylation of the nuclear transcription factor kappa B (NF-κB) and p38 mitogen-activated protein kinase (p38MAPK), inducing the production of inflammatory factors such as tumor necrosis factor-α (TNF-α), IL-1β, IL-6 and IL-8, these cytokines play an important role in the pathogenesis of COPD10,14.
The GOLD score of COPD patients can reflect objective severity of the disease (the higher the score, the more serious the disease). Our study showed that there was a strong positive correlation between serum IL-32 concentration and GOLD score, which suggested that IL-32 might be a molecular biomarker that reflects the severity of COPD. Previous studies show that down-regulating the concentration of IL-32 leads to the increase of some inflammatory factors, including TNF-α, IL-6, IL-8 and cell adhesion factor (ICAM)-1, this phenomenon indicates that IL-32 is an important pro-inflammatory cytokine8,11. The GOLD score is only an indicator of respiratory function, and there is still a certain degree of limitation for evaluating COPD as a systemic disease. The mMRC score is another indicator to evaluate the quality of life of patients with COPD. It has been widely used in the evaluation of the condition and therapeutic efficacy of patients with COPD and the prediction of the risk of death in patients15. Our study showed that serum IL-32 concentration in patients with stable COPD was positively correlated with mMRC score, suggesting that IL-32 was related to the severity of patients with COPD. However, the clinical evaluation of serum IL-32 to COPD still needs to be confirmed by further research. Elevated serum IL-32 concentration in patients with long clinical medical history of COPD also reflects that IL-32 could be associated with the chronic development of COPD. Because IL-32 is involved in the COPD immune response, researchers have proposed to treat COPD by inhibiting the secretion of IL-327. Controlling the inflammatory response mediated by IL-32 may be another theoretical basis for the development of new drugs for the treatment of COPD in the future.
It is well known that FEV1% and FEV1/FVC are important indicators for evaluating respiratory function in patients with COPD, and the decrease in both indicates the progression of COPD and the increase risk in mortality16. In our study, the linear correlation analysis showed that serum IL-32 concentration in patients with stable COPD was negatively correlated with FEV1% and the FEV1/FVC, suggesting that changes in serum IL-32 can reflect the severity of COPD disease progression. The theory of airway inflammation is an important mechanism of the pathogenesis of COPD, and excessive production of inflammatory factors induces immune damage, leading to deterioration of lung function. Previous studies shows that increased IL-32 was negatively correlated with decreased FEV1, FEV1/FVC and oxygenation index in patients with acute exacerbation of COPD, confirming that IL-32 has a direct pro-inflammatory effect and also is the cause of chronic airway inflammation persisting and progressively worsening7,17,18. We did a multiple linear regression and found that COPD grading, clinical medical history and FEV1/FVC value in stable COPD affected the serum concentration of IL-32, and the FEV1/FVC had the most obvious influence on IL-32. As a pro-inflammatory cytokine, IL-32 not only involves in the continuous chronic inflammation of the airway but also reflects the severity of patients with COPD, and therefore it can be used as an evaluation indicator of disease progression and treatment effect in patients with COPD.
To further understand whether IL-32 has a reference value in the diagnosis of COPD, we conducted further diagnostic analysis. Through a ROC analysis, we found that the sensitivity and specificity for distinguishing patients with COPD from healthy individuals were 85.34% and 64.29%, and the area under the curve reached 0.808, indicating that IL-32 can reflect the disease occurrence of COPD and has a good value for diagnosis of COPD. It is reported that IL-32 is significantly elevated in patients with chronic inflammatory diseases of the airways, and IL-32 has the effect of inhibiting airway remodeling7,8,10,17,18,19. However, the effect of IL-32 on the inhibition of airway remodeling and the promotion of airway inflammatory response remains to be confirmed. Our findings may suggest a hypothesis that serum IL-32 may be involved in the inflammatory process of COPD, inhibition of IL-32 may cut off IL-32 mediated inflammatory response, thereby reducing airway remodeling and airflow limitation in patients with COPD, and alleviating symptoms in patients. This will be a new direction for clinical treatment of COPD.
In summary, serum IL-32 concentration is higher in patients with stable COPD than in healthy individuals, and increased IL-32 is positively correlated with GOLD grade, mMRC score and clinical medical history of patients but negatively with FEV1/FVC and FEV1% predicted, suggesting that IL-32 is involved in the chronic disease process of COPD and changes in IL-32 can be used to assess the severity of COPD and contributes to the diagnosis of COPD. However, there are also some shortcomings in this study. Firstly, the study only included Chinese patients and might have a geographical and ethnographic bias. Secondly, this study did not involve patients with acute exacerbation. Thirdly, this study did not investigate the specific intrinsic molecular mechanism of IL-32 elevation in COPD. Fourthly, this study did not involve the phenotype matter for example bronchitic vs. other phenotypes, nor did it give a definitive conclusion about the relationship between BMI and IL-32. Fifthly, there were less smokers in the control arm and non-smokers are more likely to have healthier habits like exercise and diet that may positively affect their health thus introduce a bias. In the future, the in-depth research to explain these issues should be conducted.
Methods
Ethics statement
This is an observational retrospective analysis (on patient’s blood specimen for test program). Informed consent was obtained from each of the recruited patients prior to entering the study in accordance with the approved ethical guidelines. The study was approved by the Research Ethics Committees of research institutes (Jining NO.1 People’s Hospital, Jining, China; Shenmu Hospital, Shenmu, China; Zhangye Second People Hospital, Zhangye, China; Minle County People’s Hospital, Minle, China; Minqin County People’s Hospital, Minqin, China; Gansu Provincial Hospital, Lanzhou, China; The First Affiliated Hospital, Xi’an Medical University, Xi’an, China).
Objects
Between May 2015 and May 2019, a total of 116 patients from 7 hospitals (patients recruited from an indicated Ethics statement centers) were included in the observation group. At the same time, 70 outpatient health individuals distributed in the above-mentioned medical institutions were included in the control group. The demographic characteristics between the observation group and the control group, including gender, age, and smoking status, were not statistically significant (p > 0.05) (Table 5).
Diagnostic criteria for patients enrolled and severity rating of COPD
The diagnosis and severity evaluation of COPD were performed by the diagnostic criteria of the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD)1,3,6. Objective criteria: after inhalation of 300 to 400 μg of salbutamol, the ratio of forced expiratory volume (FEV1) in the first second to forced vital capacity (FVC) is less than 0.70. The severity of COPD: GOLD-1, FEV1% predicted is more or equal to 80%; GOLD-2, the FEV1% predicted is more or equal to 50%, but less than 80%; GOLD-3, the FEV1% predicted is more or equal to 30%, but less than 50%; and GOLD-4, the FEV1% predicted is less than 30%.
Inclusion criteria
Inclusion criteria: (1) must meet the diagnostic criteria of COPD and be a stable COPD (the patient's cough, expectoration and shortness of breath are in stable condition or just show a mild symptoms or the condition is basically restored to the state before acute exacerbation)1,3; (2) must have a lung function test within 3 days before enrollment; (3) no antibiotics, glucocorticoids and theophylline were used within 2 weeks before enrollment; (4) without laboratory and imaging evidences of pulmonary infection and pneumonia.
Exclusion criteria
Exclusion criteria: (1) patients who used immunosuppressive drugs 1 week before blood collection; (2) acute infection occurred within 1 month before enrollment; (3) known etiology or pathological manifestations of airflow-limited diseases such as bronchiectasis, tuberculosis, lung cystic fibrosis and tumors; and (4) combined with serious heart, liver, kidney, hematopoietic system, endocrine system and other primary diseases, or mental illness and skin disease.
Collection and processing of blood specimens
On the next day after the patient was enrolled, 5 mL of fasting elbow venous blood was taken in the morning, centrifuged (centrifugal radius 5 cm, rotation speed 3,000 r/min) for 10 min, and the supernatant was aspirated and stored in a − 70 °C refrigerator.
Enzyme-linked immunosorbent assay
The concentration of IL-32 (Lanji Biological Co., Ltd., Shanghai, China) in serum was measured by sandwich-type ELISA according to the method provided by the kit: (1) added sample dilution of 100 μL on test samples and standard samples, and incubated for 2 h at room temperature; (2) after washing, added test antibody of 100 μL and incubated for 2 h at 37 °C; (3) after washing, added streptavidin-HRP working solution of 100 μL and incubated at room temperature for 20 min in the dark; (4) after washing, added substrate solution of 100 μL and incubated at room temperature for 20 min in the dark; (5) added stop solution of 50 μL and thoroughly mixed; (6) the optical density of each well was measured with a microplate reader set at 450 nm.
Modified British Medical Research Council (mMRC) score
Dyspnea of patients was evaluated by the modified british medical research council (mMRC) scale that divided into 5 points15. 0 points: dyspnea will only occur after excessive activity; 1 point: dyspnea will occur after rapid walking; 2 points: dyspnea is more likely to occur during normal walking than normal people; 3 points: need to stop and breath after walking about a few minutes on even ground; 4 points: obvious breathing difficulty that affects normal life and work.
Objective evaluation
All included patients must undergo three lung function tests before collecting the specimens and cannot be performed on the same day (three days before including into the study, the lung function test was performed once a day). The average of the three tests was calculated as statistical data. This study selected two data directly related to the diagnosis and classification of COPD: FEV1/FVC and FEV1% predicted.
Statement for strengthening the reporting of observational studies in epidemiology (STROBE)
This study was a retrospective case–control study and was divided into seven sections: headings, abstracts, background presentations, methods, results, discussion, and other information. The study clearly described the grouping of the study and the sample size, study design, and test indicators. The statistical methods were described in detail, and the systematic errors and data offsets of the variables were analyzed. The data of the study were detailed in the results section, and the main findings of the study were explained in discussion. The keywords and terminology used in this study help to ensure the correct indexing of article in the electronic database.
Statistical processing of research data
The statistical software used in this study included IBM SPSS Statistics and MedCalc statistical software. The count data was expressed in terms of rate and analyzed by chi-square test and Fisher exact probability method. The measurement data was expressed in the form of mean ± standard deviation, and the comparison between groups was performed by analysis of variance and T test. The receiver operating characteristic (ROC) curve was used to determine the threshold of observed indicator and evaluate the diagnostic efficacy. Levene's Test for Equality of Variances was performed to determine whether the data is from a normal distribution or not. A statistical p value of less than 0.05 was considered to be statistically significant.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1:
-
The value of forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- GOLD:
-
The Global Initiative for Chronic Obstructive Pulmonary Disease
- ICAM:
-
Cell adhesion factor
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IL-32:
-
Interleukin 32
- IL-18:
-
Interleukin 18
- NF-κB:
-
Nuclear transcription factor kappa B
- mMRC:
-
The modified british medical research council
- p38MAPK:
-
P38 mitogen-activated protein kinase
- ROC:
-
Receiver operating characteristic
- STROBE:
-
Statement for strengthening the reporting of observational studies in epidemiology
- TNF-α:
-
Tumor necrosis factor-α
References
Vogelmeier, C. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 195, 557–582. https://doi.org/10.1164/rccm.201701-0218PP (2017).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 394, 1145–1158. https://doi.org/10.1016/s0140-6736(19)30427-1 (2019).
Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187, 347–365. https://doi.org/10.1164/rccm.201204-0596PP (2013).
Cortopassi, F., Gurung, P. & Pinto-Plata, V. Chronic obstructive pulmonary disease in elderly patients. Clin. Geriatr. Med. 33, 539–552. https://doi.org/10.1016/j.cger.2017.06.006 (2017).
Roos, A. B. et al. IL-17A and the promotion of neutrophilia in acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 192, 428–437. https://doi.org/10.1164/rccm.201409-1689OC (2015).
Bellinger, C. R. & Peters, S. P. Outpatient chronic obstructive pulmonary disease management: Going for the GOLD. J. Allergy Clin. Immunol. Pract. 3, 471–478. https://doi.org/10.1016/j.jaip.2015.04.010 (2015) (quiz 479-480).
Caramori, G., Adcock, I. M., Di Stefano, A. & Chung, K. F. Cytokine inhibition in the treatment of COPD. Int. J. Chro. Obstruct. Pulmon. Dis. 9, 397–412. https://doi.org/10.2147/copd.s42544 (2014).
Gasiuniene, E., Lavinskiene, S., Sakalauskas, R. & Sitkauskiene, B. Levels of IL-32 in serum, induced sputum supernatant, and bronchial lavage fluid of patients with chronic obstructive pulmonary disease. COPD 13, 569–575. https://doi.org/10.3109/15412555.2016.1145201 (2016).
Eapen, M. S., Myers, S., Walters, E. H. & Sohal, S. S. Airway inflammation in chronic obstructive pulmonary disease (COPD): A true paradox. Expert Rev. Respir. Med. 11, 827–839. https://doi.org/10.1080/17476348.2017.1360769 (2017).
Kim, S. H., Han, S. Y., Azam, T., Yoon, D. Y. & Dinarello, C. A. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity 22, 131–142. https://doi.org/10.1016/j.immuni.2004.12.003 (2005).
Kudo, M. et al. Oxidative stress induced interleukin-32 mRNA expression in human bronchial epithelial cells. Respir. Res. 13, 19. https://doi.org/10.1186/1465-9921-13-19 (2012).
Hong, J. T. et al. Interleukin 32, inflammation and cancer. Pharmacol. Therap. 174, 127–137. https://doi.org/10.1016/j.pharmthera.2017.02.025 (2017).
Rabe, K. F. & Watz, H. Chronic obstructive pulmonary disease. Lancet 389, 1931–1940. https://doi.org/10.1016/s0140-6736(17)31222-9 (2017).
Duffy, S. P. & Criner, G. J. Chronic obstructive pulmonary disease: Evaluation and management. Med. Clin. N. Am. 103, 453–461. https://doi.org/10.1016/j.mcna.2018.12.005 (2019).
Launois, C. et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: A pilot study. BMC Pulmon. Med. 12, 61–61. https://doi.org/10.1186/1471-2466-12-61 (2012).
Mohamed Hoesein, F. A., Zanen, P. & Lammers, J. W. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: An evidence-based review. Respir. Med. 105, 907–915. https://doi.org/10.1016/j.rmed.2011.01.008 (2011).
Rong, Y., Xiang, X. D., Li, Y. M., Peng, Z. Y. & Li, J. X. IL-32 was involved in cigarette smoke-induced pulmonary inflammation in COPD. Clin. Respir. J. 9, 430–435. https://doi.org/10.1111/crj.12157 (2015).
Calabrese, F. et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178, 894–901. https://doi.org/10.1164/rccm.200804-646OC (2008).
Dinarello, C. A. & Kim, S. H. IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis. 65(Suppl 3), iii61–iii64. https://doi.org/10.1136/ard.2006.058511 (2006).
Acknowledgements
We appreciate the great help of Dr. W DL and Miss Z M as interviewers.
Author information
Authors and Affiliations
Contributions
B.X.R., T.F., C.X.R., W.L., K.L. and H.L. participated in the design and coordination of the study, carried out the critical appraisal of studies, statistical analysis of studies and wrote the manuscript. All authors have read and approved the manuscript, and ensure that this is the case.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rong, B., Fu, T., Rong, C. et al. Correlation between serum IL-32 concentration and clinical parameters of stable COPD: a retrospective clinical analysis. Sci Rep 10, 12092 (2020). https://doi.org/10.1038/s41598-020-69000-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69000-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.