Abstract
Robot-Assisted Radical Prostatectomy (RARP) is one of the standard treatment options for prostate cancer. However, controversy still exists on its added value. Based on a recent large-sample retrospective cluster study from the Netherlands showing significantly improved long-term urinary functioning after RARP compared to Laparoscopic RP (LRP), we evaluated the cost-effectiveness of RARP compared to LRP. A decision tree was constructed to measure the costs and effects from a Dutch societal perspective over a ~ 7 year time-horizon. The input was based on the aforementioned study, including patient-reported consumption of addition care and consumed care for ergonomic issues reported by surgeons. Intervention costs were calculated using a bottom-up costing analysis in 5 hospitals. Finally, a probabilistic-, one-way sensitivity- and scenario analyses were performed to show possible decision uncertainty. The intervention costs were €9964 for RARP and €7253 for LRP. Total trajectory costs were €12,078 for RARP and €10,049 for LRP. RARP showed higher QALYs compared to LRP (6.17 vs 6.11). The incremental cost-utility ratio (ICUR) was €34,206 per QALY gained, in favour of RARP. As a best-case scenario, when RARP is being centralized (> 150 cases/year), total trajectory costs decreased to €10,377 having a higher utilization, and a shorter procedure time and length of stay resulting in an ICUR of €3495 per QALY gained. RARP showed to be cost-effective compared to LRP based on data from a population-based, large scale study with 7 years of follow-up. This is a clear incentive to fully reimburse RARP, especially when hospitals provide RARP centralized.
Similar content being viewed by others
Introduction
Radical prostatectomy is recommended as one of the front-line treatments for men diagnosed with localized prostate cancer who have a life expectancy greater than 10 years1,2. In many countries, this procedure is currently performed using Robot-Assisted Radical Prostatectomy (RARP), showing improvements compared to Open (ORP) and Laparoscopic (LRP) radical prostatectomy in urinary incontinence, erectile functioning, hospital stay, and blood loss3,4,5, but showing no benefits on oncological outcomes6. Additionally, RARP showed improved ergonomics compared to ORP and LRP7. However, based on the current evidence base, systematic reviews and meta-analyses concluded that the quality of the evidence is too limited to draw definite conclusions on the advantages of RARP compared to LRP8,9,10,11. For the Dutch National Health Care Institute and many other national reimbursement bodies, this is the reason to reimburse RARP not for its actual costs but for costs of ORP or LRP. Therefore, hospitals are faced with substantial additional costs, money that otherwise could be used for improvements in quality of care within a hospital.
Aiming at filling this research gap, a retrospective cluster study was conducted evaluating real-world data from 12 hospitals in the Netherlands (n = 1370) to evaluate long-term (median follow-up of 7.08 years) functional and oncologic outcomes and besides evaluate perioperative outcomes, and healthcare usage12. This study showed similar survival and oncologic outcomes, but better perioperative outcomes and significantly improved urinary functioning after RARP compared to LRP.
As a part of this retrospective cluster study, the present analysis aimed to comprehensively evaluate the intervention costs of RARP and LRP, and evaluate the cost-effectiveness of RARP compared to LRP from a Dutch societal perspective.
Methods
Research design and study sample
The design of this study follows the aforementioned retrospective cluster study12. In total 1370 patients were included undergoing either RARP or LRP between 2010 and 2012 in 12 hospitals in the Netherlands12. In this study, data were collected at one moment in time at least 5 years after surgery.
A decision tree was constructed in Microsoft Excel (Supplement A) starting with prostate cancer patients undergoing RARP or LRP. As no significant differences in oncologic outcomes and prostate cancer-specific survival were found12, the analysis focussed on functional outcomes. After RARP and LRP, patients could end up in the following health states: “continent and potent”, “continent and impotent” and “incontinent and impotent”.
The analysis was performed from a societal perspective in the Netherlands and the time horizon corresponds with the median follow-up period of 7.08 (range: 5.27 – 9.86) years12.
Input parameters
All input parameters are presented in Table 1.
Transition probabilities
To define whether a patient ended-up in a certain health state the following definitions were used: patients using no pads (EPIC-26 question 27) were considered continent, patients having a score of ≥ 17 on the Sexual Health Inventory for Men (SHIM) questionnaire were considered potent. Since no cut-off value is known for the EPIC-26 Sexual domain (primary outcome of the retrospective cluster study) to define patients having erectile dysfunction, the SHIM questionnaire was also included in the survey12. Supplement B shows the observed scores on the SHIM. The analysis assumed that patients were in those states for the complete time horizon.
As the combination of being incontinent and potent was not common according to our experts and this group was too small to perform separate analyses on (2.6%), this combination was not taken into account.
We also incorporated the risk of having complications, receiving homecare after surgery, use of additional care for incontinence and erectile dysfunction complaints directly after surgery (e.g. physiotherapy, sphincter placement), and for a longer period (e.g. pad use and pharmaceuticals)12.
Utility values
Utilities, values between 0 and 1 where a higher score indicates better health, were evaluated by the EQ5D-5L questionnaire. For each health state, a utility value was calculated (Table 1). The utility value was assumed to be stable over the follow-up period. The utility values were multiplied with the median follow-up time of 7.08 years to obtain the Quality Adjusted Life Years (QALYs).
Surgeon effects
As part of the retrospective study, a questionnaire (Supplement C) was distributed among surgeons (n = 20) that operated in the selected hospitals between 2010 and 2012 evaluating complaints of back and neck pain after or related to LRP and RARP. Supplement D shows the results of the questionnaire, and Supplement E describes how these effects were translated in monetary values to incorporate the effects in the analysis per treatment arm.
Intervention costs
The intervention costs were evaluated bottom-up by an Activity-Based Costing (ABC) analysis in 5 hospitals, 2 performing LRP, and 3 performing RARP15. The following cost categories were included: personnel, material, use of the OR, medical devices, hospitalization, and overhead costs. Because an additional lymph node dissection (LND) resulted in a longer procedure time, and the percentage differed between interventions12, the costs were calculated with and without LND. The cost categories personnel, material, and medical devices were evaluated per hospital. The costs for using the OR were based on a previous study from a Dutch perspective16. The hospitalization costs were calculated by taking the average length of stay per intervention multiplied with the reference costs for an admission day13. Finally, a weighted mean of the intervention costs with and without LND was calculated12. Table 2 shows the input parameters for the intervention costs. In Supplement E more detailed information for the calculation of several cost categories (e.g. health state costs, homecare costs) is provided.
Costs of additional care directly after surgery
Costs for complications were based on expert opinion and a previous evaluation by National Institute for Health and Care Excellence14. For homecare costs, a weighted average of the unit costs for personal care, and nursing care was calculated13.
For costs using additional care for complaints of incontinence and erectile dysfunction after surgery, the activities and/or pharmaceuticals taking into account the duration and/or frequency of activities were linked to unit costs or costs for DRGs which were corrected for inflation13,18 (Table 1). For pharmaceuticals, an initial starting dose of 5 tablets or injections was assumed based on expert opinion.
Health state costs
The health state costs included the use of pads and pharmaceuticals used for erectile dysfunction complaints (see Supplement E for more information).
Analysis and sensitivity analyses
In the analysis, the costs were discounted at a rate of 4%, and effects at a rate of 1.5% according to Dutch guidelines. The outcome of the decision tree is the incremental cost-utility ratio (ICUR) calculated by dividing the incremental costs by the incremental QALYs. Furthermore, a Deterministic Sensitivity Analysis (DSA) and a Probabilistic Sensitivity Analysis (ProbSA) were performed to evaluate the impact of parameter uncertainty. For the DSA, all parameters were varied over their upper and lower limits to evaluate the impact on the ICUR. Besides, two different definitions of having no erectile dysfunction (SHIM > 22) and being continent (0–1 pad used) were evaluated.
For the ProbSA, Table 1 shows the distributions used for the parameters in the Monte Carlo simulation (drawing 1000 random samples). All potential outcomes are plotted in a cost-effectiveness (CE-) plane. Furthermore, cost-effectiveness acceptability curves (CEAC) were drafted, indicating the probability that RARP is cost-effective compared to LRP given a certain Willingness To Pay (WTP) ratio. In the Netherlands, the informal WTP ratio is €80,000 per QALY19.
Scenario analysis
Finally, in a scenario analysis, three scenarios were evaluated. The first scenario evaluated the best-case scenario (centralization) by evaluating data from the two hospitals performing > 150 RARPs per year, including potential effects on clinical outcomes. Supplement F shows the detailed calculation and input used for this scenario. In the second scenario, the same intervention costs were included but the potential improved clinical outcomes were not taken into account as the accompanied study showed no linear relationship between hospital volume and improved functional outcomes12. In the third scenario, the Da Vinci robot was also used for other indications, evaluating the ICUR over a range of 100 to 850 procedures a year, by only adjusting the medical device costs.
Ethics approval and consent to participate
The study was approved by the medical ethical committee of the Netherlands Cancer Institute and was judged as a “non-WMO-applicable” research. Patients completed an informed consent form, which explained how their data would be used and reported. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Reporting guidelines
The CHEERS guideline was used.
Results
Base case analysis results
Total intervention costs were €9,964 for RARP and €7,253 for LRP. The categories medical devices (26%) and material (28%) contributed the most to the intervention costs of RARP. For LRP, the categories material (30%-35%), personnel (18%), and hospitalization (22%-29%) contributed the most.
Total trajectory costs were €12,078 for RARP and €10,049 for LRP. Regarding the follow-up costs, incontinence complaints accounted for the largest difference between LRP and RARP (€629) (Table 3). Total QALYs found for RARP were 6.17 and 6.11 after LRP. Showing incremental costs of €2,029 and incremental QALYs of 0.059 for RARP. RARP shows to be cost-effective at an ICUR of €34,206 as this is below the informal WTP threshold of €80,000 (Table 4).
Sensitivity analyses
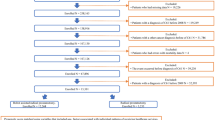
Figure 1 shows that the ICUR was most sensitive to uncertainty surrounding the utility values, intervention costs, and the two other definitions used. Although using another definition for incontinence (€44,596) and erectile dysfunction (€42,867) would show a substantial higher ICUR, it did not alter our conclusion. Uncertainty surrounding other parameters such as surgeon effects and additional care used for incontinence and erectile dysfunction had a limited effect.
Results from the one-way sensitivity analysis. This figure presents the results of the deterministic one-way sensitivity analysis. This figure shows the influence of the observed uncertainty (lower and upper value) surrounding a specific parameter on the main outcome measure. All parameters starting with a “p” indicate a probability. From this figure we learn that the uncertainty surrounding the intervention costs, definitions and utility value showed the largest deviation from the base case ICUR. However this uncertainty does not affect our conclusion. ICUR = incremental cost-utility ratio. * the uncertainty from this parameter was a combined value, the uncertainty surrounding the chance of using 1, 2 and 3 or more pads were changed at the same time. The SE surrounding these parameters can be found in Table 1.
The ProbSA showed that all possible outcomes indicate that RARP is more effective at higher costs (Fig. 2). According to the CEAC, RARP had a 99.8% probability to become cost-effective at a WTP threshold of €80,000.
Results from the probabilistic sensitivity analysis. (a) presents all potential outcomes given the distribution surrounding the parameter. The trend lines show the WTP thresholds. All potential outcomes are below the WTP threshold of €80,000. The majority of outcomes also fall below the WTP threshold of €50,000. (b) shows the probability of RARP being cost-effective, given a certain WTP threshold. The probability of RARP being cost-effective at a WTP threshold of €80,000 is 99.8%.
Scenario analyses
Table 4 shows the results of scenario 1 and 2. Total trajectory costs of scenario 1 were €10,377 and we found 6.20 QALYs for RARP, resulting in an ICUR of €3,495. For scenario 2, we found total trajectory costs of €10,600 and 6.17 QALYs, resulting in an ICUR of €9,291. Figure 3 shows that when a hospital performs ≥ 250 procedures with the Da Vinci robot, the ICUR comes below €20,000, when a hospital has ≥ 800 procedures a year, RARP is becoming cost-saving compared to LRP.
Results from scenario 3. This figure presents the incremental cost-utility ratio (ICUR) when the Da Vinci is used more often. For example when also used for other indications. Showing an ICUR below €20,000 when ≥ 250 procedures are performed per year with the Da Vinci robot. When the robot is fully used, RARP even shows the potential to be cost-saving compared to LRP.
Discussion
RARP showed to be cost-effective compared to LRP when evaluating long-term functional outcomes, presenting an ICUR of €34,206. These results strengthen the conclusions from the clinical study showing that RARP was more effective compared to LRP on the long-term12. These results can be used to inform reimbursement decisions of RARP.
The costs found for RARP (€9,964) and LRP (€7,253) were in line with previously published estimates20,21. Compared to LRP, the OR costs, personnel costs, and hospitalization costs were lower for RARP due to shorter procedure times and length of stay. In evaluating the intervention costs of RARP we created a rather negative scenario by assuming the use of the Da Vinci robot only for prostatectomies, although many hospitals use the robot in multiple indications where it also suggests to be cost-effective22,23. When increasing the utilization of the robot, the ICUR decreased substantially because of lower per-patient costs as seen in the scenario analysis. Based on our data, centralization of RARP (Table 4) resulted in a decreased length of stay, shorter procedure times, and better outcomes, as has been suggested by literature24. We should mention that these scenarios represent a best case example: results from a large volume hospital (> 150 procedures/year) and experienced surgeons, showing ICURs between €3,495 and €9,291. The effect of centralization on the cost-effectiveness may even be underestimated because we evaluated data from the early introduction phase of the Da Vinci robot25 and outcomes are expected to improve with surgeon experience26,27. Finally, as the material costs are a large driver of the intervention costs, critical appraisal of the instruments used per surgery may be useful. This could result in a cost reduction of ~ €250 per surgery28, with substantial influence on the cost-utility (Fig. 1).
The influence of surgeon effects on the cost-effectiveness was limited, although surgeons experienced substantially more pain complaints after LRP compared to RARP (69% vs 21%) (Supplement C). As similar attempts to incorporate ergonomic differences of interventions on physicians in cost-effectiveness analyses are scarce, we (pragmatically) translated the costs per surgeon having sick leave to costs per patient. In this method the costs for one surgeon having sick leave was divided over ± 38 patients. Although we used the most common approach to incorporate ergonomic effects as financial effect29, it could be argued that our approach underestimates its impact, especially when one would adopt a hospital perspective.
The QALY values identified for both interventions were rather high, representing a positive outcome for both treatment options. The QALY difference found, in favor of RARP, was neither statistically nor clinically relevant which is in line with the clinical results where the authors identified no statistically significant difference on overall QALYs measured with the EQ5D-5L12. Contrary, they showed a statistically significant and clinically relevant difference on urinary functioning (measured with the EPIC-2612). This can be explained by the fact that the EQ5D-5L is not a disease specific questionnaire and therefore less sensitive to specific functional problems. As urinary functioning is an important functional outcome after RP we consider both on the clinical analysis and on the present analysis that the effectiveness is in favor of RARP.
Our findings and conclusions seem to be in line with previous literature showing that RARP was more costly ($7,504–$9,737) compared to LRP ($6,320–$10,991), resulting in ICURs ranging between $28,801–$31,67321. Comparison with the findings from another review (including 38 cost-effectiveness studies) was more challenging because in these studies various methods were used to incorporate the costs (e.g. evaluation of the costs based on cost-to-charge ratios or hospital charges) and/or authors only presented incremental costs or savings11. However, in general, their results seem to point in the same direction: RARP could be cost-saving when optimal outcomes can be achieved, and the medical equipment is optimally used11. Yet, we should note that when the cost-effectiveness of RARP was compared to ORP, RARP is expected to show a smaller chance to be cost-effective, as the costs of ORP are lower compared to LRP11,21 but outcomes are expected to be similar to LRP30.
The strength of the present analysis is that it is the first analysis comparing RARP to LRP using long-term functional outcome data and incorporating additional care for complaints of incontinence and erectile dysfunction. Besides, this is one of the few analyses adopting a societal perspective11, and as far as we know, the first analysis incorporating costs related to homecare and ergonomic complaints of surgeons. A final strength is the bottom-up cost analysis of the intervention and follow-up costs as this provides an accurate and transparent overview of the costs31.
Several limitations should be acknowledged. First, the generalizability of our results may be limited by the focus on the Dutch healthcare system. We, therefore, presented all cost input parameters transparently to enable calculation of reliable estimates for other countries as well. Furthermore, the cost-effectiveness of RARP may be underestimated because we had no data on the recovery of functional outcomes in the years after surgery, and the recovery duration was suggested to be in favor of RARP32,33. Also we did not include costs of hormonal therapy, although a higher proportion of patients received hormonal treatment after LRP compared to RARP12. Contrary, the functional outcomes found for LRP could be underestimated due to the chosen time frame, since the larger hospitals – having more advanced urologists on average – are expected to have shifted earlier to RARP. However, incorporating several confounders in the clinical analysis, did not alter our conclusion12, for which we are confident that our results point in the right direction.
We conclude that RARP is cost-effective compared to LRP when evaluating long-term health and economic effects at most acceptable WTP ratios. When RARP is centralized and surgeons are experienced with the Da Vinci robot and/or the Da Vinci robot is used in multiple indications, RARP becomes cost-effective at all WTP ratios and has the potential to be cost-saving. Therefore, our results are a clear incentive to fully reimburse RARP, especially when hospitals provide RARP centralized.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABC:
-
Activity based costing
- CEA:
-
Cost-effectiveness analysis
- CE:
-
Cost-effectiveness
- CEAC:
-
Cost-effectiveness acceptability curve
- DSA:
-
Deterministic sensitivity analysis
- EPIC-26:
-
Expended prostate index composite short form 26
- ICUR:
-
Incremental cost utility ratio
- LND:
-
Lymph node dissection
- LRP:
-
Laparoscopic radical prostatectomy
- ORP:
-
Open radical prostatectomy
- ProbSA:
-
Probabilistic sensitivity analysis
- RARP:
-
Robot-assisted radical prostatectomy
- SHIM:
-
Sexual health inventory for men
- WTP:
-
Willingness to pay
References
European Association of Urology. Guidelines Prostate Cancer 6. treatment. https://uroweb.org/guideline/prostate-cancer/#6_6.
NHS. Guidelines for the management of prostate cancer. https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/05/guidelines-for-the-management-of-prostate-cancer.pdf (2019).
Basto, M. et al. Patterns-of-care and health economic analysis of robot-assisted radical prostatectomy in the Australian public health system. BJU Int. 117, 930–939 (2016).
Nyberg, M. et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur. Urol. Oncol. 1, 353–360 (2018).
Herlemann, A. et al. Community-based outcomes of open versus robot-assisted radical prostatectomy. Eur. Urol. 73, 215–223 (2018).
Ritch, C. R. et al. Biochemical recurrence-free survival after robotic-assisted laparoscopic vs open radical prostatectomy for intermediate- and high-risk prostate cancer. Urology 83, 1309–1315 (2014).
Bagrodia, A. & Raman, J. D. Ergonomic considerations of radical prostatectomy: physician perspective of open, laparoscopic, and robot-assisted techniques. J. Endourol. 23, 627–633 (2009).
Ramsay, C., Pickard, R., Robertson, C., et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol. Assess. (Rockv). 16, 313 (2012).
Health Quality Ontario. Ontario health technology assessment series: Robotic surgical system for radical prostatectomy: A health technology assessment. Ont. Health Technol. Assess. Ser. 17, 1–172 (2017).
Ilic, D., Evans, S. M., Allan, C. A., et al. Laparoscopic and robot-assisted vs open radical prostatectomy for the treatment of localized prostate cancer: a Cochrane systematic review. BJU International (2018).
Schroeck, F. R. et al. Cost of new technologies in prostate cancer treatment: Systematic review of costs and cost effectiveness of robotic-assisted laparoscopic prostatectomy, intensity-modulated radiotherapy, and proton beam therapy. Eur. Urol. 72, 712–735 (2017).
Lindenberg, M. A., Retèl, V. P., Kieffer, J. M., et al. Long-term functional outcomes after robot-assisted prostatectomy compared to laparoscopic prostatectomy: Results from a national retrospective cluster study. Eur. J. Surg. Oncol. (2021).
Hakkaart-van Roijen, L., van der Linden, N., Bouwmans, C., et al. Manual for cost research: methods and standard cost prices for economic evaluations in health care. (2015).
Ramsay, C. et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol. Assess. 16, 313 (2012).
Lievens, Y., Van Den Bogaert, W. & Kesteloot, K. Activity-based costing: A practical model for cost calculation in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57, 522–535 (2003).
Patel, S., Lindenberg, M., Rovers, M. M., et al. Understanding the costs of surgery: A bottom-up cost analysis of both a hybrid operating room and conventional operating room. Int. J. Heal. Policy Manag. (2020).
Dutch Federation of Academic Medical Centers. Collective labor agreement 2018–2020 for academic medical centers. https://www.nfu.nl/img/pdf/19.2084_Umcs_Uitgave_2019_NL_Cao_umc_2018-2020_v3-4-2019.pdf (2018).
Dutch Healthcare Authority (NZa). Open data from the Dutch health authority on DRGs (title is translated).
Versteegh, M. M., Ramos, I. C., Buyukkaramikli, N. C., et al. Severity-adjusted probability of being cost effective. Pharmacoeconomics (2019).
Close, A. et al. Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: A health technology assessment from the perspective of the UK natio. Eur. Urol. 64, 361–369 (2013).
Tandogdu, Z. et al. A systematic review of economic evaluations of the use of robotic assisted laparoscopy in surgery compared with open or laparoscopic surgery. Appl. Health Econ. Health Policy 13, 457–467 (2015).
Kukreja, J. B. et al. Cost-effectiveness of robot-assisted radical cystectomy using a propensity-matched cohort. Eur. Urol. Focus 6, 88–94 (2020).
Ho, C., Tsakonas, E., Tran, K., et al. Robot-assisted surgery compared with open surgery and laparoscopic surgery: Clinical effectiveness and economic analyses. Canadian Agency for Drugs and Technologies in Health (2011).
Xia, L. et al. associations between hospital volume and outcomes of robot-assisted radical prostatectomy. J. Urol. 203, 926–932 (2019).
Abrishami, P., Boer, A. & Horstman, K. Understanding the adoption dynamics of medical innovations: Affordances of the da Vinci robot in the Netherlands. Soc. Sci. Med. 117, 125–133 (2014).
Fossati, N. et al. Assessing the impact of surgeon experience on urinary continence recovery after robot-assisted radical prostatectomy: Results of four high-volume surgeons. J. Endourol. 31, 872–877 (2017).
Bravi, C. A. et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: A learning curve study. J. Urol. 202, 108–113 (2019).
Ramirez, D. et al. Reducing costs for robotic radical prostatectomy: Three-instrument technique. Urology 95, 213–215 (2016).
Tompa, E., Dolinschi, R., De Oliveira, C., et al. A systematic review of workplace ergonomic interventions with economic analyses. J. Occup. Rehabil. (2010).
Nossiter, J. et al. Robot-assisted radical prostatectomy vs laparoscopic and open retropubic radical prostatectomy: functional outcomes 18 months after diagnosis from a national cohort study in England. Br. J. Cancer 118, 489–494 (2018).
Najjar, P. A., Strickland, M. & Kaplan, R. S. Time-driven activity-based costing for surgical episodes. JAMA Surg (2017).
Koike, H. et al. Health-related quality of life after robot-assisted radical prostatectomy compared with laparoscopic radical prostatectomy. J. Robot. Surg. 11, 325–331 (2017).
Huang, X. et al. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: a systemic review and meta-analysis. Surg. Endosc. 31, 1045–1060 (2017).
Acknowledgements
We want to acknowledge the five hospitals that agreed to participate in the cost analysis. Furthermore, we want to thank Intuitive Surgical for providing a research fund (round of 2019-2020) to perform the current study.
Funding
W.H. van Harten received funding from Intuitive surgical to continue and finish this study. The sponsor had no influence on the design of the study, the methodologies used and the results presented.
Author information
Authors and Affiliations
Contributions
M.L. conceptualized the research and manuscript together with W.v.H., V.R. and H.v.d.P. H.v.d.P., M.L., W.v.H. and V.R. had discussions regarding the methodology. The analysis was performed by M.L. and was supervised by W.v.H., V.R. and H.v.d.P. Data was collected and curated by M.L. The manuscript was written by M.L. under supervision of W.v.H., V.R. and H.v.d.P. The manuscript was reviewed and edited by all authors (W.v.H., V.R., H.v.d.P., F.B., C.W.). Funding for the research was acquired by efforts from M.L., V.R. and W.v.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindenberg, M.A., Retèl, V.P., van der Poel, H.G. et al. Cost-utility analysis on robot-assisted and laparoscopic prostatectomy based on long-term functional outcomes. Sci Rep 12, 7658 (2022). https://doi.org/10.1038/s41598-022-10746-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10746-3
This article is cited by
-
Robotic-assisted surgery for prostatectomy – does the diffusion of robotic systems contribute to treatment centralization and influence patients’ hospital choice?
Health Economics Review (2023)
-
A systematic review of full economic evaluations of robotic-assisted surgery in thoracic and abdominopelvic procedures
Journal of Robotic Surgery (2023)
-
Surgical outcomes and cost analysis of a multi-specialty robotic-assisted surgery caseload in the Australian public health system
Journal of Robotic Surgery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.