Abstract
Sarcopenia is associated with poor prognosis of patients with hepatocellular carcinoma (HCC). We investigated the association of skeletal muscle volume (SMV) and its change in HCC patients taking lenvatinib. In 130 HCC patients, psoas mass index (PMI) was calculated as the left–right sum of the major × minor axis of psoas muscle at the third lumbar vertebra, divided by height squared. Patients were classified into two groups (low and normal PMI) based on indices of < 6.0 cm2/m2 for man and < 3.4 cm2/m2 for women. Change in PMI per month during the lenvatinib administration period (ΔPMI/m) was calculated; and patients were classified into two groups (severe and mild atrophy) based on the ΔPMI/m rate, as ≥ 1% or < 1%, respectively. There was no significant difference in Overall survival (OS) between the low and normal PMI groups at the start of lenvatinib administration. OS was significantly lower in the severe atrophy group than in the mild atrophy group (median; 15.2 vs. 25.6 months, P = 0.005). Multivariate analysis revealed a significant association of severe atrophy with OS (hazard ratio 1.927, P = 0.031). Progressive loss of SMV is a strong predictor of poor prognosis in HCC patients taking lenvatinib.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide1. Until 2019, the clinical guidelines of the Japan Society of Hepatology (JSH)2. specified therapy with molecular targeting agents (MTAs), including sorafenib (SOR)3, regorafenib4, lenvatinib (LEN)5. and ramucirumab6, as the standard treatment for patients with unresectable HCC (u-HCC) and vascular invasion or extrahepatic metastasis. In 2020, immune checkpoint inhibitor (ICI) therapy including the combination of atezolizumab and bevacizumab7. was approved in Japan as the first-line treatment for unresectable HCC8. However, MTAs are still used as the second-line treatment and remain important because of the convenience of the administration method.
Skeletal muscle volume (SMV) loss is an important factor of sarcopenia that is associated with liver disease. Hanai et al. reported that SMV decreased by 2.2% per year in patients with liver cirrhosis, and the rate of decrease increased in parallel with the severity of cirrhosis9. Sarcopenia has a negative impact on mortality in patients with chronic liver disease (CLD)10. and u-HCC treated with SOR11,12. and LEN13,14. Several studies have reported that decline of SMV during the treatment period is a critical factor in patients with HCC treated with SOR or LEN12,15. However, these reports estimated change in SMV only over a short period from the start of LEN.
We have previously reported that change in the skeletal muscle index (SMI) was a risk factor in the patients with HCC patients treated with transarterial chemoembolization16. In that study, we used the psoas muscle index (PMI) as a simpler method for determining SMI, calculated as the vertical × horizontal diameter of the psoas muscle at the third lumbar vertebra (L3) on computed tomography (CT), divided by height squared17.
The present study aimed to evaluate the long-term effects of SMV loss in patients with u-HCC by examining changes in PMI at the start of LEN administration (PMI-Pre), at the time of the first judgement of therapeutic effect (PMI-1st), and at the end of LEN administration (PMI-Post).
Results
Baseline characteristics
Table 1 shows the baseline (Pre) characteristics of patients. Median age of the 130 patients was 70 years (range, 38–89 years) and 107 patients (82.3%) were men. The median observation period after the start of LEN administration was 11.0 months (range, 1.9–32.7 months). The median HCC treatment period before administration of LEN was 14.8 months (range, 0–132.8 months). The causative disease of HCC was hepatitis B virus (HBV; n = 28), hepatitis C virus (HCV; n = 35), alcohol (n = 37), non-alcoholic fatty liver disease (NAFLD; n = 26), and other (n = 4). Fourteen patients (10.8%) had moderate loss of liver function reserve (Child–Pugh class B). The median albumin-bilirubin (ALBI) score was − 2.38 and 51 patients (39.2%) had loss of liver function reserve (modified ALBI [mALBI] grade 2b or 3). Forty-nine patients (37.7%) had macrovascular invasion (MVI) and 36 patients (27.7%) had extrahepatic metastasis. Extrahepatic metastasis was to lymph nodes (n = 11), lung (n = 19, bone (n = 7), adrenal glands (n = 7), and carcinomatous peritonitis (n = 5). One-hundred-eleven patients (85.3%) had received other treatment prior to administration of LEN. The breakdown of treatment was as follows: surgery (n = 25), local treatment (n = 12), hepatic intra-arterial therapy (n = 70), radiation therapy (n = 2), and other treatment (n = 2). The median administration period was 7.7 months (range, 0.3–32.7 months). The initial dose of LEN was reduced in 37 patients and the dose was reduced during LEN treatment in 64 patients. Ninety-seven patients (74.6%) discontinued LEN, most commonly due to progressive disease (PD) (n = 44). Fifty-two patients (40.0%) died during the follow-up period.
The median PMI-Pre by etiology was 5.84 cm2/m2 for HBV, 5.52 cm2/m2 for HCV, 6.39 cm2/m2 for alcohol, 5.32 cm2/m2 for NAFLD, and 5.16 cm2/m2 for other. The median change in PMI per month (ΔPMI/m) rate (ΔPMI*100/PMI-Pre) by etiology was 0.55% for HBV, 0.56% for HCV, 1.35% for alcohol, 2.67% for NAFLD, and 1.87% for other. There was no significant difference in PMI-Pre or ΔPMI/m rate in terms of etiology (P = 0.214 and P = 0.083, respectively).
Change in PMI during the LEN administration period
For all patients, median PMI-Pre, PMI-1st, and PMI-Post were 5.62, 5.61, and 4.94 cm2/m2, respectively. A significant difference was found between each of PMI-Pre and PMI-1st (P = 0.014), PMI-Pre and PMI-Post (P < 0.001), and PMI-1st and PMI-Post (P < 0.001) (Fig. 1). Median change in PMI per month during the LEN administration period (ΔPMI/m) was 0.05 cm2/m2 (interquartile range [IQR], − 0.01 to 0.19). And median rate of decrease of PMI per month (ΔPMI/m rate) was 0.99% (IQR, − 0.12 to 3.80), respectively (Table 1).
Comparison between the low and normal PMI groups at baseline
Of the 130 patients, 63 (48.5%) were classified as having low PMI. There was no significant difference between the low and normal PMI groups in terms of age, liver function reserve (median ALBI score, − 2.42 vs. − 2.35; P = 0.889), stage of HCC including MVI and extrahepatic metastasis, or treatment (the presence, absence, or breakdown of other treatments received before LEN treatment). Significantly more men had low PMI than normal PMI (P = 0.005) (Table 2). Fewer patients in the low PMI group (PMI < 6.0 cm2/m2 for men and < 3.4 cm2/m2 for women) received other treatments after discontinuing LEN compared with those in the normal PMI group (PMI ≥ 6.0 cm2/m2 for men and ≥ 3.4 cm2/m2 for women) (P = 0.006). There was no significant difference in ΔPMI/m (P = 0.226) or in ΔPMI/m rate (P = 0.539) between the low and normal PMI groups.
Assessment of prognosis
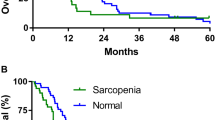
For all patients, median survival time (MST) was 21.8 months, and the survival rate at 12 and 24 months was 70.2% and 45.9%, respectively. There was no significant difference in MST between the low and normal PMI groups (18.1 month vs. 22.0 months, P = 0.199) (Fig. 2A).
Univariate analysis revealed the following as significantly associated with overall survival (OS, months): mALBI grade 2b or 3, tumor node metastasis (TNM) stage, alpha fetoprotein (AFP) ≥ 56.7 ng/dL, receiving other treatments after discontinuing LEN, and severe muscle atrophy (ΔPMI/m ≥ 1%) (Table 3). Multivariate analysis indicated the following as significantly associated with OS: severe muscle atrophy (hazard ratio [HR], 1.927; 95% confidence interval [CI], 1.061–3.500; P = 0.031), mALBI grade 2b or 3 (HR, 2.431; 95% CI, 1.333–4.434; P = 0.003), and AFP ≥ 56.7 ng/dL (HR, 2.563; 95% CI, 1.352–4.859; P = 0.004). Prognosis was significantly worse in the severe atrophy group (n = 65) than in the mild atrophy group (ΔPMI/m < 1%) (MST: 15.2 months vs. 25.6 months, P = 0.005) (Fig. 2B). Survival rates at 12 and 24 months in the severe and mild atrophy groups were 56.1%/31.8% and 83.0%/58.1%, respectively.
For all patients, the median post progression survival (PPS) was 7.8 months, and the survival rate at 12 and 24 months was 35.5% and 24.8%, respectively. There was no significant difference in the median PPS between the low and normal PMI groups (7.6 month vs. 9.1 months, P = 0.481). On the other hand, PPS was significantly worse in the severe atrophy group than in the mild atrophy group (4.6 months vs. 11 months, P = 0.04).
Characteristics of the severe and mild atrophy groups during the administration period
Table 4 shows the comparison of characteristics between the mild and severe atrophy groups during the administration period. The observation period was significantly shorter in the severe atrophy group than in the mild atrophy group (median; 8.7 months vs. 13.8 months, P < 0.001). The Pre values of choline-esterase (ChE; P = 0.024) were lower in the severe atrophy group than in the mild atrophy group. In contrast, the Post values of liver function reserve including Child–Pugh and ALBI score, albumin, and ChE were significantly worse in the severe atrophy group than in the mild atrophy group.
During the LEN administration, the LEN dose was reduced in fewer patients in the severe atrophy group compared with the mild atrophy group (P = 0.033). Fewer patients in the severe atrophy group received other treatments after discontinuing LEN compared with those in the mild atrophy group (P = 0.001). There was no significant difference in other HCC-related factors such as TNM stage and tumor markers between the groups at the start or end of administration of LEN.
Adverse events
An adverse event (AEs), regardless of grade, was experienced by 123 patients (94.6%), and severe AEs (grade 3 or worse) occurred in 37 patients (28.5%). There was no significant difference in any AEs, regardless of grade, between the low and normal PMI groups (Table S1). There was a significant difference in severe hypertension between the severe and mild atrophy groups (Table S2). There was no significant difference in the rate of severe AEs between the low and normal PMI groups (28.6% vs. 28.3%, P = 0.975) or between the severe and mild muscle atrophy groups (23.1% vs. 33.8%, P = 0.14). There was no significant difference in BMI between patients with and without severe AEs (P = 0.73).
There was no difference in PMI-Pre or ΔPMI/m rate between patients who discontinued due to adverse events or progressive disease (P = 0.367 and P = 0.15, respectively).
Discussion
In this study, we assessed change in SMV in patients with HCC who were treated with LEN. We found that SMV decreased during the administration period and that change in SMV mass during the administration period was significantly associated with poor prognosis in these patients.
Sarcopenia is associated with poor prognosis in patients with cirrhosis or HCC, independent of liver function reserve9,10,11,12,13,14. Patients with HCC receive various treatments, along with assessment of both TNM stage and liver function reserve. In terms of treatment of HCC, sarcopenia is associated with a negative impact in HCC patients who undergo curative treatments such as hepatectomy and radiofrequency ablation18. Most of these previous studies assessed SMV prior to initiation of treatment. Other non-curative treatment is often continued or repeated until response failure or intolerance to treatment occurs. The results of the present study suggest that because SMV can change during non-curative treatment (Fig. 1), the impact of SMV may be less at the start of non-curative treatment compared with its impact during curative treatment. Several studies have reported no significant association between sorafenib and hepatic intra-arterial therapy with SMV loss at baseline or OS, but identified change in SMV during these treatments as a significant prognostic factor for OS15,16. Furthermore, Roch et al. reported that the dynamic change in SMI (a decrease of 5% or more of the L3 SMI) during the first eight weeks appeared as a stronger prognostic factor than sarcopenia at start of ICI19. In contrast, Imai et al. reported an association of SMV loss with poor prognosis, both at baseline and during treatment12. These findings imply that change in SMV during treatment may be a useful predictor of prognosis in patients with HCC receiving non-curative treatment, including LEN.
Several studies have identified change in SMV during treatment as an impact factor in HCC patients with treated with MTAs12,15. However, these reports did not consider the impact of long-term change in SMV. In the present study, the median administration period of LEN was 7.7 months, and the impact of long-term change in SMV over at least 6 months was investigated in more than half of the enrolled patients.
Tsung et al. reported that pre-sarcopenia was associated with worse PPS and patients who sustained normal muscle mass from the beginning of sorafenib treatment to treatment failure had better PPS than those who developed pre-sarcopenia20. In this study as well, patients in the severe atrophy group had significantly worse PPS than those in the mild atrophy group, which was similar to the previous reported results. Prevention of SMV loss was associated to the better post progression outcome.
In patients with HCC, SMV is affected by both liver function reserve and tumor-related factors in addition to age or sex21. In fact, in the present study, median PMI-Pre, PMI-1st, and PMI-Post were 5.62, 5.61, and 4.94 cm2/m2, respectively, and SMV had decreased significantly between Pre and Post. Furthermore, there was no significant difference in ΔPMI/m between the low and normal PMI groups. Similarly, Uchikawa et al. reported a significant decrease in SMV during administration of MTAs, with or without muscle volume loss at baseline22. Roch et al. suggest that cachexia (a body-weight loss of 5% or more) might be a determinant of poor outcome of ICI19. In the present study, liver function reserve was worse and ChE and albumin levels were lower in the severe atrophy group than in the mild atrophy group (Table 4). These results suggest that maintaining nutritional status and hepatic reserve prevents SMV loss. Takada et al. reported that branch-chain amino acids (BCAAs) were useful for maintaining the serum albumin level, which helped to avoid early discontinuance of sorafenib therapy23. Another study reported that in HCC patients treated with LEN, there was significant correlation between the plasma acyl carnitine-to-free carnitine ratio and change in the Brief Fatigue Inventory score, and mentioned that LEN affected carnitine insufficiency and fatigue24. Accordingly, we should consider the early introduction of nutrition therapy, including BCAAs and levocarnitine, in HCC patients treated with MTAs.
In this study, non-mALBI 1 or 2a at the start of LEN was a significant risk factor for OS (Table 3). Furthermore, the median ALBI score at baseline was − 2.48 (mALBI grade 2a) in patients with mild atrophy and − 2.31 (mALBI grade 2a, but close to 2b) in those with severe atrophy (Table 4). Hiraoka et al. reported that mALBI grade 1 or 2a were better prognostic factors in LEN treatment 25, and similar results were obtained in this study. The above findings suggest that a decrease in hepatic reserve leads to a rapid decrease in SMV, leading to a worsening of prognosis.
In the present patients with hypertension, severe AEs (grade 3 or worse) occurred more frequently in those in the mild than those in the severe atrophy group (Table S2), despite no significant difference in any grade of hypertension between the two groups. It is possible that blood pressure control was originally worse in more of the patients in the mild atrophy group compared with the severe atrophy group. There was no significant difference in any of the other AEs that affect patients’ nutritional status such as diarrhea, anorexia, or body weight loss between either of the low and normal PMI groups or the severe and mild atrophy groups. Uojima et al. have reported low SMV as a risk factor associated with severe AEs in patients treated with LEN, and that SMV was more important than body weight in those patients14. In the present study, the initial dose of LEN and dose of LEN during administration were reduced as appropriate at the discretion of each researcher, and accordingly, AEs were unlikely to occur. However, doses of LEN during administration were less reduced in patients in the severe atrophy group compared with those in the mild atrophy group. Hiraoka et al. reported that the AE of appetite loss was more frequent in patients with lower body mass index (BMI) when a set dose was used and proposed the need for dose adjustment according to the conditions26. Although the cause of our result could not be determined because the decision to reduce the dose was left to each researcher, it is possible that more appropriate volume adjustment was performed according to the patient's condition in the mild atrophy group than the severe atrophy group. In the present study, lower BMI was not a risk factor for OS (Table 3) and there was no significant difference in BMI in terms of the severity of AEs. However, BMI tended to be low in the patients with severe atrophy than in those with mild atrophy (Table 4). Furthermore, the Pre values of ChE were lower in the severe atrophy group than in the mild atrophy group. These results suggest that patients with low BMI or poor nutritional status need to adjust their LEN dose more carefully.
The present study had several limitations in addition to its retrospective design and small sample size. First, as markers of muscular strength such as hand grip strength and walking speed were not evaluated, a diagnosis of sarcopenia was impossible according to the JSH guidelines. Second, we measured PMI by manual tracing on the CT images, which could have introduced errors due to inconsistency among the researchers. Third, we evaluated nutritional status only with respect to albumin, sugar metabolism, lipid metabolism and ChE, and BCAAs supplementation was not examined. Sano et al. have reported a significant association of BCAAs levels with sarcopenia, and a significant correlation of Δleucine with ΔSMI (R = 0.256, P < 0.001)27. Okubo et al. have reported that sarcopenia was diagnosed significantly more frequently in patients with vitamin D deficiency compared with those without vitamin D deficiency28. In addition, we did not evaluate liver fibrosis by hyaluronic acid, Mac-2 binding protein glycosylated isomer, or elastography (e.g., transient elastography, magnetic resonance). To prevent sarcopenia and thus improve prognosis in HCC patients treated with LEN, prospective large-scale and interventional studies are needed to overcome the above-mentioned limitations.
In conclusion, progressive loss of SMV was associated with OS in patients with HCC during administration of LEN. Prevention of SMV loss, including nutrition therapy, is essential for improving prognosis in HCC patients treated with LEN.
Methods
Patients
We retrospectively analyzed 171 patients with advanced u-HCC who were treated with LEN between April 2018 and July 2020 at one of the following hospitals in Japan: Fukushima Medical University Hospital, Iwate Medical University Hospital, Tohoku University Hospital, Yamagata University Hospital, Akita University Hospital, Hirosaki University Hospital, and National Hospital Organization Sendai Medical Center. Patients who had received other treatments such as surgery, local therapy, hepatic intra-arterial therapy, radiation therapy, and chemotherapy including sorafenib before the start of LEN administration were included in the study. Excluded were 39 patients in whom a large amount of data were missing at the start of LEN, or in whom SMI could not be measured because magnetic resonance imaging (MRI) was performed instead of CT. Propensity score matching was performed, and 130 patients were enrolled in the study.
All enrolled patients had been treated only with LEN from the start of LEN administration (Pre) to the end of LEN administration (Post). If a patient’s liver function was good enough to permit administration of other treatments (MTAs, hepatic arterial infusion chemotherapy, or radiation therapy) at Post, these treatments were added to the regimen. The study protocol conformed to the ethics guidelines of the Declaration of Helsinki. The study protocol was reviewed and opt-out consent was approved by the Ethics Committee of Fukushima Medical University (No. 2019–233). The need to obtain informed consent from the participants was waived by the Ethics Committee of Fukushima Medical University due to the retrospective nature of the study.
Evaluation of HCC
The diagnosis of HCC was performed by CT or MRI. TNM stage was determined according to the Japanese criteria29. The size and number of HCCs were evaluated using the Up-to-7 criteria30. Therapeutic effect of LEN was assessed by CT at 1–3 months after the start of administration of LEN according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST)31. Afterwards, patients were followed up by CT every 3 months.
Assessment of liver function reserve
Liver function reserve was evaluated by the Child–Pugh classification32, ALBI score, and mALBI grade33,34.
Treatment
All patients received 4 mg/8 mg/12 mg LEN once daily based on their body weight and liver function reserve. The initial dose of LEN was reduced as appropriate, at the discretion of each researcher. When any grade 3 or worse severe AEs or any unacceptable grade 2 AEs occurred, the dose of LEN was reduced or withdrawn until symptoms subsided, according to the guidelines provided by the manufacturer. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The administration period (months) was defined as the period between the times of the start and end of LEN administration. If any of the following criteria were met, the administration of LEN was discontinued: (i) development of any unacceptable or serious AEs, (ii) progressive disease (PD) of the tumor as defined by mRECIST, (iii) worsening liver function reserve, or (iv) request by the patient to discontinue LEN.
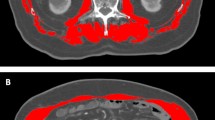
Evaluation of change in skeletal muscle volume and characteristics
The JSH guidelines for sarcopenia in liver disease recommend evaluation by SMI as the total muscle volume of four limbs measured by bioelectrical impedance analysis (BIA) divided by height squared17. A simpler method for determining SMI is by PMI, calculated as the left–right sum of the major × minor axis of the psoas muscle at L3 on CT, divided by height squared 16, which is indirectly correlated with SMI obtained using BIA17. Thus, we used PMI in the present study. We measured PMI on CT images obtained at the start of LEN administration (PMI-Pre), at the time of the first judgement of therapeutic effect (PMI-1st), and at the end of LEN administration (PMI-Post). Patients were classified into two groups based on the cut-off values specified by JSH guidelines for sarcopenia in liver disease: low PMI group (PMI < 6.0 cm2/m2 for men and < 3.4 cm2/m2 for women) and normal PMI group (PMI ≥ 6.0 cm2/m2 for men and ≥ 3.4 cm2/m2 for women)17.
As an index of progressive muscle atrophy, we calculated ΔPMI/m with the following formula: (PMI-Pre – PMI-Post)/administration period16. The rate of decrease in PMI per month (ΔPMI/m rate; ΔPMI*100/PMI-Pre) was also calculated. Since the median ΔPMI/m rate was 0.99%, we set the cutoff value to 1%. Patients were classified into two groups (severe and mild atrophy groups) according to ΔPMI/m of ≥ 1% or < 1%, respectively.
Characteristics including age, liver function reserve, stage, laboratory findings and prognosis including OS (months), MST, and survival rate were evaluated.
Propensity score matching
As this study was a retrospective study, several selection biases or cofounding factors existed. Propensity score matching was performed to exclude biases of confounding factors. We selected the following 4 factors: age, gender, ALBI score, and the stage of HCC, which were considered as prognostic factors for HCC patients by previous study21. The calculation of propensity scores determined as 0.2 times a standard deviation of all patients’ propensity scores.
Statistical analysis
Continuous variables are expressed as the median and interquartile range (IQR). Statistical analyses were performed using the χ2 test, Fisher’s exact test, the Mann–Whitney U test, Kruskal–Wallis rank sum test, or Wilcoxon signed rank test, as appropriate. OS was evaluated by Kaplan–Meier analysis, using the log-rank test. Cox proportional hazard analysis was used to determine the risk factors for OS. In the case of missing values, statistical analysis was performed with the available data. All P-values were two-tailed, and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using Easy R (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html)35.
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Parki, D. M., Bray, F., Ferlay, J. & Pisani, P. Estimating the world cancer burden: GLOBOCAN 2000. Int. J. Cancer. 94, 153–156 (2001).
Kokudo, N. et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 49, 1109–1113 (2019).
Llovet, J. M. et al. Sorafenibb in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Zhu, A. X., Finn, R. S., Galle, P. R., Llovet, J. M. & Kudo, M. Ramucirumab in advanced hepatocellular carcinoma in REACH-2: the true value of alpha-fetoprotein. Lancet Oncol. 20, e191 (2019).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Kudo, M. Recent advances in systemic therapy for hepatocellular carcinoma in an Aging Society: 2020 update. Liver Cancer. 9, 640–662 (2020).
Hanai, T. et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 46, 743–751 (2016).
Hiraoka, A. et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J. Gastroenterol. 50, 1206–1213 (2015).
Hiraoka, A. et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 47, 558–565 (2017).
Imai, K. et al. Rapid depletions of subcutaneous fat mass and skeletal muscle mass predict worse survival in patients with hepatocellular carcinoma treated with sorafenib. Cancer 11, 1206 (2019).
Hiraoka, A. et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: analysis adjusted with inverse probability weighting. J. Gastroenterol. Hepatol. https://doi.org/10.1111/jgh.15336 (2020).
Uojima, H. et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. 9, 193–206 (2020).
Yamashima, M. et al. Significance of psoas muscle thickness as an indicator of muscle atrophy in patients with hepatocellular carcinoma treated with sorafenib. Mol. Clin. Oncol. 7, 449–453 (2017).
Fujita, M. et al. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol. Res. 49, 778–786 (2019).
Nishikawa, H. et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 46, 951–963 (2016).
Chang, K. V. et al. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 7, 90–103 (2018).
Roch, B. et al. Cachexia – sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 143, 19–26 (2020).
Tsung, Y. C. et al. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci. Rep. https://doi.org/10.1038/s41598-020-75198-z (2020).
Imai, K. et al. Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: The role of liver functional reserve and tumor-related factors in loss of skeletal muscle volume. Nutrients https://doi.org/10.3390/nu9101054 (2017).
Uchikawa, S. et al. Skeletal muscle loss during tyrosine kinase inhibitor treatment for advanced hepatocellular carcinoma patients. Liver Cancer. 9, 148–155 (2020).
Takeda, H. et al. Effect of treatment with branched-chain amino acids during sorafenib therapy for unresectable hepatocellular carcinoma. Hepatol. Res. 44, 302–312 (2014).
Okubo, H. et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 15, e0229772 (2020).
Hiraoka, A. et al. Post-progression treatment eligibility of unresectable hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. 9, 73–83 (2020).
Hiraoka, A. et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions—Multicenter analysis. Cancer Med. 8, 3719–3728 (2019).
Sano, A. et al. Plasma free amino acids are associated with sarcopenia in the course of hepatocellular carcinoma recurrence. Nutrition https://doi.org/10.1016/j.nut.2020.111007 (2020).
Okubo, T. et al. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol. Res. 50, 588–597 (2020).
The Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th English edition. 26 (Tokyo: Kanehara & CO., Ltd 2020).
Mazzaferro, V. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 10, 35–43 (2009).
Lencioni, R. & Llovet, J. M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 52–60 (2010).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649 (1973).
Hiraoka, A. et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 6, 325–336 (2017).
Hiraoka, A. et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer 8, 121–129 (2019).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Acknowledgements
We would like to thank all participants for their cooperation in data collection and for providing information during the study period.
Author information
Authors and Affiliations
Contributions
M.F. conceived and designed the study; K.A., H.K., T.O., M.N., K.O, T.K., W.S., T. E., N. T. and H. N. collected the data; M.F. analyzed the data; M.F., K.A. and H.O. drafted the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, M., Abe, K., Kuroda, H. et al. Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: a multicenter study in Tohoku, Japan. Sci Rep 12, 6479 (2022). https://doi.org/10.1038/s41598-022-10514-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10514-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.