Abstract
Many second-line therapies are recently approved for patients with advanced hepatocellular carcinoma (HCC), in whom protein malnutrition is prevalent that would affect treatment outcomes. In this study, we aimed to investigate the role of pre-sarcopenia and muscle restoration in patients with sorafenib-failed advanced HCC. From August 2012 to March 2017, 385 patients who developed radiology-proven HCC progression after sorafenib treatment were enrolled in the study. Pre-sarcopenia is defined as transverse psoas muscle thickness per body height < 16.8 mm/m, which was prevalent (64.7%) in our patients. Age > 60 years, female gender, and body mass index < 22 kg/m2 were independent predictors to the development of pre-sarcopenia. Patients with muscle depletion had significantly worse post-progression survival (PPS) compared with their counterparts (median PPS: 3.8 vs. 5.8 months, p = 0.003), particularly in those with intermediate liver reserves (Child–Pugh class B or Albumin-bilirubin grade 2). Besides, pre-sarcopenia independently predicted post-progression mortality in sorafenib-failed HCC (hazard ratio: 1.340, p = 0.012). In patients who developed pre-sarcopenia before sorafenib treatment, muscle restoration was associated with a longer PPS compared with their counterparts (6.3 vs. 3.6 months, p = 0.043). In conclusion, pre-sarcopenia independently determined the outcomes of sorafenib-failed HCC. Nutrition support to restore muscle mass would prolong survival for higher-risk patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer and the fourth cause of cancer-related deaths worldwide, with increased incidence to nearly 750,000 new cases per year1,2. In general, the prognosis of HCC is relatively dismal because only 46% of patients can be diagnosed at an early stage, and most patients require systemic therapy for the unresectable, advanced-staged disease2,3. Sorafenib is the first approved systemic treatment for advanced HCC; but its effects are still modest4,5. Recently, several positive results from the first and second-line phase 3 trials enable HCC patients to new treatment options6,7,8. Maintenance of good liver reserve and performance status at disease progression, which are generally measured by Child–Pugh class, ALBI grade or Eastern Cooperative Oncology Group (ECOG) status, are important for longer survival with second-line therapy2,6,9,10.
Depletion of muscle and decline of muscle strength are common in patients with advanced liver disease and malignancies, which are reported in association with cachexia and indicated as prognostic factors for them11,12,13,14,15. A significant proportion of patients with HCC were observed as malnourished or at a high risk of malnutrition, which remarkably affected clinical outcomes16. In parallel with liver dysfunction and enlargement of tumor size, skeletal muscle decreases profoundly in patients with advance HCC17. Presence of muscle depletion can lead to physical disability and is associated with poor prognosis in these patients18,19,20. According to previous studies, pre-sarcopenia, which is defined as low skeletal muscle mass assessed by computed tomography (CT) scan, can predict the prognosis of HCC in patients treated with sorafenib18,19,20,21,22,23. However, whether this objective, quantitative surrogate marker of performance status can predict post-progression prognosis in sorafenib-failed patients is still unclear. In addition, factors linked to pre-sarcopenia must be identified. In this study, we aimed to investigate the role of muscle mass depletion in HCC patients who failed sorafenib treatment as well as the risk factors for the presence of pre-sarcopenia in these patients.
Results
Demographic characteristics of the study cohort
According to the value of transverse psoas muscle thickness per body height (TPMT/BH), which was measured from the CT scan image at the level of umbilicus, 249 patients (64.7%) showed pre-sarcopenia at the time of sorafenib treatment failure. Compared with patients with normal muscle mass, patients with the presence of pre-sarcopenia at HCC progression were significantly older (patients with pre-sarcopenia vs. patients with normal muscle mass: 64.0 ± 12.4 vs. 61.4 ± 13.5 years old, p = 0.029), predominantly female (25.7% vs. 14.0%, p = 0.009), lower in body mass index (BMI) (23.0 ± 3.8 vs. 25.0 ± 4.1, p < 0.001), more prevalent in chronic hepatitis C infection (30.1% vs. 16.9%, p = 0.005), with larger tumor size (6.6 vs. 5.5 cm, p = 0.015), and associated with shorter duration of sorafenib treatment (median treatment duration: 67 vs. 80 days, p = 0.026).
Regarding functional liver reserve, presence of pre-sarcopenia was significantly associated with prolonged prothrombin time (international normalized ratio [INR]: 1.15 vs. 1.09, p = 0.001), lower serum albumin level (3.2 vs. 3.4 g/dL, p = 0.011), and a higher incidence of ascites formation (49.2% vs. 39.7%, p = 0.018). After sorafenib treatment failure, most patients received the best supportive care from August 2012 to March 2017. No significant difference in post-progression treatment was identified according to the patients’ muscle status. Detailed characteristics of patients with progressive disease (PD) of HCC after sorafenib treatment are presented in Table 1.
Factors associated with pre-sarcopenia in sorafenib-failed HCC
In Table 2, age > 60 years (odds ratio [OR]: 1.796; 95% CI 1.126–2.863, p = 0.014), female gender (OR: 1.877; 95% CI 1.774–3.220, p = 0.045), and BMI < 22 kg/m2 (OR: 4.116; 95% CI 2.216–7.646, p < 0.001) were independent predictors of pre-sarcopenia in sorafenib-failed HCC by multivariate analysis.
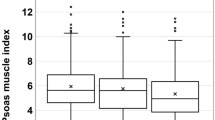
According to the beta coefficient values in multivariate analysis, a scoring system was established to predict the presence of pre-sarcopenia in sorafenib-failed HCC by incorporating gender, age, and BMI at HCC progression. Patients with scores > 0 had a significantly higher risk of pre-sarcopenia (p < 0.001). In addition, the risk and prevalence of pre-sarcopenia increased in alignment with these scores (Fig. 1).
Post-progression survival (PPS) Associated with Pre-sarcopenia
Following progression of 4.2 months (interquartile range: 1.9–9.6), 358 deaths occurred with the median PPS as 4.2 months (95% CI: 3.6–4.9). Patients presented with pre-sarcopenia while HCC progression had significantly worse PPS than others without remarkable muscle depletion (median PPS: 3.8 vs. 5.8 months, p = 0.003) (Fig. 2). Among patients who had normal muscle mass before sorafenib treatment (n = 194), the presence of pre-sarcopenia at PD suggested a shorter PPS than muscle maintainers (4.1 vs. 5.6 months, p = 0.112). In patients with the presence of pre-sarcopenia before sorafenib treatment (n = 191), reversal of pre-sarcopenia while tumor progression was significantly associated with a longer PPS compared with their counterparts (6.3 vs. 3.6 months, p = 0.043) (Supplementary Fig. 1).
As shown in Fig. 3A–C, the presence of pre-sarcopenia at PD significantly determined PPS in patients at Child–Pugh class B while HCC progression (3.4 vs. 2.2 months, p = 0.016). However, PPS was not significantly different based on muscle status in patients at Child–Pugh A or C. Similar results were also noted by evaluating liver function according to the albumin-bilirubin (ALBI) grade. The PPS was significantly better in patients with ALBI grade 2 with normal muscle mass versus those with the presence of pre-sarcopenia (6.3 vs. 4.2 months, p = 0.009). Nevertheless, the muscle-dependent survival difference was not observed in patients at ALBI grade 1 or 3 while tumor progression (Fig. 3D–F).
Prognostic factors associated with PPS
Independent factors associated with PPS were determined by multivariate analysis. To avoid the effect of collinearity, Child–Pugh class and ALBI grade were not included in the same multivariate model. For the analysis of model 1 (Table 3), presence of pre-sarcopenia at PD (hazard ratio [HR]: 1.404; 95% CI 1.112–1.773, p = 0.004), maximal tumor size > 7 cm (HR: 1.722; 95% CI 1.356–2.186, p < 0.001), serum level of alpha-fetoprotein (AFP) > 400 ng/mL (HR: 1.322; 95% CI 1.044–1.673, p = 0.020), Child–Pugh class C (HR: 5.429; 95% CI 3.351–8.706, p < 0.001), early PD within 4 months of sorafenib treatment (HR: 1.388; 95% CI 1.058–1.822, p = 0.018), and the presence of new extrahepatic metastasis (HR: 1.783; 95% CI 1.414–2.248, p < 0.001) were independent risk factors for worse PPS in patients who failed sorafenib treatment for advanced HCC. In model 2 of multivariate analysis, ALBI grade 3 (HR: 4.209; 95% CI 2.864–6.186, p < 0.001), presence of pre-sarcopenia, larger tumor size, higher AFP, early PD, and new extrahepatic metastasis, were independent survival predictors.
Subgroup analysis
The status of muscle depletion was significantly related to worse PPS irrespective of age (Fig. 4). This association was significant in male gender, patients with lower BMI, extrahepatic metastasis, progressive macrovascular invasion, early PD, larger tumor size, and Child–Pugh classes B/C or ALBI grades 2/3.
Discussion
This is the first study to investigate the role of muscle wasting in sorafenib-failed HCC. Unlike a previous study that enrolled patients at different tumor stage19, our patients were homogenous in tumor status. The results of this large cohort study indicated that the presence of pre-sarcopenia was associated with a poor outcome in patients with sorafenib-failed advanced HCC. In addition, we identified older age (> 60 years), female gender, and lower BMI value (< 22 kg/m2) while sorafenib failure were independent predictors for the presence of pre-sarcopenia. These findings implied nutrition replacement to maintain muscle mass could prolong survival for higher-risk patients.
Malnutrition is common in patients with cancer or advanced liver disease and could significantly affect their prognosis11,24. To evaluate nutrition status in patients with advanced liver disease or cancer, body composition is more important than body weight because the increased weight could be composed of additional water in the form of ascites or edema24. Muscle status is a reliable surrogate for nutrition status and physical activity. Unlike ECOG performance status and the subjective classification of ascites and hepatic encephalopathy in the Child–Pugh system, which are traditionally adopted in the assessment and management of HCC, muscle mass status is an objective and quantitative factor to evaluate the patients’ general condition. According to the clinical trials in France and Canada, 27.5–30% of HCC patients had muscle depletion25,26. In advanced HCC, the prevalence of muscle wasting was reported as 49% and 65.1% in the Italian and Japanese cohorts, respectively19,20. According to previous studies, skeletal muscle depletion did not influence overall survival among patients with early or intermediate-staged HCC15. However, it was suggested as an independent predictor of mortality in patients with unresectable HCC, who were undergoing sorafenib therapy18,19,20,21,22. In our study with much more patient numbers, the prevalence of pre-sarcopenia (muscle depletion) was 49.6% at the initiation of sorafenib treatment, and increased to 64.7% with the development of progressive disease. Presence of pre-sarcopenia also independently predicted a poor PPS in HCC patients who failed sorafenib treatment. In addition, the survival-predictive role of pre-sarcopenia was more significant in patients with larger tumor size, early PD, extrahepatic metastasis, and progressive vascular invasion of advanced HCC. These findings suggested that muscle depletion deteriorates in line with tumor stage and has to get more serious concern in patients with advanced tumors.
Similar to previous Japanese studies, in which unremarkable association between Child–Pugh class and muscle mass depletion was reported15,18, no significant differences in liver function could be observed according to our patients’ muscle status. Muscle wasting was found to predict mortality in cirrhotic patients at Child–Pugh class A and B27. In our cohort, the presence of pre-sarcopenia was associated with a significantly worse PPS, particularly in patients at Child–Pugh B or ALBI grade 2 while sorafenib failure. Independent of liver reserves, we still identified older age, female gender, and lower BMI as predictors of pre-sarcopenia in HCC patients who failed sorafenib treatment. The prevalence of muscle depletion was similarly demonstrated to increase with older age and decreased BMI28,29. In our cohort, significant reduction of BMI and increased ascites formation were observed in pre-sarcopenic patients. The presence of ascites could overestimate the value of BMI; but it did not affect the risk of muscle loss in patients with BMI ≥ 22 kg/m2. In contrast, a significant risk of pre-sarcopenia was identified in patients whose BMI was less than 22 kg/m2, even along with the bias of ascites. On the other hand, muscle depletion was more prevalent in female HCC patients in our study. This finding was similar to the results from previous studies15,20. Yet, we observed that the association between the presence of pre-sarcopenia and worse PPS was much more significant in the male patients. Female patients, usually with an abundance of adipose tissue, generate energy preferentially from fat that might account for the ostensible resistance to muscle loss compared to the male patients17,30.
In our cohort, patients who sustained normal muscle mass from the beginning of sorafenib treatment to treatment failure had better PPS than those who developed pre-sarcopenia. Impressively, the initially pre-sarcopenic patients who increased muscle mass during sorafenib treatment and relieved from pre-sarcopenia were observed to have significantly longer PPS compared with others who kept in muscle depletion. These findings suggested the potential therapeutic strategy of nutrition intervention to prevent muscle wasting and improve clinical outcomes of advanced HCC. In addition to hand-foot syndrome, gastrointestinal adverse events, such as anorexia, vomiting and dyspepsia are also common in sorafenib-treated patients4,5. Besides, sorafenib might inhibit carnitine absorption and also lead to pre-sarcopenia31. All of these adverse events could exacerbate protein energy malnutrition and associate with poorer survival of HCC patients16. Therefore, aggressive nutrition intervention and lifestyle modification are important in patients with sorafenib-treated HCC to improve their prognosis. Several studies have suggested that supplementation of branched-chain amino-acids may be useful in maintaining liver reserves and beneficial to patients treated with sorafenib32,33. Exercise therapy might be also promising in preventing skeletal muscle depletion34, but further investigations with large cohorts are still needed.
Imaging-based definitions of muscle depletion were diverse and not yet standardized until now. Status of muscle mass could be measured by a cross-sectional area of the psoas muscle35, TPMT at the level of the umbilicus normalized by body height36, and the third lumbar vertebra muscle index37,38. Among these indices, the psoas cross-sectional area and the third lumbar vertebral muscle index could only be measured by the commercialized software with limited accessibility. In contrast, measurement of TPMT is accessible on most CT scan images without special software. According to previous studies36,39, muscle depletion defined by the value of TPMT/height less than 16.8 mm/m indicated a higher mortality rate in cirrhotic patients independent of the MELD and MELD-Na scores. This measurement was strongly correlated with the third lumbar vertebral muscle index. In addition to CT scan, measurement of muscle mass from the magnetic resonance imaging (MRI) at the level of the third vertebrae was recently reported to have good survival prediction in decompensated cirrhotic patients40. In our study, we measured TPMT/height on CT scan or MRI to evaluate muscle status of our patients. As this measurement could be obtained from radiological images regularly performed for staging purposes or follow-up, it could be promoted in clinical practice to overcome intrinsic limits of bioimpedance and anthropometric measurements, which were hampered by elevated BMI and ascites20.
There are several limitations in this study. First, this is a retrospective study that only enrolled patients treated in a single hospital. However, our hospital is the leading tertiary medical center in Taiwan with strict regulations. The information bias would be ameliorated by regular tumor reassessment by radiological images and clinical evaluation. In addition, it is so far the largest real-life Asian cohort of patients with advanced HCC who failed first-line sorafenib treatment. It is also the first study to demonstrate the prognosis-predicting role of pre-sarcopenia in these patients. Second, the muscle strength evaluation, such as hand grip strength and walking speed12, which is usually regarded as a diagnostic criterion for sarcopenia was not assessed due to the retrospective design of our study. Third, we could not obtain information about daily calorie intake or nutrition support from our patients. Even dietitian consulting would be applied for all patients at high risk of malnutrition, some of them could restore muscle mass during cancer treatment, but others kept muscle wasting. This finding suggested the diverse implementation rate and effectiveness of nutrition support, and highlighted the unmet need of nutrition intervention in these patients. Fourth, most of our patients had chronic hepatitis B or C. Whether our results could be applied to other patients with underlying alcoholic liver disease is still undetermined.
In conclusion, pre-sarcopenia independently determined the prognosis of sorafenib-failed HCC particularly in patients with intermediate liver reserves. Building muscle mass would be important for patients at higher risk of pre-sarcopenia to improve survival.
Materials and methods
Selection of patients
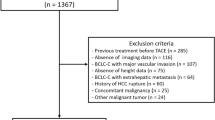
From August 2012 to March 2017, 385 consecutive patients who experienced progressive disease after sorafenib treatment for advanced HCC in Taipei Veterans General Hospital were retrospectively reviewed. All patients were initially diagnosed according to the criteria of American Association for the Study of Liver Diseases (AASLD) treatment guidelines for HCC41. According to the strict reimbursement regulations in Taiwan, patients at Barcelona Clinic Liver Cancer (BCLC) stage C and Child–Pugh class A with portal vein invasion or extrahepatic metastasis were approved for sorafenib treatment9. PD was defined according to the followed CT scan or MRI which was performed every two months after the start of sorafenib treatment42. Patterns of PD were classified as intrahepatic or extrahepatic tumor growth (> 20% increase in tumor size of the preexisting lesions), and new intrahepatic or extrahepatic lesions43. Progressive macrovascular invasion was defined as PD in vascular tumor thrombus without newly developed intrahepatic or extrahepatic lesion or progression of existed tumor. This study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. It was approved by the Institutional Review Board of the Taipei Veterans General Hospital (IRB No.: 2019-07-038BC). Informed consent was waived by the Institutional Review Board of the Taipei Veterans General Hospital due to the retrospective design and most enrolled patients had died.
Definition, clinical assessments and outcomes
Muscle mass status was assessed by measuring TPMT on CT scan or MRI at the level of the umbilicus36,44. The value of TPMT/BH less than 16.8 mm/m was defined as pre-sarcopenia36. In this study, we retrospectively calculated TPMT/BH at the beginning of sorafenib treatment and at tumor PD recognized by image studies. Anthropometric measurements, laboratory exams, including hemogram, serum chemistry, AFP level, as well as Child–Pugh class, ALBI grade, and ECOG performance status were evaluated when PD was confirmed. The PPS was measured from the date of radiology-proven PD to the date of death.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile ranges), while categorical variables were analyzed as frequency and percentages. The Pearson chi-square analysis or Fisher’s exact test was used to compare categorical variables, while the Student t-test or Mann–Whitney U test was applied for continuous variables. The predictive power of a score to predict pre-sarcopenia was assessed using the area under receiver operating characteristic curves (AUROC). Survival was estimated by the Kaplan–Meier method and compared by the log-rank test. Additionally, Cox’s proportional-hazard model was used to identify prognostic factors of survival. For all analyses, p < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS Statistics v. 17.0 for Windows, Armonk, NY, USA).
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse events
- AFP:
-
Alpha-fetoprotein
- ALBI grade:
-
Albumin-bilirubin grade
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona-Clinic-Liver-Cancer
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitors
- INR:
-
International normalized ratio
- LD:
-
Liver decompensation
- Mets:
-
Metastasis
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- MRI:
-
Magnetic resonance image
- MVI:
-
Macroscopic vascular invasion
- NA:
-
Not adopted
- NEH:
-
New extrahepatic metastasis
- NS:
-
Not significant
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PPS:
-
Post-progression survival
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236. https://doi.org/10.1016/j.jhep.2018.03.019 (2018).
Njei, B., Rotman, Y., Ditah, I. & Lim, J. K. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 61, 191–199. https://doi.org/10.1002/hep.27388 (2015).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. https://doi.org/10.1056/NEJMoa0708857 (2008).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34. https://doi.org/10.1016/S1470-2045(08)70285-7 (2009).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66. https://doi.org/10.1016/S0140-6736(16)32453-9 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63. https://doi.org/10.1056/NEJMoa1717002 (2018).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296. https://doi.org/10.1016/S1470-2045(18)30937-9 (2019).
Lee, P. C. et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int. 38, 321–330. https://doi.org/10.1111/liv.13527 (2018).
Takada, H. et al. Baseline and early predictors of good patient candidates for second-line after sorafenib treatment in unresectable hepatocellular carcinoma. Cancers (Basel) 11, 1256. https://doi.org/10.3390/cancers11091256 (2019).
Hanai, T. et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 46, 743–751. https://doi.org/10.1111/hepr.12616 (2016).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. https://doi.org/10.1093/ageing/afq034 (2010).
Prado, C. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 9, 629–635. https://doi.org/10.1016/S1470-2045(08)70153-0 (2008).
Tan, B. H., Birdsell, L. A., Martin, L., Baracos, V. E. & Fearon, K. C. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15, 6973–6979. https://doi.org/10.1158/1078-0432.CCR-09-1525 (2009).
Iritani, S. et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 50, 323–332. https://doi.org/10.1007/s00535-014-0964-9 (2015).
Schutte, K. et al. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC). Clin Nutr 34, 1122–1127. https://doi.org/10.1016/j.clnu.2014.11.007 (2015).
Imai, K. et al. Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: The role of liver functional reserve and tumor-related factors in loss of skeletal muscle volume. Nutrients 9, 1054. https://doi.org/10.3390/nu9101054 (2017).
Hiraoka, A. et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 47, 558–565. https://doi.org/10.1111/hepr.12780 (2017).
Nishikawa, H. et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 14, 1637–1647. https://doi.org/10.3892/ol.2017.6287 (2017).
Antonelli, G. et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United European Gastroenterol. J. 6, 1039–1048. https://doi.org/10.1177/2050640618781188 (2018).
Imai, K. et al. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci 16, 9612–9624. https://doi.org/10.3390/ijms16059612 (2015).
Imai, K. et al. Rapid depletions of subcutaneous fat mass and skeletal muscle mass predict worse survival in patients with hepatocellular carcinoma treated with sorafenib. Cancers (Basel) https://doi.org/10.3390/cancers11081206 (2019).
Takada, H. et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE 13, e0198812. https://doi.org/10.1371/journal.pone.0198812 (2018).
Bruggeman, A. R. et al. Cancer cachexia: Beyond weight loss. J. Oncol. Pract. 12, 1163–1171. https://doi.org/10.1200/JOP.2016.016832 (2016).
Mir, O. et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 7, e37563. https://doi.org/10.1371/journal.pone.0037563 (2012).
Meza-Junco, J. et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J. Clin. Gastroenterol. 47, 861–870. https://doi.org/10.1097/MCG.0b013e318293a825 (2013).
Merli, M., Riggio, O. & Dally, L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi). Hepatology 23, 1041–1046. https://doi.org/10.1002/hep.510230516 (1996).
Chen, L. K. et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101. https://doi.org/10.1016/j.jamda.2013.11.025 (2014).
Bravo-Jose, P. et al. Prevalence of sarcopenia and associated factors in institutionalised older adult patients. Clin. Nutr. ESPEN 27, 113–119. https://doi.org/10.1016/j.clnesp.2018.05.008 (2018).
Riggio, O. et al. Malnutrition is not related to alterations in energy balance in patients with stable liver cirrhosis. Clin. Nutr. 22, 553–559. https://doi.org/10.1016/s0261-5614(03)00058-x (2003).
Amanuma, M., Nagai, H. & Igarashi, Y. Sorafenib might induce sarcopenia in patients with hepatocellular carcinoma by inhibiting carnitine absorption. Anticancer Res. 40, 4173–4182. https://doi.org/10.21873/anticanres.14417 (2020).
Takeda, H. et al. Effect of treatment with branched-chain amino acids during sorafenib therapy for unresectable hepatocellular carcinoma. Hepatol. Res. 44, 302–312. https://doi.org/10.1111/hepr.12125 (2014).
Imanaka, K. et al. Impact of branched-chain amino acid supplementation on survival in patients with advanced hepatocellular carcinoma treated with sorafenib: A multicenter retrospective cohort study. Hepatol. Res. 46, 1002–1010. https://doi.org/10.1111/hepr.12640 (2016).
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 70(172–193), 2019. https://doi.org/10.1016/j.jhep.2018.06.024 (2019).
Englesbe, M. J. et al. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg. 211, 271–278. https://doi.org/10.1016/j.jamcollsurg.2010.03.039 (2010).
Durand, F. et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J. Hepatol. 60, 1151–1157. https://doi.org/10.1016/j.jhep.2014.02.026 (2014).
Montano-Loza, A. J. et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 10, 166–173, 173.e1. https://doi.org/10.1016/j.cgh.2011.08.028 (2012).
DiMartini, A. et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 19, 1172–1180. https://doi.org/10.1002/lt.23724 (2013).
Gu, D. H. et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin. Mol. Hepatol. 24, 319–330. https://doi.org/10.3350/cmh.2017.0077 (2018).
Praktiknjo, M. et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology 67, 1014–1026. https://doi.org/10.1002/hep.29602 (2018).
Bruix, J., Sherman, M. & American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 53, 1020–1022. https://doi.org/10.1002/hep.24199 (2011).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750. https://doi.org/10.1002/hep.29913 (2018).
Reig, M. et al. Postprogression survival of patients with advanced hepatocellular carcinoma: Rationale for second-line trial design. Hepatology 58, 2023–2031. https://doi.org/10.1002/hep.26586 (2013).
Huguet, A. et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study. Nutrition 51–52, 73–79. https://doi.org/10.1016/j.nut.2018.01.008 (2018).
Acknowledgements
We gratefully acknowledge the patients and their families/caregivers, the independent data monitoring committee, and the nursing staff of Division of Gastroenterology and Hepatology, Taipei Veterans General Hospital.
Author information
Authors and Affiliations
Contributions
Study concept and design: T.-Y.C., P.-C.L., Y.-H.H.; Acquisition of data: T.-Y.C., P.-C.L.; Analysis and interpretation of data: T.-Y.C., P.-C.L.; Drafting of manuscript: T.-Y.C., P.-C.L., Y.-H.H.; critical revision: M.-C.H., Y.C.; Study supervision: Y.C., M.-C.H., Y.-H.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, TY., Lee, PC., Chen, YT. et al. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci Rep 10, 18375 (2020). https://doi.org/10.1038/s41598-020-75198-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75198-z
This article is cited by
-
Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: an updated meta-analysis
Scientific Reports (2023)
-
Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: a multicenter study in Tohoku, Japan
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.