Abstract
The objective is to assess the impact of anticoagulant treatment in non-valvular atrial fibrillation (AF) and different categories of renal dysfunction in real world. Electronic Health recordings of patients with diagnosis of AF and renal function collected throughout 5 years and classified according to KDIGO categories. Stroke, transitory ischemic attack (TIA), intracranial hemorrhage and all-cause mortality were identified. Anticoagulant treatments during the study period were classified in untreated (never received therapy), VKA, NOAC and Aspirin. The risk of events was calculated by Cox-proportional hazard models adjusted by confounders. A total of 65,734 patients with AF, mean age 73.3 ± 10.49 years old and 47% females and follow-up of 3.2 years were included. KDIGO classification were: G1 33,903 (51.6%), G2 17,456 (26.6%), G3 8024 (12.2%) and G4 6351 (9.7%). There were 8592 cases of stroke and TIA, 437 intracranial hemorrhage, and 9603 all-cause deaths (incidence 36, 2 and 38 per 103 person/year, respectively). 4.1% of patients with CHA2DS2-VASc Score 2 or higher did not receive anticoagulant therapy. Risk of stroke, TIA, and all-cause mortality increased from G1 to G4 groups. Anticoagulant treatments reduced the risk of events in the four categories, but NOAC seemed to offer significantly better protection. Renal dysfunction increases the risk of events in AF and anticoagulant treatments reduced the risk of stroke and all-cause mortality, although NOAC were better than VKA. Efforts should be done to reduce the variability in the use of anticoagulants even in this high risk group.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia in adults, is a frequent condition in aged populations increasing the risk for stroke, heart failure, dementia, and mortality1,2. Anticoagulant treatment3,4,5 had demonstrated a significant reduction in the risk of stroke and today is mandatory in subjects with a CHA2DS2-VASc score equal to or higher than 26. Recommendations for the use of anticoagulants or antiplatelet agents in patients with CKD have been released for scientific societies3,5, and randomized trials have provided information about the efficacy and tolerance of treatments. Despite the evidence on the efficacy and side effects of anticoagulant treatments, their use in daily clinical practice is far from perfect due to several reasons such as lack of anticoagulant prescription despite guidelines recommendations, changes of anticoagulants during the follow-up and lack of adherence to long-term prophylactic therapies or the presence of comorbidities. Atrial Fibrillation and CKD usually coexist, and CKD not only increases the cardiovascular risk but also can alter the pharmacokinetics of the anticoagulants. Moreover, both AF and CKD share common risk factors, in fact all conditions included in the CHA2DS2-VASc score increase the risk of chronic kidney disease (CKD)7.
The impact of anticoagulant therapy in AF using real-world data (RWD) has been addressed in multiple studies8,9,10,11,12,13,14,15,16,17, but information about the scenario of AF in CKD is scarce18. Therefore, the study’s objective is to assess the impact of anticoagulant treatment on the risk of stroke, all-cause mortality, and major bleedings in patients with non-valvular AF and different categories of renal dysfunction.
Subjects and methods
Study population and baseline data collection
The sample was recruited from beneficiaries of the Valencian Health Agency’s universal health care system. The Valencian Community is a Mediterranean region located on the East-coast of Spain, with a population of 3,799,885 people older than 18 years in 2012. Every patient has a unique personal identification number for the health system, so there is one unique electronic centralized clinical record per patient. Total population data was extracted using the health information exchange function of ABUCASIS for the period of time between 1st January 2012 and 31st December 2016. ABUCASIS includes information on patient demographics, medications, vital status, past medical history and laboratory data, among others. Patients' data collected from the system during the study were documented by a process of pseudo-anonymization, making it impossible to use this information to identify the patients since the only link between the data and the patient is a code not available to the researchers. The data generated during the study was handled according the Spanish Law 3/2018 of Data Protection and Guaranty of Digital Rights the and corresponding European norms19. The study was reviewed and approved by the Committee for Ethics and Clinical Trials of the Hospital Clinico of Valencia. The Ethical Committee approved that the study developed under exemption of informed consent.
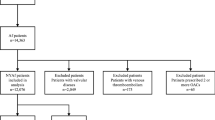
Forty-six thousand three hundred ninety-six subjects with diagnosis of Atrial fibrillation (ICD-9 427.31 and V07.39O; ICD-10 I48.1, I48.2, I48.91) and KDIGO stratification of risk20 were included in the study from 1st January 2012. Incident AF during the study period, until 31st December 2016, was also included. Information from primary care physicians, specialists, nurses’ offices, pharmacies, hospitals, and emergency departments was collected. The presence of hypertension, diabetes and dyslipidemia were collected according to previously detailed definitions21. CHA2DS2-VASc, HAS-BLED and KDIGO categories were calculated for each patient. Since at the beginning of the inclusion in the study not all the baseline variables were available to adjust for potential confounding, a 6-month window around the time of study inclusion were used.
Event definitions
Incidences of stroke, transitory ischaemic attack, haemorrhagic stroke, and all-cause mortality until 31st December 2016 were collected. Events were assigned from the ICD codes recorded at discharge from hospitalizations or the emergency room. Death was extracted from the death registry. Follow-up was calculated as the difference between the starting point of taking the medication class and the date of the event, death, or 31st of December 2016, whichever occurred first.
Renal assessment and KDIGO categories
Serum creatinine was measured and estimated glomerular filtration rate (eGFR) was calculated from creatinine, age and sex using the CKD-EPI22. Albuminuria and/or proteinuria was assessed in first voiding urine in the morning and expressed as the ratio with urinary creatinine (mg/g). KDIGO stratification of riskx20 considering eGFR and urinary albumin excretion, at baseline of the observational period study, was performed. The stratification used was the following: KDIGO 1 (G1) eGFR > 60 ml/min/1.73 m2 and UAE < 30 mg/g creatinine; KDIGO 2 (G2) eGFR > 60 ml/min/1.73 m2 and UAE 30–299 mg/g creatinine or eGFR 45–59 ml/min/1.73 m2 and UAE < 30 mg/g creatinine; KDIGO 3 (G3) eGFR 30–44 ml/min/1.73 m2 and UAE < 30 mg/g creatinine, or eGFR 45–59 ml/min/1.73 m2 and UAE 30–299 mg/g creatinine, or eGFR > 60 ml/min/1.73 m2 and UAE > 300 mg/g creatinine; KDIGO 4 (G4) eGFR 15–29 ml/min/1.73 m2 or eGFR 30–44 ml/min/1.73 m2 and UAE 30–299 mg/g creatinine or eGFR 45–59 ml/min/1.73 m2 and UAE > 299 mg/g creatinine.
Anticoagulant treatment
Treatment was collected from the prescription repository of the EHR with the ATC-codes. Anticoagulant treatment was grouped in no treatment, vitamin K antagonists (VKA), B01AA (acenocumarol, warfarin), non-vitamin K antagonist Oral Anticoagulant (NOAC), B01AE (dabigatran, ribaroxaban, apixaban, edoxaban) and B01AC [aspirin (acetylsalicylic acid)]23. The initial treatment was the one prescribed at the time of starting the observational period or after diagnosis for incident cases, and the last treatment was the current prescription when the event occurred or at the end of the observational period. If anticoagulants were not dispensed during three months, patients were considered that were not taking treatment. For each subject, the persistence with a treatment was estimated and the time without discontinuation previous to one event or until the end of the treatment was calculated. In addition, duration of prescription stratified in those with less than 20 months, between 20 and 40 and more than 40. Subjects without any of these prescriptions during the whole follow-up period were considered untreated.
Statistical analysis
Values are mean plus minus standard deviation and values of incidence per 1000 person/year. Incidence and survival by groups of treatment were analysed using Cox proportional hazard regression models. The risk of events based on therapy groups was evaluated by means of cumulative survival rates and Cox proportional hazards regression models. Individual time-period taking the different anticoagulants was also considered. A death is counted only toward the drug group the patient was taking at the time of death. The analysis was adjusted by potential confounders including age, sex, hypertension, diabetes, coronary heart disease, dyslipidemia, heart failure and duration of treatments. KDIGO categories were also included in some of the analysis. Chi-square was used to compare the incidence rates across groups of persistence with treatment. The statistical R-package was used for analysis.
Results
General characteristics of the study population
A total of 65,734 patients with AF, mean age 73.3 ± 10.49 years old and 47% females and follow-up of 3.2 years, were included. Hypertension was present in 87.8%, dyslipidemia in 74.7%, diabetes in 44.1% heart failure 37.4%, and coronary heart disease 27.8%. At baseline, mean CHA2DS2-VASc Score was average 3.43 (IQ 1.71–2.50) and HAS-BLED average 2.36 (IQ 1.00–2.39). According to the KDIGO classification the distribution of patients was as follows: G1 33,903 (51.6%), G2 17,456 (26.6%), G3 8024 (12.2%) and G4 6351 (9.7%). The study population’s main characteristics by the KDIGO categories are shown in Table 1 and by the ever exposure to an anticoagulant during the study in Table 2. The distribution of patients according to the CHA2DS2-VASc Score and KDIGO categories is shown in Fig. 1. The total follow-up was 2,761,922 person-months.
Anticoagulant treatment and events in all patients
The distribution of treatments during the observational period is shown in Fig. 2. During the study period, 6247 subjects were untreated, 42,790 (65.1%) received VKA, 14,588 (22.2%), (NOAC) and 22,372 (34.0%) aspirin. The mean CHA2DS2-VASc Score was significantly lower in the untreated group, (Table 2) p < 0.001. Selecting those with a CHA2DS2-VASc Score equal to or greater than two, 4.1% (2626 subjects) were not receiving anticoagulant therapy in the study period, being the majority in the G1 group. The total time of treatment for each of the anticoagulant groups was 229,894 person-months in untreated subjects, 1,486,150 person-months in the VKA, 299,965 person-months in NOAC and 551,246 person-months in the aspirin group.
A total of 8592 patients suffered stroke and TIA. The overall incidence of stroke and TIA combined was 36 events/1000 patients/year. All treatments reduced the rate of incident ischemic stroke and TIA as compared to the absence of treatment, after adjusting by age, sex, CHA2DS2-VASc score, hypertension, diabetes, dyslipidemia, coronary heart disease, heart failure, KDIGO categories and time of anticoagulation, (Table 3). Those treated with NOAC had the lowest risk, followed by the aspirin group and VKA. The incidence of ischemic stroke and TIA grouped by the number of months in which the treatment was maintained is in Table 4. The higher of time under treatment was associated with lower incidence of stroke and TIA and the differences among the treated groups disappeared.
Intracranial hemorrhage was diagnosed in 437 subjects, incidence 2/1000 patients/years, being the risk higher in the VKA compared to those treated with NOAC and aspirin, Tables 3 and 4. A total of 2003 patients needed hospitalization due to gastrointestinal hemorrhage, 8 events/1000 patients/year, being the incidence higher in those untreated. Among those treated, the risk was higher in the VKA group.
There were 9603 deaths, incidence of all-cause mortality 38/1000 patients/year, in the study period. Treatments reduced the risk of death as compared to the absence of treatment after adjusted by potential confounders, being the lowest risk NOAC treatment followed by aspirin, Tables 3 and 4.
Anticoagulant treatment and events by KDIGO categories
The distribution of the anticoagulant treatments by different KDIGO categories is shown in Table 2. The proportion of untreated or treatment modality was similar in the four categories. The incidence of ischemic stroke plus TIA according the KDIGO categories and CHA2DS2-VASc Score is shown in Fig. 3 (left panel). Risk increases across the CHA2DS2-VASc Score and for each value the risk did not change for the KDIGO category. The three group of treatments, NOAC, VKA and aspirin reduce the risk in all the KDIGO groups. The reduction of risk across KDIGO categories was significantly higher for the NOAC, Table 3.
The incidence of hemorrhagic stroke, 2/1000 patients/year increased twice in the G4. Anticoagulant treatment with VKA did not reduce significantly the risk, while it was reduced by NOAC and the aspirin group, Table 3 and 4. The risk of gastrointestinal hemorrhage increased across the four KDIGO categories [HR G-2 1.41 (1.25–1.58), G-3 1.58 (1.38–1.81), G-4 2.06 (1.80–2.36)], Table 5 despite that HAS-BLED score was not significant different among the KDIGO categories (p < 0.05). The highest incidence was observed in patients treated with VKA.
In contrast with the incidence of neurologic events, all-cause mortality is dependent on both KDIGO category and CHA2DS2-VASc Score, Fig. 3 (right panel). The three groups of the anticoagulant treatment reduced the risk in the four KDIGO categories, an improvement that was less in the more advanced KDIGO categories. The NOAC offered better protection than the other treatment groups in each of the KDIGO categories, Table 3 and Fig. 4.
Accumulate risk of events over time in untreated and treated with VKA, NOAC or Aspirin by KDIGO categories adjusted by age, sex, CHA2DS2-VASc Score, hypertension, diabetes, dyslipidaemia, coronary heart disease, heart failure and time of treatment. Stroke and TIA (panel A), all-cause mortality (panel B) and haemorrhagic stroke (panel C). Hazard rate reduction of each anticoagulant treatment with the untreated as a referent are in Table 3. Lines for each treatment are Green untreated; blue VKA; Brown aspirin; Red NOAC.
Discussion
In a real-world setting of a large cohort of patients with the diagnosis of non-valvular atrial fibrillation, the renal disease stage largely influences the risk and the beneficial impact of the different anticoagulant treatment. Although all the anticoagulants reduce the risk of stroke, TIA, and all-cause mortality, the NOAC seem to offer significantly better protection than the treatment with VKA without increment in hemorrhagic events. Furthermore, significant variability in the use of anticoagulants was observed. 4.7% of patients with a CHA2DS2-VASc Score equal to or greater than 2 did not receive any anticoagulant therapy along the study period.
Randomized Clinical Trials (RCT) are the gold standard to assess drugs’ efficacy and safety, but RWD data may reflect a broader picture of clinical settings10. The EHR of the present study covers 92% of the population in our community and guarantees the interoperability of all sources of information. In addition, information about urinary albumin excretion was available in all subjects16,17. We exclude patients in KDIGO G5 or in renal replacement therapy, since the information of this group in the EHR is incomplete.
Several studies9,10,11,12, systematic reviews13,14, and meta-analysis15,16 have been published describing different aspects of the anticoagulation of non-valvular AF using RWD data. However, among many studies analyzing the anticoagulation in AF patients with renal dysfunction24,25,26,27,28,29,30,31,32,33,34,35,36,37,38, meta-analyses focus on RWD34,36 are scarce. In this increasing population, the efficacy and protection level achieve with treatments and the risk of side effects have to be considered. The meta-analysis of Dahal et al.34, which includes 11 primary cohorts and 2 sub studies, concluded that the use of warfarin in non-end-stage CKD resulted in a lower risk of ischemic stroke and mortality without effect on major bleedings. The beneficial impact of treatment was not present for patients in end-stage kidney disease (ESKD). Ding et al.36, compared a register, the Murcia AF Project, with the AMADEUS clinical trial in terms of the impact of VKAs in the incidence of ischemic stroke, major bleeding, all-cause mortality, myocardial infarction, and intracranial hemorrhage in subjects with eGFR < 60 ml/min/1.73 m2. The annual risk of ischemic stroke, major bleeding and all-cause mortality was significantly higher, more than twice, in the register compared with those in the clinical trial. The authors conclude that risk may be under-estimated in the environment of randomized control trials. Another registry37 concludes that the incidence rates of stroke, mortality, and bleeding increase with reducing eGFR across the whole range of renal function. In this study, anticoagulant treatment reduced stroke and mortality risk at one year compared with untreated patients. Likewise, in a Nationwide Observational cohort study38, non-ESKD was associated with a higher risk of stroke/thromboembolism across risk strata in AF patients. Those with high-risk, CHA2DS2-VASc Score equal to or higher than two, benefited from warfarin treatment for stroke prevention. In contrast patients in ESKD, treatment with warfarin increases the risk of hemorrhagic stroke39.
The present study expands the information available on the renal function’s impact on the risk of AF complications in real world. We have observed that: (i) incidence of neurological events, stroke and TIA, increases across the CHA2DS2-VASc Score and for each value the risk did not change for the KDIGO categories; (ii) anticoagulant treatment reduces the risk of neurological events and mortality across the spectrum of renal dysfunction in subjects with GFR > 15 ml/min/1.73 m2; (iii) NOAC were superior to VKA across the KDIGO categories, while superiority over aspirin was present in the lowest renal damage categories, KDIGO 1 and 2; (iv) aspirin also reduced risk of stroke and TIA and mortality across the renal dysfunction categories, as well as reduced risk of hemorrhagic stroke in the lowest renal damage categories, KDIGO 1–3 and (v) hemorrhagic events, neurologic and in the gastrointestinal tract were lower in the NOAC group. Results concerning gastrointestinal hemorrhage may have selection bias due to the choice of treatment was influenced by the bleeding risk of patients. The fact that the gastrointestinal hemorrhage was higher in the untreated subjects is probably because many of these patients had high bleeding risk and therefore were not treated with anticoagulants.
Several studies have compared the NOAC and VKA in terms of stroke risk reduction or major bleeding events with better results for NOAC in some studies26,32 but no others33. In the present study performed in patients with KDIGO 1–4 and in the absence of ESKD we confirm the superiority of NOAC. Superiority of NOAC in reduction of stroke over to VKA disappeared in patients with a very low GFR or in dialysis, although experienced fewer bleeding events with NOAC40,41. In fact, pivotal RCT have demonstrated a net clinical benefit for NOACs versus VKA with mild-moderate CKD, but there is little evidence in patients with AF and stages 442. Further benefit of NOAC over VKA is in the incidence of anticoagulant-related nephropathy, mainly described after the introduction of VKA treatment although some case with the use of NOAC have been reported43. Finally, it is worthy to comment the fact that the difficulty to achieve anticoagulation in range for the VKA is greater when lowest is the GFR. In the present study, the relatively low use of NOAC depends on the study period, 2012–2016, and prescription limitations within the EHR due to their cost.
Less attention had been paid to the treatments with aspirin. What is relevant of the present data is the fact that aspirin also reduce the risk of stroke and TIA and mortality without increasing the risk of intracranial bleeding in the present study in KDIGO 1–3 but not in KDIGO 4. Protection it was inferior to NOAC but not to VKA. Whether or not the benefit of aspirin is due to a selection bias at the time of inclusion needs to be considered. Despite this observation, patients with a CHA2DS2-VASc Score 2 or higher needs anticoagulant treatment44.
Lack of persistence is a relevant point when RWD are analyzed. One study observed suboptimal persistence to NOAC in patients with AF, with 1 in 3 patients adhering to their NOAC < 80% of the time and the lack of persistence was associated with poor clinical outcomes13. In the present study, it is worthy to comment, the longer the duration of anticoagulant treatment largely reduced the risk of stroke and mortality. The inclusion of RWD in meta-analyses could help evaluate the effectiveness of health care interventions15 and RW cost-effectiveness studies could assist policy-makers for an optimal allocation of resources45.
Strengths and limitations of the study should be contemplated. A large number of patients with AF was analyzed with a long follow-up accounting for potential confounders such age, sex, and major cardiovascular risk factors. Moreover, the time of drug prescription was considered. Some limitations such as the presence of a high percentage of missing values are inherent to the EHR. To minimize its impact, only patients with the required variables for the analysis were selected. Renal parameters, eGFR and albuminuria were assessed at the time of starting the study or when the incident AF occurs, then it is possible that during the study some changes in the renal status have been produced. The reasons for the lack of treatment, the quality of VKA control, and the dosage and type of NOAC were not analyzed. Challenging patients with End Stage Renal Disease were excluded. Finally, even that the time period of the study was in which NOAC were progressively introduced, there is a large number of patients treated with this drug class.
The 2020 ESC guidelines on diagnosis and management of AF had upgraded treatment recommendations for switching from VKAs to NOAC therapy5. It is recommended or indicated for patients on VKA who have a time in the therapeutic range below 70%, mainly in subjects with reduced eGFR. According to the present data, anticoagulant treatment reduced stroke and TIA risk, as well mortality, in patients with KDIGO stage 4 including patients with GFR > 15 ml/1.73 m2, then NOAC treatment could be recommended but prospective control studies are required, but renal function should be monitored. Finally, a major goal is to increase the percentage of treated patients and their adherence to the treatment.
In conclusion, the present study demonstrated that the use of anticoagulant treatment in real life is far from guidelines’ recommendations. A high proportion of high-risk untreated subjects was detected. Although potential advantages of NOAC compared to VKA were present across different degrees of renal dysfunction, we must increase the prescription rate to reduce mortality and stroke incidence.
References
Michaud, G. F. & Stevenson, W. G. Atrial Fibrillation. N Engl J Med. 384, 353–361 (2021).
John, R. M., Michaud, G. F. & Stevenson, W. G. Atrial fibrillation hospitalization, mortality, and therapy. Eur Heart J. 39, 3958–3960 (2018).
January, C. T. et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 74, 104–132 (2019).
Chao, T. F., Nedeljkovic, M. A., Lip, G. Y. H. & Potpara, T. S. Stroke prevention in atrial fibrillation: comparison of recent international guidelines. Eur Heart J Suppl. 22(Suppl O), O53–O60 (2020).
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom- Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio- Thoracic Surgery (EACTS). Eur Heart J 2020(42), 373–498 (2020).
Lip, G. Y. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why?. Eur Heart J. 34, 1041–1049 (2013).
Lai, A. C. et al. A Personalized Approach to Chronic Kidney Disease and Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 77(11), 1470–1479 (2021).
Shen NN, Wu Y, Wang N, Kong LC, Zhang C, Wang JL, Gu ZC, Chen J. Direct Oral
Anticoagulants vs. Vitamin-K Antagonists in the Elderly With Atrial Fibrillation: A Systematic Review Comparing Benefits and Harms Between Observational Studies and Randomized Controlled Trials. Front Cardiovasc Med. 2020;7:132.
Bang OY, On YK, Lee MY, Jang SW, Han S, Han S, Won MM, Park YJ, Lee JM, Choi HY, Kang S, Suh HS, Kim YH. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: Results from a real-world data analysis. PLoS One. 2020;15:e0242922.
Domek, M., Gumprecht, J., Ding, W. Y., Lip, G. Y. H. & Lane, D. A. Practice-derived data on non-vitamin K antagonist oral anticoagulant therapy to complement observations from randomized trials. Eur Heart J Suppl. 22, I1–I12 (2020).
Anghel, L., Sascău, R., Trifan, A., Zota, I. M. & Stătescu, C. Non-Vitamin K Antagonist Oral Anticoagulants and the Gastrointestinal Bleeding Risk in Real-World Studies. J Clin Med. 9, 1398 (2020).
Sun, X. et al. Hemorrhage Risk Profiles among Different Antithrombotic Regimens: Evidence from a Real-World Analysis of Postmarketing Surveillance Data. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-07110-w (2020).
Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SS, Jackevicius CA. Real-World Adherence and Persistence to Direct Oral Anticoagulants in Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2020;13:e005969.
Hill, N. R. et al. A Systematic Review of Network Meta-Analyses and Real-World Evidence Comparing Apixaban and Rivaroxaban in Nonvalvular Atrial Fibrillation. Clin Appl Thromb Hemost. 26, 1076029619898764 (2020).
Briere, J. B. et al. Impact of methodological choices on a meta-analysis of real-world evidence comparing non-vitamin-K antagonist oral anticoagulants with vitamin K antagonists for the treatment of patients with non-valvular atrial fibrillation. Curr Med Res Opin. 35, 1867–1872 (2019).
Coleman, C. I. et al. Meta-analysis of real-world evidence comparing non-vitamin K antagonist oral anticoagulants with vitamin K antagonists for the treatment of patients with non-valvular atrial fibrillation. J Mark Access Health Policy. 7, 1574541. https://doi.org/10.1080/20016689.2019.1574541 (2019).
Bowrin K, Briere JB, Fauchier L, Coleman C, Millier A, Toumi M, Clay E, Levy P. Real-world cost-effectiveness of rivaroxaban compared with vitamin K antagonists in the context of stroke prevention in atrial fibrillation in France. PLoS One. 2020;15:e0225301.
Ashton V, Kerolus-Georgi S, Moore KT. The Pharmacology, Efficacy, and Safety of Rivaroxaban in Renally Impaired Patient Populations. J Clin Pharmacol. 2021: doi: https://doi.org/10.1002/jcph.1838.
The EU General Data Protection Regulation GDPR. https://gdpr-info.eu/
Murton, M. et al. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv Ther. 38, 180–200 (2021).
Holgado, J. L. et al. Acute kidney injury in heart failure: a population study. ESC Heart Fail. 7, 415–422 (2020).
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612.
ATC/DDD Index. WHO Collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs, 2021. Oslo, Norway 2020.
White, E. M. & Coons, J. C. Direct Oral Anticoagulant Use in Special Populations: Elderly, Obesity, and Renal Failure. Curr Cardiol Rep. 23, 27. https://doi.org/10.1007/s11886-021-01456-9 (2021).
Lau WL. Controversies: Stroke Prevention in Chronic Kidney Disease. J Stroke Cerebrovasc Dis. 2021:105679. doi: https://doi.org/10.1016/j.jstrokecerebrovasdis.
Chen, C. et al. Effect of Rivaroxaban or Apixaban in Atrial Fibrillation Patients with Stage 4–5 Chronic Kidney Disease or on Dialysis. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-021-07144-8 (2021).
Stefil, M., Nabrdalik, K. & Lip, G. Y. H. Renal Disease and Atrial Fibrillation. Card Electrophysiol Clin. 13, 95–112 (2021).
Proietti M, Vitolo M, Lip GYH. Integrated care and outcomes in patients with atrial fibrillation and comorbidities. Eur J Clin Invest. 2021:e13498. doi: https://doi.org/10.1111/eci.13498.
Wetmore, J. B. et al. CKD Progression in Medicare Beneficiaries With Nonvalvular Atrial Fibrillation Treated With Apixaban Versus Warfarin. Am J Kidney Dis. S0272–6386(20), 31203–31208. https://doi.org/10.1053/j.ajkd.2020.12.004 (2021).
Magnocavallo, M. et al. Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy. J Clin Med. 10, 83. https://doi.org/10.3390/jcm10010083 (2020).
Agarwal, M. A. et al. Clinical Outcomes of Warfarin Initiation in Advanced Chronic Kidney Disease Patients With Incident Atrial Fibrillation. JACC Clin Electrophysiol. 6, 1658–1668 (2020).
Bansal N, Zelnick LR, Soliman EZ, Anderson A, Christenson R, DeFilippi C, Deo R, Feldman HI, He J, Ky B, Kusek J, Lash J, Seliger S, Shafi T, Wolf M, Go AS, Shlipak MG; CRIC Study Investigators. Change in Cardiac Biomarkers and Risk of Incident Heart Failure and Atrial Fibrillation in CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2020:S0272–6386(20)31137–9. doi: https://doi.org/10.1053/j.ajkd.2020.09.021.
Heleniak, Z., Papuga-Szela, E., Krzysztof, P. & Anetta, U. Efficacy and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and Chronic Kidney Disease Stage G4: A Single-Center Experience. J Cardiovasc Pharmacol. 76, 671–677 (2020).
Dahal, K., Kunwar, S., Rijal, J., Schulman, P. & Lee, J. Stroke, Major Bleeding, and Mortality Outcomes in Warfarin Users With Atrial Fibrillation and Chronic Kidney Disease: A Meta-Analysis of Observational Studies. Chest 149, 951–959 (2016).
Ding, W. Y., Lip, G. Y. H., Pastori, D. & Shantsila, A. Effects of Atrial Fibrillation and Chronic Kidney Disease on Major Adverse Cardiovascular Events. Am J Cardiol. 132, 72–78 (2020).
Ding WY, Rivera-Caravaca JM, Shantsila A, Marin F, Gupta D, Roldán V, Lip GYH. Outcomes in VKA-treated patients with atrial fibrillation and chronic kidney disease: Clinical trials vs 'real-world'. Int J Clin Pract. 2020:e13888. doi: https://doi.org/10.1111/ijcp.13888.
Banerjee, A. et al. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest 145, 1370–1382 (2014).
Bonde, A. N. et al. Net Clinical Benefit of Antithrombotic Therapy in Patients With Atrial Fibrillation and Chronic Kidney Disease: A Nationwide Observational Cohort Study. J Am Coll Cardiol 64, 2471–2482 (2014).
Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, Dhar G. Association Between Use of Warfarin for Atrial Fibrillation and Outcomes Among Patients With End-Stage Renal Disease: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e202175.
Chan, K. E., Lazarus, J. M., Thadhani, R. & Hakim, R. M. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 20, 872–881 (2009).
Van Der Meersch, H., De Bacquer, D. & De Vriese, A. S. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Am Heart J. 184, 37–46 (2017).
Alhousani, M. et al. Using oral anticoagulants among chronic kidney disease patients to prevent recurrent venous thromboembolism: A systematic review and meta-analysis. Thromb Res. 198, 103–114 (2021).
De Aquino Moura, K. B. et al. Anticoagulant-related nephropathy: systematic review and meta-analysis. Clin Kidney J. 12, 400–407 (2019).
Lip, G. Y. H. et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 154, 1121–1201 (2018).
Bowrin, K., Briere, J. B., Levy, P., Toumi, M. & Millier, A. Use of real-world evidence in meta-analyses and cost-effectiveness models. J Med Econ. 23, 1053–1060 (2020).
Acknowledgements
We acknowledge the contribution of the Health Authorities of the “Conselleria de Salud Universal” Generalitat Valenciana for allowing to use the data.
Funding
This work was supported by the BigData@heart (IMI2-FPP116074-2); BIGMEDILYTICS (ICT-15-780495); PI16/01402, CIBERObn Institute of Health Carlos III.
Author information
Authors and Affiliations
Contributions
J.C., J.R. and M.F. were in charge of the conceptualization. F.M., J.R. and J.C organized the Methodology. A.F., I.S., J.D., R.U. and J.T did the Formal analysis. J.R and M.F. performed the Investigation. A.F., I.S. and J.C. curated Data. J.C., M.F. and J.R. wrote the main manuscript: original draft preparation. M.F., J.R., J.D., R.U. and J.T. wrote the main manuscript: review and editing. All authors have read and agreed to the published version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calderon, J.M., Martinez, F., Fernandez, A. et al. Real world data of anticoagulant treatment in non-valvular atrial fibrillation across renal function status. Sci Rep 12, 6123 (2022). https://doi.org/10.1038/s41598-022-10164-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10164-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.