Abstract
Sustained forms of atrial fibrillation (AF) may be associated with a higher risk of adverse outcomes, but few if any long-term studies took into account changes of AF type and co-morbidities over time. We prospectively followed 3843 AF patients and collected information on AF type and co-morbidities during yearly follow-ups. The primary outcome was a composite of stroke or systemic embolism (SE). Secondary outcomes included myocardial infarction, hospitalization for congestive heart failure (CHF), bleeding and all-cause mortality. Multivariable adjusted Cox proportional hazards models with time-varying covariates were used to compare hazard ratios (HR) according to AF type. At baseline 1895 (49%), 1046 (27%) and 902 (24%) patients had paroxysmal, persistent and permanent AF and 3234 (84%) were anticoagulated. After a median (IQR) follow-up of 3.0 (1.9; 4.2) years, the incidence of stroke/SE was 1.0 per 100 patient-years. The incidence of myocardial infarction, CHF, bleeding and all-cause mortality was 0.7, 3.0, 2.9 and 2.7 per 100 patient-years, respectively. The multivariable adjusted (a) HRs (95% confidence interval) for stroke/SE were 1.13 (0.69; 1.85) and 1.27 (0.83; 1.95) for time-updated persistent and permanent AF, respectively. The corresponding aHRs were 1.23 (0.89, 1.69) and 1.45 (1.12; 1.87) for all-cause mortality, 1.34 (1.00; 1.80) and 1.30 (1.01; 1.67) for CHF, 0.91 (0.48; 1.72) and 0.95 (0.56; 1.59) for myocardial infarction, and 0.89 (0.70; 1.14) and 1.00 (0.81; 1.24) for bleeding. In this large prospective cohort of AF patients, time-updated AF type was not associated with incident stroke/SE.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is classified into paroxysmal, persistent or permanent AF1. This classification into different AF types is useful in clinical practice, as more sustained forms of the arrhythmia are less amenable to rhythm control treatment strategies. For example, catheter ablation is less successful among patients with persistent AF compared to paroxysmal AF2.
Patients with AF face an increased risk of stroke, congestive heart failure (CHF) and death3,4,5. To what extent the risk of these and other outcome events is influenced by AF type is still debated. A recent meta-analysis found a higher risk of thromboembolism and death in patients with non-paroxysmal AF than in patients with paroxysmal AF during 1 to 2.8 years of follow-up6. This raises the possibility that the arrhythmia itself is involved in the disease process and may at least in part explain the increased risk for adverse outcome events. On the other hand, patients with non-paroxysmal AF are usually older and have a higher burden of risk factors and co-morbidities than patients with paroxysmal AF7,8, suggesting that co-morbidities and not AF type may be key drivers for the increased risk of adverse outcome events.
AF type9 and co-morbidities10 change over time in a relevant number of AF patients. However, none of the previous studies took these changes into account in their analyses. In this study we therefore assessed the risk of stroke/systemic embolism (SE) and other adverse outcome events according to AF type, taking into consideration changes of AF type and co-morbidities in models with time-varying covariates.
Methods
To increase sample size and power, we combined data from two ongoing prospective, observational, multicenter cohort studies with similar inclusion and exclusion criteria.
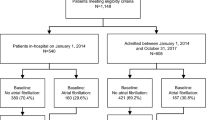
Between 2010 and 2014 a total of 1553 patients with documented AF were included in the Basel Atrial Fibrillation (BEAT-AF) study across 7 centers in Switzerland11. The Swiss Atrial Fibrillation (Swiss-AF) study enrolled 2415 AF patients between 2014 and 2017 across 14 centers in Switzerland12,13. In both cohorts, patients were required to have previously documented AF. Main exclusion criteria for both cohorts were the inability to sign informed consent, the presence of exclusively short transient episodes of AF (e.g., secondary after cardiac surgery, thyrotoxicosis or severe sepsis), as well as any acute illness within the last 4 weeks. Patients enrolled in BEAT-AF were not eligible for participation in Swiss-AF.
For the present study, we excluded 77 (1.9%) patients from the combined dataset without any follow-up information and 41 (0.9%) because of missing baseline AF type or co-morbidities. Seven (0.2%) patients with accidental inclusion in both cohorts were only counted once, leaving 3843 (97.0%) patients for this analyses, which used follow-up data up to April 17, 2019. The study protocols of both studies were approved by the local ethics committees (Ethikkommission Nordwest und Zentralschweiz), and informed written consent was obtained from all participants. All methods were performed in accordance with relevant guidelines and regulations.
Baseline and follow-up assessments
Patients completed detailed questionnaires about personal, medical, nutritional and lifestyle factors in both cohorts. Smoking status was categorized as current smokers or non-current smokers (past or never smokers). Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. AF was classified according to the guidelines of the European Society of Cardiology into the three main categories paroxysmal (self-terminating, usually within 48 h), persistent (episodes lasting longer than seven days or requiring termination by electrical or medical cardioversion) or permanent AF (AF is accepted by the patient and the physician and no further attempts to restore sinus rhythm are performed)1. Stroke risk was categorized using the CHA2DS2-VASc score as recommended by current guidelines1. The CHA2DS2-VASc score ranges from 0 to 9 by giving one point for a history of hypertension, CHF, diabetes, vascular disease, age between 65 and 74 years and female sex, and two points for a history of stroke/TIA or an age ≥ 75 years. Coronary artery disease was defined as either a history of myocardial infarction, percutaneous transluminal coronary angioplasty and/or coronary bypass graft surgery.
After a face-to-face baseline examination in both cohorts, yearly follow-up examinations in BEAT-AF were performed by mail and phone interviews, while in Swiss-AF all patients had yearly clinical follow-up visits. During these yearly follow-ups, we obtained updated information about risk factors, co-morbidities, AF type, and intercurrent adverse outcome events. The current AF type was assessed in both cohorts during each baseline and follow-up visit based on the study related information, as well as all available clinical information and medical reports.
Outcome events and definitions
The same outcome definitions were used in both cohorts. They are provided in Supplementary Table S1. Each event in each cohort was independently validated by two physicians. In case of discrepancy, a third physician was consulted for a final decision. The primary outcome for this analysis was a composite of stroke or SE. Secondary outcomes were myocardial infarction, hospitalization for CHF, bleeding, major adverse cardiovascular events (MACE) and all-cause mortality.
Statistical analysis
Baseline characteristics were stratified by AF type at study entry. Categorical variables were presented as numbers (percentages) and compared using χ2-tests. The distribution of continuous variables was checked using kurtosis, skewness and visual inspection of the histogram. Continuous variables were presented as mean ± standard deviation or median (interquartile range) and compared using analysis of variance or Kruskal–Wallis tests, as appropriate.
To assess the association of clinical predictors with adverse outcome events, we constructed Cox regression models with time-varying covariates to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) and to adjust for potential confounders. All regression models were adjusted for a pre-defined set of baseline covariates, including age, sex, and heart rate, and time-updated information on smoking (current vs. history/never smoker), BMI, diabetes, coronary artery disease, hypertension, CHF, stroke/transient ischemic attack (TIA), renal failure, oral anticoagulation, antiplatelet therapy, electrical cardioversion and pulmonary vein isolation. We used the most recent update of the AF type before the outcome event or the latest follow-up, as appropriate.
A sensitivity analysis was performed using a binary AF type classification of paroxysmal vs. non-paroxysmal (persistent or permanent) AF as these types can be difficult to be distinguished. An interaction analysis using a multiplicative interaction between cohort and AF type was performed for all outcome events. As information on BMI has not been collected during the first two follow-ups in BEAT-AF, we imputed weight, whenever missing, based on a linear mixed effects model which included age, sex, height and a natural spline for time since recruitment as fixed effects, and patient ID as random intercept. The assumption of proportional hazards was assessed visually by plotting log(-log(survival)) against log(time) for categorical variables. Schoenfeld-residuals were plotted for continuous variables. All analyses were conducted using R version 3.5.1 (R Core Team 2018). A two-sided p value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics stratified by baseline AF type are shown in Table 1. Mean (± SD) age of the total population was 71 ± 10 years and 1080 (28%) participants were women. At baseline, 1895 (49%) patients had paroxysmal AF, 1046 (27%) persistent AF and 902 (24%) permanent AF. Patients with persistent or permanent AF were older, less often women, and more often had a history of coronary artery disease, hypertension, CHF, diabetes and renal failure than patients with paroxysmal AF (all p < 0.001). Among patients with paroxysmal, persistent and permanent AF, mean (± SD) CHA2DS2-Vasc Score was 3.0 (± 1.8), 3.1 (± 1.7) and 3.9 (± 1.6) respectively (p < 0.001), and 76%, 91% and 94% of patients received oral anticoagulation (p < 0.001).
During 11,920 patient-years of follow-up, we observed a total of 1177 (14.6%) changes in the AF type. Any AF progression was observed among 490 (12.8%) patients, any AF regression in 343 (8.9%) patients. The overall incidence of stroke/SE was 1.0 per 100 patient-years of follow-up. It was 0.8, 1.0 and 1.5 per 100 patient-years in patients with paroxysmal, persistent and permanent AF (Table 2). In age and sex adjusted models, the HR (95% CI) for stroke/SE was 1.22 (95% CI 0.76; 1.94, p = 0.41) for time-updated persistent and 1.35 (95% CI 0.88; 2.05, p = 0.17) for time-updated permanent AF. The multivariable adjusted HRs were 1.13 (95% CI 0.69; 1.85), p = 0.64 and 1.27 (95% CI 0.84; 2.00), p = 0.28, respectively.
Table 3 shows absolute and relative risks for secondary outcome events according to AF type. The incidence per 100 patients-years was higher among patients with time-updated persistent and permanent AF for all outcome events. In age and sex adjusted models, presence of time-updated persistent AF was significantly associated with CHF hospitalizations (HR 1.47 [95% CI 1.12; 1.93], p < 0.001). Presence of time-updated permanent AF was significantly associated with a higher risk of CHF hospitalization (HR 1.78 [95% CI 1.39; 2.26], p < 0.001), MACE (HR 1.75 [95% CI 1.37; 2.23], p < 0.001), all-cause mortality (HR 1.80 [95% CI 1.40; 2.30], p < 0.001), clinically relevant non-major bleeding (HR 1.38 [95% CI 1.07; 1.77], p = 0.01) and any bleeding (HR 1.28 [95% CI 1.05; 1.57], p = 0.02). The associations were attenuated in multivariable models that adjusted for time-updated co-morbidities, but time-updated persistent AF remained associated with CHF hospitalizations (p = 0.05) and presence of time-updated permanent AF remained significantly associated with CHF hospitalization (p = 0.04), MACE (p = 0.008) and all-cause mortality (p = 0.005) (Table 3). All AF type by cohort p-values for interaction in the multivariable models were > 0.05 (data not shown).
Independent predictors for the primary outcome stroke/SE were history of stroke/TIA (HR 2.70 [95% CI 1.88; 3.89], p < 0.001), increasing age (HR 1.05 per year [95% CI 1.03; 1.08], p < 0.001), history of CHF (HR 1.51 [95% CI 1.01; 2.24], p = 0.04) and oral anticoagulation (HR 0.59 [95% CI 0.35; 1.00], p = 0.05). HRs for all covariates of primary and secondary outcomes are presented in the Supplementary Table S2.
The results remained broadly consistent when persistent and permanent AF were combined in time-updated non-paroxysmal AF for a binary sensitivity analysis (Supplementary Table S3).
Discussion
In this large study of well-treated patients with AF, the incidence rate of stroke/SE among patients with time-updated permanent AF was almost twice as high compared with patients with paroxysmal AF. Adjustment for age and sex weakened this association and after taking into account the effect of risk factors, co-morbidities and their changes over time, the association of AF type and stroke/SE was further attenuated. Major confounding was also observed for all secondary outcome events, although time-updated AF type remained significant association with some of these outcomes. These results suggest that co-morbidities have a significant impact on the relationships between AF type and risk of adverse outcomes.
With an incidence of 1.0 cases per 100 patient-years the risk of stroke/SE was low and overall comparable to stroke rates in well-treated patients from contemporary clinical trials14,15,16,17. Overall, stroke rates across different cohort studies are highly heterogeneous18. The low incidence in our study can be explained at least in part by the high prevalence of oral anticoagulation. At baseline more than 84% of the whole study population and more than 90% of patients classified as having non-paroxysmal AF received oral anticoagulation, and this prevalence tends to go up over time19.
In adjusted models with time-varying covariates, time-updated persistent and permanent AF were not significantly associated with stroke/SE. A recent meta-analysis found a pooled adjusted HR for thromboembolism in patients with non-paroxysmal AF of 1.38 (95% CI 1.91; 1.61), p < 0.0016. It is important to note that none of the studies that has been included in the meta-analysis updated AF type nor co-morbidities. Especially not updating co-morbidities and risk-factor might exacerbate the problem of residual confounding and may lead to an overestimation of the effect of AF type, given the frequent changes in patients with AF10. Also, the effect was strongest among patients without oral anticoagulation6. Taken together, our results suggest that the independent effect of AF type on stroke risk is modest at best.
On the other hand, several risk factors of the CHA2DS2-VASc Score were independently associated with stroke/SE in our study (Table S2). Yoon et al. assessed dynamic changes of the CHA2DS2-VASc Score and the risk of ischemic stroke in a nationwide cohort of more than 160,000 AF patients. They found an annual increase of the CHA2DS2-VASc Score of 0.14 points and rates of stroke increased when patients accumulated risk factors10. Another study on changes in risk factors over time among AF patients showed that 52% of the whole cohort and 90% of patients who experienced a stroke during follow-up developed at least one new CHA2DS2-VASc risk factor. The most common new-onset risk factor was hypertension, followed by CHF20. Therefore clinicians should be aware of the clinical importance to account for dynamic changes of the co-morbidities when assessing the stroke risk in AF patients. Taken together, while presence of persistent and permanent AF might be a mirror of a higher risk factor burden, it should not be used to guide anticoagulation treatment in AF patients.
In multivariable models, presence of time-updated permanent AF was significantly associated all-cause mortality, hospitalization for CHF and MACE. The association with mortality could be driven by more severe strokes, which have been described among patients with persistent and permanent AF21,22, but also through new-onset or worsening CHF4. In addition, cardiovascular risk factors and co-morbidities such as renal failure, diabetes, history of CHF and age were more prevalent in non-paroxysmal AF patients (Table 1) and also strongly associated with all outcome events (Table S2). Therefore, optimal risk factor control and optimal treatment of co-morbidities remains a major target to prevent adverse outcome events among AF patients.
The absolute risks of CHF hospitalizations, all-cause mortality and bleeding were much higher than the risk of stroke/SE. While better risk prediction and effective treatment with oral anticoagulation led to a significant decline of stroke risk over the last decades23, the improvement in mortality was only modest23,24 and the outcomes of CHF have not improved at all25. It is well known that AF is an independent risk factor for all-cause, cardiovascular and non-cardiovascular death5 and the majority of deaths among anticoagulated AF patients were related to CHF26. Previous data showed that modifiable risk factors, including obesity, smoking, hypertension and diabetes accounted for the majority of CHF risk in AF patients27. Importantly, once AF patients developed CHF, the risk of death significantly increased27. Time-updated persistent and permanent AF were independently associated with hospitalization for CHF and time-updated permanent AF was additionally associated with all-cause mortality and MACE suggesting that the AF type might be helpful in risk assessment of these secondary outcome events. In order to address the excess in mortality and morbidity beyond thromboembolism a more holistic and integrated approach to AF management has been advocated28,29 and been incorporated into the current guidelines for AF management30, aiming to not only avoid stroke, but also improve management of symptoms as well as cardiovascular risk factors and co-morbidities. Adherence to this pathway has shown to significantly reduce major adverse outcome events, especially among frail patients31.
One of the key strengths of this study is the large sample size, the fact that all outcome events were independently validated by two physicians, and that co-morbidities and AF type were updated on a yearly basis. On the other hand, some potential limitations need to be taken into account in the interpretation of our results. Due to the observational study design, we are not able to prove causality and residual confounding may persist despite comprehensive multivariable adjustment. Sex-specific analyses were not performed given the relatively low number of women enrolled in our cohorts. The assessment of the clinical AF type is challenging, and does not well correlate with AF burden32. However, assessment of AF type is very commonly performed in clinical practice, and our results therefore are of clinical interest.
Conclusion
In this large, prospective study of contemporary AF patients, presence of time-updated persistent or permanent AF was not associated with an increased risk of stroke/SE in models that accounted for changes in AF type and co-morbidities. While time-updated AF type remained significantly associated with all-cause mortality and CHF hospitalizations, taking into account co-morbidities and risk factors attenuated these results. Therefore, better risk-factor control and management of co-morbidities might help to reduce the incidence of cardiovascular outcomes and mortality among AF patients.
Data availability
The patient informed consent forms, as approved by the responsible ethics committee (Ethikkommission Nordwest und Zentralschweiz), do not allow the data to be made publicly available. The participants signed a consent form, which states that their data, containing personal and medical information, are exclusively available for research institutions in an anonymized form. Researchers interested in obtaining the data for research purposes can contact the Swiss-AF scientific lead. Contact information is provided on the Swiss-AF website (http://www.swissaf.ch/contact.htm). Authorization of the responsible ethics committee is mandatory before the requested data can be transferred to external research institutions.
References
Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart. J. 37, 2893–2962. https://doi.org/10.1093/eurheartj/ehw210 (2016).
Chen, H. S., Wen, J. M., Wu, S. N. & Liu, J. P. Catheter ablation for paroxysmal and persistent atrial fibrillation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD007101.pub2 (2012).
Wolf, P. A., Abbott, R. D. & Kannel, W. B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 22, 983–988 (1991).
Wang, T. J. et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 107, 2920–2925 (2003).
Conen, D. et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 305, 2080–2087. https://doi.org/10.1001/jama.2011.659 (2011).
Ganesan, A. N. et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur. Heart J. 37, 1591–1602. https://doi.org/10.1093/eurheartj/ehw007 (2016).
Chiang, C. E. et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ. Arrhythm. Electrophysiol. 5, 632–639. https://doi.org/10.1161/circep.112.970749 (2012).
Nieuwlaat, R. et al. Atrial fibrillation management: a prospective survey in ESC member countries: The Euro Heart Survey on Atrial Fibrillation. Eur. Heart J. 26, 2422–2434. https://doi.org/10.1093/eurheartj/ehi505 (2005).
Blum, S. et al. Incidence and predictors of atrial fibrillation progression: A systematic review and meta-analysis. Heart Rhythm https://doi.org/10.1016/j.hrthm.2018.10.022 (2018).
Yoon, M. et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in asian patients with atrial fibrillation: A nationwide cohort study. Thromb. Haemost. 118, 1296–1304. https://doi.org/10.1055/s-0038-1651482 (2018).
Blum, S. et al. Prospective assessment of sex-related differences in symptom status and health perception among patients with atrial fibrillation. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.005401 (2017).
Conen, D. et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J. Am. Coll. Cardiol. 73, 989–999. https://doi.org/10.1016/j.jacc.2018.12.039 (2019).
Conen, D. et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): Structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med. Wkly. 147, w14467. https://doi.org/10.4414/smw.2017.14467 (2017).
Connolly, S. J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151. https://doi.org/10.1056/NEJMoa0905561 (2009).
Granger, C. B. et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365, 981–992. https://doi.org/10.1056/NEJMoa1107039 (2011).
Patel, M. R. et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891. https://doi.org/10.1056/NEJMoa1009638 (2011).
Giugliano, R. P. et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 369, 2093–2104. https://doi.org/10.1056/NEJMoa1310907 (2013).
Quinn, G. R., Severdija, O. N., Chang, Y. & Singer, D. E. Wide variation in reported rates of stroke across cohorts of patients with atrial fibrillation. Circulation 135, 208–219. https://doi.org/10.1161/circulationaha.116.024057 (2017).
Zimny, M. et al. Uptake of non-vitamin K antagonist oral anti coagulants in patients with atrial fibrillation—A prospective cohort study. Swiss Med. Wkly. 147, w14410 (2017).
Chao, T.-F. et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J. Am. Coll. Cardiol. 71, 122–132. https://doi.org/10.1016/j.jacc.2017.10.085 (2018).
Staszewski, J., Brodacki, B., Tomczykiewicz, K., Kotowicz, J. & Stepien, A. Strokes in paroxysmal atrial fibrillation have more favorable outcome than in permanent atrial fibrillation. Acta Neurol. Scand. 119, 325–331. https://doi.org/10.1111/j.1600-0404.2008.01100.x (2009).
Deguchi, I. et al. Clinical outcomes of persistent and paroxysmal atrial fibrillation in patients with stroke. J. Stroke Cerebrovasc. Dis. 23, 2840–2844. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.010 (2014).
Schnabel, R. B. et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386, 154–162. https://doi.org/10.1016/s0140-6736(14)61774-8 (2015).
Lane, D. A., Skjoth, F., Lip, G. Y. H., Larsen, T. B. & Kotecha, D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.005155 (2017).
Miyasaka, Y. et al. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: A community-based study over two decades. Eur. Heart J. 27, 936–941. https://doi.org/10.1093/eurheartj/ehi694 (2006).
Marijon, E. et al. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 128, 2192–2201. https://doi.org/10.1161/CIRCULATIONAHA.112.000491 (2013).
Chatterjee, N. A. et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail. 5, 552–560. https://doi.org/10.1016/j.jchf.2017.04.004 (2017).
Romiti, G. F. et al. Adherence to the “atrial fibrillation better care” pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb. Haemost. https://doi.org/10.1055/a-1515-9630 (2021).
Lip, G. Y. H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 14, 627–628. https://doi.org/10.1038/nrcardio.2017.153 (2017).
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 42, 373–498. https://doi.org/10.1093/eurheartj/ehaa612 (2021).
Yang, P. S. et al. Application of the simple atrial fibrillation better care pathway for integrated care management in frail patients with atrial fibrillation: A nationwide cohort study. J. Arrhythm. 36, 668–677. https://doi.org/10.1002/joa3.12364 (2020).
Charitos, E. I., Purerfellner, H., Glotzer, T. V. & Ziegler, P. D. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J. Am. Coll. Cardiol. 63, 2840–2848. https://doi.org/10.1016/j.jacc.2014.04.019 (2014).
Acknowledgements
All BEAT-AF and Swiss-AF investigators and contributors are listed below.
Funding
BEAT-AF has been supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Roche Diagnostics, Boehringer Ingelheim, Sanofi-Aventis, MerckSharp&Dome, Bayer, Daiichi-Sankyo, Pfizer/Bristol-Myers Squibb and the Foundation for Cardiovascular Research Basel (FCVR Basel). The Swiss-AF cohort study is supported by grants of the Swiss National Science Foundation (33CS30_1148474 and 33CS30_177520), the University of Basel, Roche Diagnostics and the Foundation for Cardiovascular Research Basel (FCVR Basel). David Conen holds a McMaster University Department of Medicine Mid-Career Research Award. His work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: P.A., P.E., G.M., M.D.V., D.S., J.S., J.H.B., R.K., N.R., M.K., C.S., S.O. Data curation: S.B., S.A., P.B.M., R.M., D.C. Formal analysis: S.B., M.C., D.C. Funding acquisition: S.O., M.K., S.O. Methodology: S.B., M.C., D.C. Resources: M.K., S.O., D.C. Supervision: P.A., P.E., G.M., M.D.V., D.S., J.S., J.H.B., R.K., N.R., M.K., C.S., S.O. Writing – original draft: S.B., D.C. Writing – review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
SB: research grant from the Mach-Gaensslen Foundation outside the submitted work; DS: speaker fees from Biosense Webster, Daiichi-Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, and Bayer; consultancy honoraria from Biosense Webster; JHB: grants from the Swiss National Foundation of Science, The Swiss Heart Foundation, grants from Bayer, lecture fees from Sanofi Aventis and Amgen, to the institution outside the submitted work; RK: grants from Biotronik, BiosenseWebster, Boston Scientific, Medtronic, and Abbott; LHB: grants from the Swiss National Science Foundation (PBBSB-116873, 33CM30-124119, 32003B-156658; Berne, Switzerland), The Swiss Heart Foundation (Berne, Switzerland, and the University of Basel (Basel, Switzerland); unrestricted research grant from AstraZeneca, and consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical, and travel grants from AstraZeneca and Bayer; NR: grant from the Swiss Heart Foundation; MK: served on the speakers’ bureau for Boston Scientific, St. Jude Medical and Biotronik; lecture/consulting fees from Sorin, Boehringer Ingelheim, Bayer, Sanofi Aventis, Novartis, Medtronic, Pfizer-BMS; unrestricted grants from Bayer and Pfizer-BMS; proctor for Medtronic (Cryoballoon); CS: speaker honoraria from Biosense Webster, Boston Scientific and Medtronic; research grants from Biosense Webster, Daiichi-Sankyo, and Medtronic; proctor for Medtronic (Cryoballoon); DC: consultant/speaker fees from Servier Canada outside of the submitted work. No other author has any conflict of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blum, S., Aeschbacher, S., Coslovsky, M. et al. Long-term risk of adverse outcomes according to atrial fibrillation type. Sci Rep 12, 2208 (2022). https://doi.org/10.1038/s41598-022-05688-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05688-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.