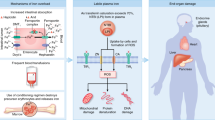
Abstract
Liver damage affects the prognosis of patients with erythropoietic protoporphyria (EPP). However, there is no radical cure for EPP patients with severe liver damage. This study aims to investigate the effectiveness of phlebotomy in patients with severe liver damage. We examined seven patients diagnosed with EPP and liver damage between 2010 and 2020. Of the 7 cases, phlebotomy was performed in 3 cases with severe hepatic disorder, and the improvement effect of hepatic disorder was observed in all cases. In addition, as an additional study, we also investigated the mechanism by which liver damage becomes more severe. Liver biopsy samples were stained with hematoxylin and eosin and immunohistochemistry was used to examine the expression of adenosine triphosphate-binding transporter G2 (ABCG2). Liver biopsies were performed in 3 of 7 patients with EPP. Of these three patients, ABCG2 expression was low in two patients, especially in the protoporphyrin (PP) deposition area. Two patients with reduced ABCG2 expression subsequently developed severe liver damage. However, the causal relationship between the decreased expression of ABCG2 and the exacerbation of liver damage has not been directly proved, and further investigation is required in the future. This study demonstrated the effectiveness of phlebotomy in EPP patients with severe liver damage.
Similar content being viewed by others
Introduction
Hereditary porphyria is a metabolic disorder that develops when any of the enzymes involved in the heme synthesis system is genetically impaired. This disease significantly impairs the patients’ quality of life; currently, there is no radical treatment for hereditary porphyria indicating that patients may develop serious sequelae, which may be fatal. Erythropoietic porphyria (EPP) is caused by mutations in the FECH gene1. In 10%–20% of patients with EPP, hepatic dysfunction is observed due to deposition of erythrocyte protoporphyrin and serum protoporphyrin in hepatocytes and bile canaliculi2,3. Approximately 2%–5% of patients with EPP die because of liver damage gradually progressing to cirrhosis or liver failure (chronic liver failure), which rapidly develops into irreversible cholestatic liver failure (acute liver insufficiency). Regarding the onset of liver dysfunction, in cases with mutations in the FECH gene that lead to loss of enzyme activity, the precursor protoporphyrin potentially accumulates, leading to an increased incidence of liver damage. In contrast, while it is reported that aggravation of liver damage correlates with protoporphyrin levels4, other study have reported cases without any correlation between liver damage and serum protoporphyrin levels5. Currently, our understanding about the association of liver damage with EPP is limited.
Ursodeoxycholic acid6, cimetidine7,8,9, cholestyramine10, and plasmapheresis11, among other compounds, have been reported to be effective for liver damage in EPP. Moreover, liver transplants have been reported to be beneficial; however, the long-term clinical course after treatment remains unclear12. Therefore, there is an urgent need to identify the most effective treatment for patients with severe liver damage.
In this study, we report the efficacy of phlebotomy in EPP patients with severe liver injury treated at our hospital.
Results
Patient background
The median age at referral was 31 years (26–64 years). Of the seven patients, six were male and one was female. Family history was observed in 6 patients and photosensitivity was observed in all 7 patients. The serum PP level at the time of referral was as high at 4059 (range 2973–14,518) µg/dL red blood cells (RBCs) (Table 1). EPP was confirmed in six patients by genetic testing (Table 2)13. All the EPP patients in which FECH mutations were identified are predicted to be caused by the pathogenic FECH mutation in combination with the low expression allele c.315-48C in trans13.
Liver function and iron metabolism test at the time of referral
Table 3 shows the results of each liver function test at the time of referral. Hepatocellular injury type was noted in one patient (No. 6), cholestatic type in two (No. 1, 4), mixed type in one patient (No. 5), and an unclassifiable type in three patients (No. 2, 3, and 7)14. In addition, two patients showed jaundice at the time of referral (No. 1, 3). No obvious anemia was observed in 7 patients at the time of referral.
Treatment and prognosis
Thorough shading was performed in all the nine patients. Ursodeoxycholic acid was used in four patients, cimetidine in five patients, and colestyramine in two patients (Table 1). Since these existing treatments were ineffective, 3 patients (No1, No5, No6) subsequently added phlebotomy. No cases of liver cancer were observed during the course of the study until March 2021.
Laboratory data immediately before phlebotomy and efficacy of phlebotomy
Blood chemistry data (collected immediately before phlebotomy) are summarized for the three patients (No. 1, 5, and 6) who underwent phlebotomy. All the three patients had overt jaundice and significantly elevated serum PP levels (Table 4).
In patient No. 1, plasma exchange was performed five times; although serum PP levels were decreased, hepatic injury did not improve. Phlebotomy was performed four times in total (400 mL was removed in the first time and 200 mL was removed thereafter). A decrease in the serum PP level and subsidence of liver injury was observed, along with a decrease in the Hb and serum ferritin levels (Fig. 1).
Clinical course after phlebotomy. In patient No. 1, plasma exchange was not performed. Phlebotomy therapy was performed three times (first time, 400 mL; following 2–3 times, 200 mL). In patient No. 5, a plasma exchange was performed five times; however, the serum PP level and liver damage did not improve; thus, the treatment was changed to phlebotomy. Phlebotomy was carefully performed four times in total (400 mL the first time; 200 mL thereafter). In patient No. 6, plasma exchange was performed four times; however, improvement in the serum PP level and hepatitis is not obtained. Thus, the treatment was changed to phlebotomy. Phlebotomy was performed four times in total (400 mL the first time; 200 mL thereafter).
In patient No. 5, plasma exchange was performed five times and the serum PP levels were decreased; however, liver injury did not improve. Phlebotomy was conducted four times in total (400 mL was removed in the first time and 200 mL removed thereafter), and a decrease in the serum PP level was observed with improvement in liver injury (Fig. 1).
In patient No. 6, phlebotomy therapy was performed three times (400 mL was removed in the first time and 200 mL was removed thereafter) from the beginning; the serum PP level increased and liver injury improved along with a decrease in the Hb and serum ferritin levels (Fig. 1).
Pathological findings
Liver biopsy was performed in 3 cases, and the Maltese cross was confirmed in all cases. In No. 5 and No. 6, PP deposits were distributed in the bile duct and hepatocytes. On the other hand, in No. 2, PP deposition was mainly in the bile duct and less was deposited in hepatocytes (Fig. 2). The stainability of ABCG2 was guaranteed in No. 2, On the other hand, it showed a marked decrease in No. 5 and No. 6 (Fig. 2).
Moreover, to evaluate damage in the cell membrane of affected hepatocytes, double staining with ABCG2 and cadherin was performed (Fig. 3). In patient No. 5, cadherin was well expressed in PP-deposited hepatocytes, whereas ABCG2 was stained, suggesting the selective loss of ABCG2 activity on the cell membrane. Although the expression of cadherin in PP-deposited hepatocytes was heterogeneously detected in patient No. 6, ABCG2 staining was reduced in hepatocytes with PP deposition, indicating the loss of ABCG2 activity on the preserved cell membrane as well as damage to the cell membrane. Contrastingly, in patient No. 2, the expression of cadherin and ABCG2 was maintained.
ABCG2 and cadherin double staining pathology. To evaluate the function of the hepatic cell membrane, cadherin staining was performed and double staining with ABCG2 was performed. In No. 5, cadherin (red) was strongly stained in PP-deposited hepatocytes, but ABCG2 (green) was weakly stained (black circles). In No. 6, both the staining properties of ABCG2 (green) and cadherin (red) were decreased in PP-deposited hepatocytes (the part indicated by the red circle). On the other hand, in No. 2, the stainability of ABCG2 and cadherin was guaranteed.
Discussion
In general, liver damage is a factor that mostly affects the prognosis of patients with EPP. Approximately 5–10% of all patients with EPP have liver damage, where 1% of these patients have fatal conditions2,3. Therefore, there is an urgent need to establish effective treatment for EPP patients with severe liver damage. EPP is caused by the accumulation of excess protoporphyrin in RBCs; hence, plasmapheresis may be useful for removing haemolyzed and spilled protoporphyrin in the blood. However, it was not effective in patient No. 5 and 6, suggesting that sufficient protoporphyrin could not be removed. Therefore, plasmapheresis was followed by phlebotomy in these patients15,16,17. In patient No. 1, phlebotomy was introduced from the beginning. Phlebotomy is theoretically suitable for the removal of protoporphyrin in erythrocytes. However, hypermyelination due to the progression of anemia caused by phlebotomy may induce further production of protoporphyrins, which may worsen the condition. Therefore, we carefully performed a 400-mL phlebotomy for the first time by monitoring the blood data (such as Hb levels) and then slowly performed a 200-mL phlebotomy once every 1–2 weeks. After phlebotomy, liver damage rapidly subsided simultaneously with the decrease in protoporphyrin in all three patients; liver injury relapse was not noted thereafter. Phlebotomy has been proposed for congenital erythropoiesis (Günter's disease), acute liver and cutaneous porphyria18. This strategy is expected to suppress heme biosynthesis through the regulation of ALAS, a pathway restriction enzyme that leads to the accumulation of porphyrins. Although the effectiveness of phlebotomy in EPP patients has been shown this time, it is necessary to investigate the mechanism such as the regulatory action of ALAS in the future.
There are also reports on the onset and exacerbation factors of liver damage in EPP patients.
It has been reported that PP has low water solubility, which is an important mechanism for the onset of liver damage; the excretion of PP into the bile duct causes inflammation due to viscosity19. Furthermore, it has been reported that PP level and liver damage are correlated4. However, there are many cases in which the PP level does not correlate with the severity of liver damage, and therefore, it is necessary to analyze the mechanism of onset and severity of liver damage. In this study, we focused on ABCG2, a type of hepatocyte transporter, because it is involved in porphyrin transport20,21,22. We have previously reported the development of different levels of liver damage between siblings with the same PP level and the association between ABCG2 staining and liver damage5.
In this study, we increased the number of patients and conducted additional studies. Liver biopsies were performed in 3 of the 7 patients (No. 2, 5, and 6) to examine ABCG2 expression. ABCG2 staining was lower in patients No. 5 and 6 who had more severe liver damage than in patients No. 2. However, we also considered the possibility that the decreased expression of ABCG2 was affected by reactive oxygen species production due to the accumulation of protoporphyrin in hepatocytes. To solve this problem, we performed double staining with cadherin, which is a tight junction. Patient No. 5 maintains cadherin expression in protoporphyrin-deposited hepatocytes, indicating a selective loss of ABCG2 in the conserved cell membrane of hepatocytes. From this result, the accumulation of porphyrins in hepatocytes with loss of ABCG2 may be important for the onset and exacerbation of liver damage. However, we have not directly proved the causal relationship between the decreased expression of ABCG2 and liver damage, and further investigation is required in the future.
In recent years, there have been very interesting reports that ABCG2 deficiency protects against EPP-related hepatotoxicity23. It is considered that one of the mechanisms of liver damage protection is that the excretion of PP into the bile duct is reduced due to the decreased expression of ABCG2. On the other hand, in actual human patients, PP accumulation in hepatocytes and apoptosis of hepatocytes with decreased ABCG2 expression can be observed, so there may be a mechanism different from that of the mouse model. In any case, further research is needed on mechanism analysis.
In conclusion, phlebotomy has proven to be an effective treatment option in EPP patients with severe liver damage. Further research is needed to elucidate the pathophysiology of EPP in order to suppress porphyrin production and improve liver damage.
Methods
Patient background
We examined seven patients (No. 1–7) who were diagnosed with EPP and liver damage.
Ethics declarations
Written informed consent were obtained from all of the patients enrolled in this study and ethical permission of this study was granted by the Review Boards of Kindai University Faculty of Medicine (approval number 25-085). All experiments were performed in accordance with the Declaration of Helsinki.
Pattern classification of liver damage
The pattern classification of liver damage was performed using the drug-induced liver damage diagnostic criteria (JDDW2004)14. Hepatocellular injury type; ALT > 2N + ALP ≤ N or ALT ratio/ALP ratio ≥ 5, cholestatic type; ALT ≤ N + ALP > 2 N or ALT ratio/ALP ratio ≤ 2, mixed type; ALT > 2 N + ALP > N and 2 < ALT ratio/ALP ratio < 5 (N: Upper limit of normal, ALT ratio = ALT value/N, ALP ratio = ALP value/N).
Phlebotomy
Phlebotomy was first performed by removing 400 mL and then by removing 200 mL several times, depending on the patient's condition.
Histological analysis
Percutaneous liver biopsy was performed in four patients. Liver biopsy samples were subjected to hematoxylin and eosin (H&E) staining. Immunohistochemical analyses were performed using mouse anti-human adenosine triphosphate-binding transporter G2 (ABCG2) antibody (Abcam, Cambridge, United Kingdom, BXP-21) and rabbit anti-human E-Cadherin (Cell Signaling Technology, Danvers, MA, 24E10). For fluorescence staining, secondary antibodies labeled with Alexa 488 or Alexa 555 were used (Life Technologies, Carlsbad, CA). Fluorescent images were taken using a confocal laser microscope (Carl Zeiss GmBH, Jena, Germany).
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Bloomer, J., Wang, Y., Singhal, A. & Risheg, H. Molecular studies of liver disease in erythropoietic protoporphyria. J. Clin. Gastroenterol. 39, S167–S175 (2005).
Anstey, A. V. & Hift, R. J. Liver disease in erythropoietic protoporphyria: Insights and implications for management. Gut 56, 1009–1018 (2007).
Todd, D. J. Erythropoietic protoporphyria. Br. J. Dermatol. 131, 751–766 (1994).
Bloomer, J. R. The liver in protoporphyria. Hepatology 8, 402–407 (1988).
Hagiwara, S. et al. Impaired expression of ATP-binding cassette transporter G2 and liver damage in erythropoietic protoporphyria. Hepatology 62, 1638–1639 (2015).
Pirlich, M., Lochs, H. & Schmidt, H. H. Liver cirrhosis in erythropoietic protoporphyria: Improvement of liver function with ursodeoxycholic acid. Am. J. Gastroenterol. 96, 3468–3469 (2001).
Horie, Y. et al. Cimetidine in the treatment of porphyria cutanea tarda. Intern. Med. 35, 717–719 (1996).
Tu, J. H., Sheu, S. L. & Teng, J. M. Novel treatment using cimetidine for erythropoietic protoporphyria in children. JAMA Dermatol. 152, 1258–1261 (2016).
Fujimori, N. et al. Cimetidine/lactulose therapy ameliorates erythropoietic protoporphyria-related liver injury. Clin. J. Gastroenterol. 10, 452–458 (2017).
Bloomer, J. R. Pathogenesis and therapy of liver disease in protoporphyria. Yale J. Biol. Med. 52, 39–48 (1979).
Do, K. D., Banner, B. F., Katz, E., Szymanski, I. O. & Bonkovsky, H. L. Benefits of chronic plasmapheresis and intravenous heme-albumin in erythropoietic protoporphyria after orthotopic liver transplantation. Transplantation 73, 469–472 (2002).
McGuire, B. M. et al. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl. 11, 1590–1596 (2005).
Nakano, H. et al. Novel ferrochelatase mutations in Japanese patients with erythropoietic protoporphyria: High frequency of the splice site modulator IVS3-48C polymorphism in the Japanese population. J. Invest. Dermatol. 126, 2717–2719 (2006).
Hanatani, T. et al. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharmacoepidemiol. Drug Saf. 23, 984–988 (2014).
Yoshida, A. et al. Erythropoietic protoporphyria-related hepatopathy successfully treated with phlebotomy. Intern. Med. 57, 2505–2509 (2018).
Egan, D. N., Yang, Z., Phillips, J. & Abkowitz, J. L. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood 126, 257–261 (2015).
Minder, E. I. & Barman-Aksözen, J. Iron and erythropoietic porphyrias. Blood 126, 130–132 (2015).
Blouin, J. M. et al. Identification of novel UROS mutations in a patient with congenital erythropoietic porphyria and efficient treatment by phlebotomy. Mol. Genet. Metab. Rep. 27, 100722 (2021).
Lyoumi, S. et al. Protoporphyrin retention in hepatocytes and Kupffer cells prevents sclerosing cholangitis in erythropoietic protoporphyria mouse model. Gastroenterology 141, 1509–1519 (2011).
Tamura, A. et al. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: Identification of alleles that are defective in porphyrin transport. Mol. Pharmacol. 70, 287–296 (2006).
Kobuchi, H. et al. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS ONE 7, e50082 (2012).
Ogino, T. et al. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol. Cell. Biochem. 358, 297–307 (2011).
Wang, P. et al. The essential role of the transporter ABCG2 in the pathophysiology of erythropoietic protoporphyria. Sci. Adv. 5, eaaw127 (2019).
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI: 18H03554, N. Nishida, 18K07922, M. Kudo, 19K08455)
Author information
Authors and Affiliations
Contributions
S.H., N.N., M.K.: Study concept and design; S.H., H.I., K.U., Y.M., M.T., T.A., M.M., Y.K., A.Y.: Patient recruitment and characterization; S.H., N.N., M.S., H.N.: Data acquisition; S.H., N.N., A.P.: Data analysis; S.H., N.N., M.K.: Article drafting; all authors provided input and critical revision and approved the final version. All the authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagiwara, S., Nishida, N., Ida, H. et al. Role of phlebotomy in the treatment of liver damage related to erythropoietic porphyria. Sci Rep 12, 6100 (2022). https://doi.org/10.1038/s41598-022-10089-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10089-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.