Abstract
Acute upper gastrointestinal bleeding (UGIB) in acute coronary syndrome (ACS) patients are not uncommon, particularly under dual antiplatelet therapy (DAPT). The efficiency and safety of early endoscopy (EE) for UGIB in these patients needs to be elucidated. This multicenter randomized controlled trial randomized recent ACS patients presenting acute UGIB to non-EE and EE groups. All eligible patients received intravenous proton pump inhibitor therapy. Those in EE group underwent therapeutic endoscopy within 24 h after bleeding. The data regarding efficacy and safety of EE were analyzed. It was early terminated because the UGIB rate was lower than expected and interim analysis was done. In total, 43 patients were randomized to non-EE (21 patients) and EE (22 patients) groups. The failure rate of control hemorrhage (intention-to-treat [ITT] 4.55% vs. 23.81%, p < 0.001; per-protocol [PP] 0% vs. 4.55%, p = 0.058) and 3-day rebleeding rate (ITT 4.55% vs. 28.57%, p = 0.033; PP 0% vs. 21.05%, p = 0.027) were lower in EE than non-EE group. The mortality, minor and major complication rates were not different between two groups. Male patients were at higher risk of minor and major complications after EE with OR (95% CI) of 3.50 (1.15–10.63) and 4.25 (1.43–12.63), respectively. In multivariate analysis, EE was associated with lower needs for blood transfusion (HR 0.13, 95% CI 0.02–0.98). Among patients who discontinued DAPT during acute UGIB, a higher risk (OR 5.25, 95% CI 1.21–22.74) of coronary artery stent re-thrombosis within 6 months was noticed. EE for acute UGIB in recent ACS patients has higher rate of bleeding control, lower 3-day rebleeding rate and lower needs for blood transfusion, but more complications in male patients. Further enrollment is mandatory to avoid bias from small sample size (ClinicalTrial.gov Number NCT02618980, registration date 02/12/2015).
Similar content being viewed by others
Introduction
Acute upper gastrointestinal bleeding (UGIB) remains challenging with significant morbidity and mortality despite advancements in pharmacological and endoscopic therapy1. Pharmacological therapy with proton pump inhibitors (PPIs) and therapeutic endoscopy using various modalities have been shown to significantly reduce re-bleeding, need for surgery and mortality of patients with acute UGIB1,2,3. Additionally, early endoscopy (EE) within 12–24 h, which not only allows early diagnosis and discharge of patients with low risk features, but also enables risk stratification and endoscopic hemostasis with less transfusion requirements and shorter hospital stay, has been recommended for medium-to-high risk patients1,2,4. However, endoscopy carries potential risk for complications. Therefore, to evaluate the pros and cons of EE is of paramount importance to treat high risk patients with acute UGIB, particularly for those with poor cardiopulmonary function5,6.
Dual antiplatelet therapy (DAPT) are the cornerstone in the management of patients with acute coronary syndrome (ACS)7,8. DAPT or other co-prescriptions further increase the major bleeding risk8,9. Consequently, serious peptic ulcer disease (PUD) complications occur in a significant proportion of ACS patients10,11,12. However, endoscopy hemostasis in ACS patients may impose risks for cardiopulmonary compromised13. Endoscopy within the first week after myocardial infarction (MI) seems to be associated with higher risk for cardiovascular events because of fragile remodeling myocardium, and the safety and timing of endoscopy is not well understood among ACS patients14,15. Given the lack of guidelines and randomized control trials (RCTs), gastroenterologists are always reluctant to perform endoscopy in ACS patients due to potential adverse events. In this study, we aimed to evaluate the efficacy and safety of EE versus pharmacological therapy alone for management of acute UGIB in ACS patients.
Materials and methods
Study design and randomization
A multicenter RCT of recent ACS patients presenting with acute UGIB was conducted in three tertiary centers (Far Eastern Memorial Hospital, Hsin-Chu Branch and Taipei Branch of National Taiwan University Hospital) in Taiwan. All methods were performed in accordance with the relevant CONSORT 2010 guidelines and regulations (ClinicalTrial.gov Number NCT02618980, registration date 02/12/2015) and was conducted according to Declaration of Helsinki. It was approved by the Research Ethics Review Committee of study institutes (FEMH IRB-103062-F, Hsin-Chu NTUH 105-001-F, Yun-Lin NTUH 201411020RIND). Patients with recent ACS, including unstable angina (UA), ST-elevation MI (STEMI) and non-ST elevation MI (NSTEMI) who presented symptoms of acute UGIB were evaluated for enrollment. The inclusion criteria were as follows: (1) age over 20-year-old, (2) ACS episodes in the past 2 weeks, (3) symptoms of UGIB including hematemesis, coffee ground emesis or tarry stool passage accompanied with a decrease in hemoglobin (Hb) level greater than 2 g/dL from baseline. Patients with any one of the following criteria were excluded: (1) malignancy or other advanced disease with a life expectancy of < 6 months, (2) pregnant or lactating women, (3) history of allergy or severe side effects from PPIs, contrast, and iodine, (4) platelet count < 80 k/μL, or prothrombin time INR > 2.0, (5) decompensated (Child-Turcotte-Pugh score B and C) liver cirrhosis, (6) stage 3–5 chronic kidney disease (CKD) (estimated Ccr < 60 mL/min/1.73 m2) using Cockcroft-Gault formula, exclusive of end-stage renal disease under renal replacement therapy16. All the authors had access to the study data and had reviewed and approved the final manuscript.
Informed consent was obtained from all eligible patients who were randomly assigned to EE or non-EE management. Patients in both groups received bolus intravenous pantoprazole 40 mg followed by continuous infusion (8 mg/h)3,17. In the EE group, patients underwent endoscopy within 24 h after onset of UGIB symptoms. All enrolled patients were monitored in cardiac intensive care unit (ICU). At endoscopy, stigmata of hemorrhage (SRH) were treated by endoscopic therapy in combination of any two of the followings: epinephrine submucosal injection, thermocoagulation, hemoclipping, and argon plasma coagulation. Hemostasis was considered initial successful if bleeding had stopped at endoscopy. Antral-biopsy specimens were obtained to a rapid urease test and histopathological examination for Helicobacter pylori (Hp) study. Patients assigned to non-EE group received medical treatment with PPIs alone and underwent esophagogastroduodenoscopy 2 weeks after enrollment to evaluate the recent SRH. All the enrolled patients in both groups were kept on oral form PPI for 3 months after 3-day infusional PPI therapy and acute UGIB controlled. Proton pump inhibitors were discontinued once peptic ulcer bleeding was excluded by endoscopy. Decision on discontinuation of DAPT was at the discretion of cardiologists depending on cardiac conditions of each enrolled patient.
Study endpoints
The primary endpoint was failure of control hemorrhage. The secondary endpoints included complication rate, length of hospital stay, units of blood transfusion, re-bleeding rate, needs for repeated intervention (endoscopic therapy, transarterial embolization (TAE), or surgery) for uncontrollable recurrent bleeding. Blood troponin-T, creatine kinase-MB, Hb, hematocrit (Hct) and complete electrocardiogram (ECG) were checked every 8 h within 24 h after enrollment. APACHE II, Rockall and Blatchford scores at intervention were calculated18. Primary care team in the cardiac ICU were blinded to the study protocol. The consulted gastroenterologists gave recommendation for management of UGIB after randomization. The clinical outcomes were recorded by primary care team as routine clinical practice.
Definition of failure to control hemorrhage
The time frame for acute bleeding episode was defined as 24 h after enrollment. Clinical failure of control bleeding was defined as: hematemesis or nasogastric tube drainage of significant fresh blood or coffee ground substance (≥ 200 mL) ≥ 2 h, or persistent hypovolemic shock after intervention; or 3 g/dL drop in Hb level (or 9% drop of Hct) within 24 h if no blood transfusion; or a decrease in Hb ≥ 2 g/dL or an increase ≤ 1 g/dL, despite 2 or more units of red blood cells (RBC) component transfusion within 24 h.
Definition of clinically significant re-bleeding
Clinically significant recurrent bleeding was defined by the followings: vomiting of fresh blood, fresh blood in the nasogastric tube (NGT) aspirate, hematochezia or melena, and a decrease in Hb ≥ 2 g/dL or an increase less than 1 g/dL, despite 2 or more units of RBC component transfusion after a normal color stool passage, and without coffee ground or fresh blood emesis or in the NGT aspirate.
Definition of major and minor complications
Major complications were defined as death and life-threatening arrhythmias within 24 h after randomization. Minor complications were defined as hypotension (< 90/60 mmHg), hypertension (> 180/100 mmHg), tachycardia (> 120 bpm), bradycardia (< 60 bpm), tachypnea (> 24/min.), oxygen desaturation (SpO2 < 90%), and minor arrhythmias.
Sample size estimation and randomization
The null hypothesis of this study was the superiority of EE over non-EE in the efficacy on bleeding control. The primary efficacy analysis used an intention-to-treat (ITT) approach that included all patients meeting the entry criteria who had completed the follow-up. Approximately 80% of UGIB patients will stop bleeding spontaneously19, and rates of hemostasis that resulted from a first endoscopic procedure exceeded 94% in most large studies20. However, there was no data demonstrating the outcome of patients under DAPT developing acute UGIB treated medically alone. Therefore, we assumed that about 70% of acute UGIB patients under DAPT would stop bleeding spontaneously without therapeutic endoscopy. As a result, we estimated a sample size of at least 78 patients in EE and non-EE groups in order to achieve a statistical power of 80% at a alpha of 0.05, beta of 0.02 significance level on a two-tailed test, with margin of error of 2% in order to detect a 24% (94% vs. 70%) difference. Sealed envelopes with computer generated randomization number (0 for non-EE, 1 for EE group) were used. The time point of randomization was the onset of UGIB symptoms and the non-stratified randomization was performed by gastroenterologists after emergent consultation from ICU. After enrollment, gastroenterologists opened the consecutive envelops for randomization.
Statistical analysis
Intention-to-treat and per-protocol (PP) analyses were done. Continuous variables were expressed as mean ± standard deviation and the comparisons between two groups were performed using the Student t test; categorical variables were summarized as count (%) and the comparisons between groups were made using the χ2 or the Fisher’s exact test when appropriate. Univariate and multivariate logistic regression models were performed for evaluation of the risk factors for outcomes in both groups. A two-tailed p value < 0.05 was considered as statistically significant. The statistical analysis was performed using STATA software (version 11.0; Stata Corp, College Station, TX, USA). Interim analysis was performed if there was statistically significant difference in primary endpoint between two groups.
Results
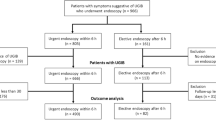
Sixty-five patients were assessed for enrollment and 43 (21 in non-EE and 22 in EE group) eligible ACS patients were randomized (Fig. 1 and Table 1). The study was terminated earlier because of slow enrollment. The age (mean ± SD) (70.67 ± 12.82 vs. 63.55 ± 12.19 years old, p = 0.069), gender (female ratio, 61.9% vs. 81.82%, p = 0.146), body mass index (23.71 ± 3.21 vs. 24.29 ± 3.38 kg/m2, p = 0.573), status of cigarette smoking (38.1% vs. 59.1%, p = 0.169), history of PUD (19.05% vs. 27.27%, p = 0.523), medications about prophylactic PPI, antiplatelet agents and non-steroid anti-inflammatory drugs (NSAIDs) and timing of UGIB after onset of ACS were not different between two groups. The most common clinical presentation of UGIB in both groups was tarry stool passage. The mean (± SD, range) timing of EE after presentation of acute UGIB was 13.56 (± 6.95, 2.23–22.68) h. The laboratory findings before intervention, cardiac function, proportion of multivessel disease, proportion of patients underwent coronary artery catheterization, and status of discontinuing DAPT were not different statistically between two groups. Patients in EE group had higher Rockall score (5.55 ± 2.70 vs. 4.14 ± 1.35, p = 0.039). Resuming any antiplatelet agent after intervention was numerically earlier in EE than non-EE group (5.13 ± 1.89 vs. 8.63 ± 5.26 days, p = 0.098).
Among five patients in the non-EE group and one patient in the EE group who failed control bleeding in the timeframe of 24 h after onset of UGIB, two of them in non-EE group underwent one time EGD and one in EE group underwent the first EGD after 4 h of the onset of UGIB then the second look EGD after 12 h of the UGIB due to persistent coffee ground substance in NGT with successful hemostasis. The remaining three patients in non-EE group refused endoscopy with PPI therapy. The failure rate of control hemorrhage (ITT/PP, 4.55% vs 23.81%/0% vs. 15.79%, p = 0.0002/0.058) and 3-day rebleeding rate (4.55% vs. 28.57%/0% vs. 21.05%, p = 0.033/0.027) were lower in EE than non-EE group (Table 2) and the trial was stopped due to significant difference in primary endpoint. None of enrolled patient underwent TAE or surgery for UGIB. Regarding the SRH, there was a higher proportion of gastric ulcer in non-EE group (61.90% vs. 36.36%/57.89% vs. 33.33%, p = 0.035/0.053), while Hp infection rate (33.33% vs. 40.91%/26.32% vs. 42.86%, p = 0.618/0.273) and length of hospital stay (11.57 ± 5.67 vs. 13.64 ± 10.99 days/11.05 ± 5.66 vs. 13.14 ± 11.01 days, p = 0.446/0.462) were not different between two groups. The units of RBC transfusion were lower after intervention (0.77 ± 1.23 vs. 2.76 ± 2.86 units/0.62 ± 1.02 vs. 2.63 ± 2.99 units, p = 0.005/0.006) in EE than non-EE group. The mortality, minor and major complication rates were not different between two groups, except for a higher proportion of patients with ECG ST-T changes in non-EE group (38.10% vs. 9.09%/41.11% vs. 9.25%, p = 0.024/0.018). One patient died of multiorgan failure due to poor cardiac function within 24 h after enrollment in EE-group.
In univariate analysis (Table 3), patients in EE group was not associated with renal function impairment [ITT/PP, hazard ratio (HR) 0.38/0.39, 95% confident interval (CI) 0.10–1.39/0.10–1.49], TnT elevation (HR 0.42/0.44, 95% CI 0.12–1.43/0.12–1.56), arrhythmias (HR 1.64/1.82, 95% CI 0.45–5.94/0.49–6.76), chest pain (HR 0.76/0.86, 95% CI 0.22–2.67/0.23–3.15), discontinuation of DAPT (HR 1.14/1.40, 95% CI 0.33–4.01/0.36–5.49), mortality (HR 0.39/0.29, 95% CI 0.08–1.85/0.05–1.75) and stent re-thrombosis (HR 0.74/0.68, 95% CI 0.19–2.91/0.17–2.73), but lower needs for blood transfusion (HR 0.23/0.23, 95% CI 0.07–0.84/0.06–0.88). In multivariate analysis, less needs for blood transfusion was also noted in EE group (HR 0.13/0.22, 95% CI 0.02–0.98/0.04–1.21) by ITT analysis. Among recent ACS patients with discontinuation of DAPT during acute UGIB, there was a higher risk [odds ratio (OR) 5.25, 95% CI 1.21–22.74)] of coronary artery stent re-thrombosis within 6 months after bleeding episode (Table 4). Male patients were at higher risk of minor and major complications after EE than female ones, with OR (95% CI) of 3.50 (1.15–10.63) and 4.25 (1.43–12.63), respectively.
Discussion
Management of acute UGIB and endoscopic examination in poor cardiac function patients are complex. To our knowledge, this study was the first multicenter RCT to evaluate the efficacy and safety of urgent endoscopy for management of acute UGIB in ACS patients. We have found that EE had higher rate of hemorrhage control, lower 3-day rebleeding rate, and lower needs for blood transfusion than PPI therapy alone. Additionally, early intervention with endoscopy did not increase risk of complications as compared with medical treatment. However, EE should be carefully considered for male patients with recent ACS, while there might be higher complication rate than female patients by logistic regression analysis in our study.
Acute UGIB remains challenging with significant morbidity, particularly composite ischemia (HR 1.94 at 30-day, p < 0.05, and 1.9 at 1-year, p < 0.001), mortality from all-cause (HR 4.87 at 30-day, p < 0.05, and 3.97 at 1-year, p < 0.05) and cardiac events (HR 5.35 at 30-day, p < 0.05, and 3.77 at 1-year, p < 0.05)1,21,22,23,24. Despite of many benefits from EE, no study has been able to demonstrate that EE leads to a reduction in mortality of acute UGIB. One of the most important reasons is probably due to that mortality of patients with acute UGIB is mainly determined by co-morbidity rather than the success of hemorrhage control1,2,25. Moreover, endoscopy carries certain potential risk for complications, including cardiopulmonary events, infectious and thromboembolic events, bleeding, instrumental (perforation, penetration and impaction), and drug reaction from premedication5,6. Thus, whether to perform EE in ACS patients presenting acute UGIB is always questionable.
Among ACS patients, DAPT remains cornerstone for the management, especially after coronary artery stenting7. Aspirin interferes platelet aggregation activity and has been demonstrated to reduce the risk of cardio-and cerebro-vascular events by as much as 30%, and 18% of all-cause mortality in the secondary prevention of cardiovascular diseases12,26,27. However, aspirin increased the risk for GI adverse effects because of inhibition of cyclooxygenase-mediated prostaglandin synthesis. Up to 60% of aspirin users develop GI mucosal lesions under endoscopic examination, especially stomach and duodenum28. Clopidogrel, another antiplatelet agent, inhibits platelet function by blocking the adenosine diphosphate receptor on platelets and the CAPRIE trial has shown that long-term clopidogrel monotherapy was more effective and better tolerated than aspirin in reducing combined risk of ischaemic stroke, MI, or vascular death29. Clopidogrel seems to be associated with fewer GI adverse effects compared with aspirin29. Nonetheless, an animal study revealed that clopidogrel impair the healing of gastric ulcers by suppressing the release of platelet-derived growth factors which are crucial for repair of mucosal defects30. Clinical studies have also disclosed that 8–12% of clopidogrel users with a history of PUD bleeding develop recurrent GI bleeding within 12 months31,32. A nationwide population-based study demonstrated that the use of clopidogrel increased the risk of UGIB with HR of 3.66 (95% CI 2.96–4.51), especially in elderly, CKD, past history of PUD, and concomitant use of aspirin and NSAIDs11. Therefore, how to deal with acute UGIB in the setting of recent ACS is still challenging to gastroenterologists and cardiologists.
In patients with recent ACS, the safety and timing of endoscopy is not well known. Dynamic changes in infarct size may occur since the loss of viable myocardium is progressive after coronary artery occlusion during several hours to days. The infarcted region which itself is a critical determinant of remodeling, incidence of arrhythmias, sudden cardiac death and thus prognosis of ACS, may further expand or contract. Therefore, endoscopy within the first week after MI seems to be associated with higher risk for cardiovascular events. Nonetheless, in several observational cohort and retrospective studies, endoscopy for post-ACS patients has been described as relatively acceptable with complication rates ranging from 7.5 to 48.4%, depending on the definition of complications, timing of endoscopy, and clinical condition of enrolled patients33,34,35,36,37,38. A systemic review of literature has shown that overall complication rate of esophagogastroduodenoscopy after MI was about 1% to 8%, with a large predominance of minor complications39. Women seem to experience more periprocedural MI than men (31% vs. 11%, p = 0.058)36, and ACS patients who are very ill (APACHE-II score ≥ 16) are more likely to develop endoscopic complications than those with relatively stable condition (21% vs. 2%)35. Another retrospective study has also revealed that patients with APACHE II scores > 16 experienced more minor complications (chest pain, abnormal vital signs, or minor arrhythmias) than those with scores ≤ 15 (54.5% vs. 24.2%, p = 0.02)37. From the result of a nationwide database involving 1,281,749 ACS patients, endoscopy after coronary arterial catheterization was not associated with a difference in mortality compared with pre-angiogram endoscopy (OR = 0.84, 95% CI 0.60–1.19)40. However, design of these studies is mostly retrospective or observational. Given the lack of guidelines and RCTs, gastroenterologists are always reluctant to perform endoscopy for UGIB in ACS patients due to potential risk of complications. In our RCT, we have demonstrated that complication rates were not increased by EE as compared with PPI therapy alone, but higher risk in male patients.
Theoretically, the drug–drug interaction between PPI and clopidogrel reduces the antiplatelet effects and increases the major composite ischemia events. However, several clinical studies and recommendations from international societies suggested prophylactic PPI use for ACS patients taking antiplatelet or antithrombotic agents, particularly those at high risk of UGIB41,42. A register-based RCT to examine the effect of screening for risk of UGIB and prophylactic PPI treatment in DAPT patients did not show a reduced incidence of UGIB (1.3% vs. 0.8%, p = 0.38) but a higher compliance with DAPT and reduced risk of recurrent cardiovascular events40. In our study, about one-third of patients discontinued DAPT in both groups which was associated with higher risk of coronary artery stent re-thrombosis (Tables 1 and 4). Given that EE could provide initial higher rate of bleeding control, resuming DAPT as early as possible might be achieved to reduce recurrent cardiac ischemic events after coronary artery stenting. According to our results, patients in EE group resumed any antiplatelet agent earlier than those in non-EE group (mean 5.13 vs. 8.63 days), although statistically insignificant (Table 1). Additionally, lower needs for blood transfusion after EE may attenuates complications from over-transfusion of component therapy, particularly in ACS patients who had heart failure and pulmonary edema.
There were some limitations in this study. First, because of more than half ACS patients receiving prophylactic PPI therapy in our institute, the acute UGIB rate was lower than our expectation. Therefore, the number of patients enrolled were smaller than estimated sample size. However, the primary endpoint has been achieved with statistically significance. Secondly, the discontinuing and resuming DAPT was at the discretion of cardiologists rather than a standardized protocol. We discontinued aspirin in the majority (76.19% and 68.18% in non-EE and EE group, respectively) of enrolled subjects because of which is more ulcerogenic than clopidogrel in terms of pharmacological mechanism. It was difficult to suggest the strategy in adjusting DAPT during acute UGIB in ACS patients from our result. Additionally, UGIB status was evaluated only according to clinical symptoms and laboratory data. Among such high-risk patients, UGIB status could be obscured by hemodilution after fluid resuscitation. Finally, we only identify male gender as the significant risk factor for complications from EE in ACS patients. This is probably due to the small sample size and we need further enrollment of eligible patients.
In conclusion, EE for acute UGIB in recent ACS patients has been demonstrated as an efficient and safe procedure for hemorrhage control with lower needs for blood transfusion in this multicenter RCT. Enrollment of more patients and longer study period are warranted to identify risk factors for complications from EE.
References
Hopper, A. D. & Sanders, D. S. Upper GI bleeding requires prompt investigation. Practitioner 255(1742), 15–19 (2011).
Rockall, T. A. et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 38(3), 316–321 (1996).
Sung, J. J. et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: A randomized trial. Ann. Intern. Med. 150(7), 455–464 (2009).
Rockall, T. A. et al. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 311(6999), 222–226 (1995).
Meier, P. N. Complications in gastrointestinal endoscopy: A summary at the first international symposium in Hannover 2009. Endoscopy 43(10), 892–896 (2011).
Blacker, D. J., Wijdicks, E. F. & McClelland, R. L. Stroke risk in anticoagulated patients with atrial fibrillation undergoing endoscopy. Neurology 61(7), 964–968 (2003).
Berger, J. S. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am. J. Cardiol. 112(5), 737–745 (2013).
Khan, S. U. et al. Meta-analysis of the safety and efficacy of the oral anticoagulant agents (apixaban, rivaroxaban, dabigatran) in patients with acute coronary syndrome. Am. J. Cardiol. 121(3), 301–307 (2018).
Chang, S. H. et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 318(13), 1250–1259 (2017).
Derry, S. & Loke, Y. K. Risk of gastrointestinal haemorrhage with long term use of aspirin: Meta-analysis. BMJ 321(7270), 1183–1187 (2000).
Lin, C. C. et al. Risk factors of gastrointestinal bleeding in clopidogrel users: A nationwide population-based study. Aliment Pharmacol. Ther. 38(9), 1119–1128 (2013).
Yeomans, D. et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol. Ther. 22(9), 795–801 (2005).
Dorreen, A. et al. Safety of digestive endoscopy following acute coronary syndrome: A systematic review. Can. J. Gastroenterol. Hepatol. 2016, 9564529. https://doi.org/10.1155/2016/9564529 (2016).
ErtlT, G. & Frantz, S. Healing after myocardial infarction. Cardiovasc. Res. 66, 22–32 (2005).
Gaudron, P. et al. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: Their inter-relation and prognostic impact. J. Am. Coll. Cardiol. 38(1), 33–40 (2001).
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 39(2 Suppl 1), S1–S266 (2002).
Lau, J. Y. et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N. Engl. J. Med. 356(16), 1631–1640 (2007).
Knaus, W. A. et al. APACHE II: A severity of disease classification system. Crit. Care Med. 13(10), 818–829 (1985).
Laine, L. & Peterson, W. L. Bleeding peptic ulcer. N. Engl. J. Med. 331, 717–727 (1994).
Van, D. J. & Brugge, W. R. Endoscopy of the upper gastrointestinal tract. N. Engl. J. Med. 341(23), 1738–1748 (1999).
Chen, Y. L. et al. Major adverse upper gastrointestinal events in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention and dual antiplatelet therapy. Am. J. Cardiol. 108(12), 1704–1709 (2011).
Shalev, A. et al. Incidence, predictors and outcome of upper gastrointestinal bleeding in patients with acute coronary syndromes. Int. J. Cardiol. 157(3), 386–390 (2012).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345(7), 494–502 (2001).
Nikolsky, E. et al. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J. Am. Coll. Cardiol. 54(14), 1293–1302 (2009).
Blatchford, O., Murray, W. R. & Blatchford, M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 356(9238), 1318–1321 (2000).
Weisman, S. M. & Graham, D. Y. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch. Intern. Med. 162(19), 2197–2202 (2002).
AT Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324(7328), 71–86 (2002).
Chowdhury, A. et al. Gastro-duodenal mucosal changes associated with low-dose aspirin therapy: A prospective, endoscopic study. Indian J. Gastroenterol. 20(6), 227–229 (2001).
CS Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348(9038), 1329–1339 (1996).
Ma, L. et al. Platelets modulate gastric ulcer healing: Role of endostatin and vascular endothelial growth factor release. Proc. Natl. Acad. Sci. U.S.A. 98(11), 6470–6475 (2001).
Chan, F. K. et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N. Engl. J. Med. 352(3), 238–244 (2005).
Ng, F. H. et al. High incidence of clopidogrel-associated gastrointestinal bleeding in patients with previous peptic ulcer disease. Aliment Pharmacol. Ther. 18(4), 443–449 (2003).
Al-Ebrahim, F. et al. Safety of esophagogastroduodenoscopy within 30 days of myocardial infarction: A retrospective cohort study from a Canadian tertiary centre. Can. J. Gastroenterol. 26(3), 151–154 (2012).
Cappell, M. S. The safety and clinical utility of esophagogastroduodenoscopy for acute gastrointestinal bleeding after myocardial infarction: A six-year study of 42 endoscopies in 34 consecutive patients at two university teaching hospitals. Am. J. Gastroenterol. 88(3), 344–350 (1993).
Cappell, M. S. & Iacovone, F. M. J. Safety and efficacy of esophagogastroduodenoscopy after myocardial infarction. Am. J. Med. 106(1), 29–35 (1999).
Lee, J. G., Krucoff, M. W. & Brazer, S. R. Periprocedural myocardial ischemia in patients with severe symptomatic coronary artery disease undergoing endoscopy: Prevalence and risk factors. Am. J. Med. 99(3), 270–275 (1995).
Lim, R. G. et al. Endoscopy after acute myocardial infarction: An evaluation of safety. South Med. J. 106(10), 545–549 (2013).
Spier, B. J. et al. Safety of endoscopy after myocardial infarction based on cardiovascular risk categories: A retrospective analysis of 135 patients at a tertiary referral medical center. J. Clin. Gastroenterol. 41(5), 462–467 (2007).
Cena, M. et al. Safety of endoscopic procedures after acute myocardial infarction: A systematic review. Cardiol. J. 19(5), 447–452 (2012).
Hoffman, G. R. et al. Safety of endoscopy for hospitalized patients with acute myocardial infarction: A national analysis. Am. J. Gastroenterol. 115(3), 376–800 (2020).
Collet, J. P. et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. (Engl Ed) 74(6), 544 (2021).
Kumbhani, D. J. et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: A report of the American College of Cardiology solution set oversight committee. J. Am. Coll. Cardiol. 77(5), 629–658 (2021).
Acknowledgements
All the authors have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: C.-S.C., C.-C.C, H.-P.W., and Y.-W.W. Generation, collection, assembly, analysis and/or interpretation of data: C.-S.C., C.-C.C, K.-C.C., Y.-J.F., W.-F.H. Y.-N.C., W.-C.T., C.-K.L., T.-H.L., H.-P.W., and Y.-W.W. Drafting or revision of the manuscript: C.-S.C. and Y.-W.W. Approval of the final version of the manuscript: C.-S.C., C.-C.C, K.-C.C., Y.-J.F., W.-F.H. Y.-N.C., W.-C.T., C.-K.L., T.-H.L., H.-P.W., and Y.-W.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, CS., Chen, CC., Chen, KC. et al. Randomized controlled trial of early endoscopy for upper gastrointestinal bleeding in acute coronary syndrome patients. Sci Rep 12, 5798 (2022). https://doi.org/10.1038/s41598-022-09911-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09911-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.