Abstract
Rheumatoid arthritis (RA) is a chronic rheumatic disease with a clear sex-bias. Recent data indicated a role for dopamine in RA pathogenesis, while dopaminergic pathways can be modulated by estrogens. As defined mechanism of action of dopamine on B cell function in RA are unclear, we aimed to elucidate this, with special focus on sex-differences. Healthy controls (HC, n = 64) and RA patients (n = 61) were recruited. Expression of D1–D5 dopamine receptors (DRs) was investigated by flow cytometry on peripheral blood mononuclear cells (PBMCs). D1-like DRs were stimulated in vitro to assess effects on B cell activation and proliferation. Secretion of cytokines and dopamine content were measured by ELISA. All DRs were expressed on PBMCs of HC and RA patients. Dopamine content in PBMCs, and frequency of D1DR expressing B cells were significantly higher in RA females (p < 0.001). Expression of D1DR on RA B cells correlated positively with disease duration and severity only in women. Combined B cell and D1-like DR stimulation induced higher IL-8 and CCL-3 secretion from PBMCs of female RA patients compared to HC. These results indicate sex-specific differences in dopaminergic pathway in RA, with a proinflammatory feature of the D1DR pathway in women.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic joint inflammation, articular bone erosion and joint destruction with decrease of function in many patients1.
In most epidemiological studies RA affects up to 1% of the population2,3 and shows a clear female predominance4. Not only the prevalence but also the course of the disease differs between men and women. One possible explanation is the differential role of sex hormones on immune reactions5 but other factors such as genetic predisposition, epigenetics and gut microbiota likely also play a role6,7,8. Although the pathogenesis of RA is not entirely clear, there is no doubt that B cells play a crucial role, since anti B cell therapy with rituximab is beneficial for some patients (11) and autoantibodies such as rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) are produced by differentiated B cells, the plasma cells9,10. Besides autoantibody production, B cells also present antigens to T cells and produce proinflammatory cytokines thereby worsening RA. Disease improvement after B cell depletion therapy in both seronegative and seropositive RA patients11 as well as other clinical evidences12 underline the importance of these antibody-independent B cell functions in RA13. For example, secretion of cytokines by activated B cells is involved in bone resorption. In this context, CCL3 which inhibits the bone matrix-producing osteoblasts14, and TNF-α which stimulates differentiation of osteoclasts15 have been identified as relevant cytokines among others.
The immune system can be affected by the nervous system and neurotransmitters. Dopamine is known as a neurotransmitter of the sympathetic nervous system that plays several important roles not only in the brain but also in the whole body. Recent evidence, however, supports a key role of dopaminergic pathways also in the modulation of immunity in the periphery16,17,18,19. Dopamine acts via five different dopamine receptors (DRs), which are expressed in most immune cells. In addition, immune cells are able to synthesize and utilize dopamine as an autocrine/paracrine transmitter20,21,22. DRs belong to G protein coupled receptors (GPCRs)23,24. Depending on their pharmacological, biochemical, and physiological profile DRs are assigned in two subclasses, namely D1- and D2-like receptors. D1 and D5 DR belong to the stimulatory D1-like and D2, D3 and D4 DR to the inhibitory D2-like receptor family25.
There are clinical evidences suggesting the involvement of the neurotransmitter dopamine in the pathogenesis of RA: the incidence of RA in Schizophrenia patients is much lower than in the general population26, and the prevalence of RA is also altered in Parkinson’s disease, even though the causal interaction of these diseases is conflicting27,28,29. Also, the incidence of restless legs syndrome is increased in RA patients compared to healthy controls (HC)30 and, of interest, patients with restless leg syndrome have increased plasma levels of dopamine31. Furthermore, high local concentration of dopamine was measured in the synovial fluid of RA patients32.
Also in vitro, dopamine modulates immune response. Previous experimental studies have suggested a pro-inflammatory role for D1-like DR and a rather anti-inflammatory role for D2-like DR, whereas the mechanisms of action have been poorly described to date. Notably, dopamine was shown to promote the interaction between B cells and T-follicular helper (TFH) cells in germinal centers of the lymph nodes, hereby promoting B cell maturation via D1DR33. Further, it was demonstrated that D2DR expression on B cells negatively correlates with disease activity as well as with inflammatory biomarkers in human RA34, and the migration of synovial fibroblasts from young RA patients was increased by D1-like DR activation in vitro35. Also, experimental studies in vivo strongly suggested a role for dopamine in arthritis36,37,38,39. However, more research is needed to better understand the role of specific DR signaling in RA.
The primary aim of this study was to investigate the possible involvement of the neurotransmitter dopamine in the systemic immune cell activation in RA40.
Since women have a higher incidence of RA than men and estrogens appear to have a direct influence on DR expression41, we investigated the expression and function of the dopaminergic pathway in peripheral immune cells of RA patients with special focus on sex differences.
Results
Study population
We recruited a total of 61 patients with confirmed diagnosis of RA, 64 healthy controls and 43 patients with chronic inflammatory rheumatic and musculoskeletal diseases (CIRMD): 20 psoriatic arthritis (PsA) and 23 axial spondyloarthritis (axSpA).
The mean age was 44.4 years (y) for HC and 53 years for RA, with an age range of 19–75 and 21–79 respectively. Mean age for PsA was 44.3 years, with an age range of 25–71, whereas mean age for axSpA was 44.6 years with an age range of 26–81. The mean disease duration of RA patients was 5.7 years, for PsA 5.3 years and for axSpA 12 years. Detailed subject characteristics are listed in Table 1. Patients with psychiatric or neurological comorbidities were excluded from the study.
Dopaminergic pathway is expressed in PBMCs of healthy controls and rheumatoid arthritis patients
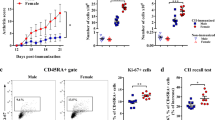
PBMCs from HC and RA subjects contained dopamine (Fig. 1a,b). Notably, PBMCs of female RA contained about three times more dopamine than HC (Fig. 1a), whereas PBMCs from male RA contained significant less dopamine than male HC (Fig. 1b). As dopamine is a precursor of noradrenaline and adrenaline, we measured the amount of these catecholamines as well, but found comparable concentrations in both groups (Suppl. Fig. 1), thus suggesting that only dopamine is specifically altered during RA. To find out if PBMCs synthesize dopamine themselves, we stained T cells, B cells, NK cells and monocytes for tyrosine hydroxylase (TH), the rate limiting enzyme in catecholamine synthesis. The gating strategy for these leukocyte subpopulations is shown in Fig. 1c. Our results revealed the presence of TH in all leukocytes (Fig. 1d,e), with no statistical differences in RA compared to HC. We further analyzed the expression of dopaminergic receptors (DRs) on leukocyte subpopulations. The gating strategy is shown in Supplementary Fig. 2a. We demonstrated the presence of D1–D5 DRs on all leukocyte subpopulations analyzed (Fig. 2), with NK cells and monocytes showing the highest amount of all DR+ cells. D5DR was very weakly expressed in all analyzed PBMC subsets. In most of them, D2-like DRs were higher expressed than D1-like DRs. In general, a tendency towards a higher expression of almost all DRs in all PBMC subsets was observed for RA women in comparison to HC. In contrast, rather reduced DR expression levels for RA men compared to healthy males indicated a possible sex bias. Of interest, a significant higher expression of D1DR was detected in B cells from female RA patients compared to HC, whereas no differences were observed in men (Fig. 2b). The gating strategy is shown in Supplementary Fig. 2a.
Dopamine content and expression of tyrosine hydroxylase in peripheral immune cells from RA patients and HC. Dopamine level was measured in PBMCs obtained from healthy control (HC) and rheumatoid arthritis (RA) patients by TriCat ELISA and TH expression was analyzed by flow cytometry. (a, b) Concentration of dopamine in 106 freshly isolated PBMCs (HC female n = 10, RA female n = 14, HC male n = 11, RA male n = 10). (c) Gating strategy to discriminate between CD3+CD56- T cells, CD3-CD56+ NK cells, CD19+ B cells and CD14+ monocytes d) Quantification of TH expression in aforementioned PBMC subsets from HC and RA patients (HC female n = 24, RA female n = 27, HC male n = 16, RA male n = 15). Welch’s t test was used to compare catecholamine levels in PBMCs from HC and RA patients; Mixed-effects analysis with Geisser-Greenhouse correction and Sidak multiple comparison test was used for comparing TH expression between groups; *p ≤ 0.05.
Expression of dopaminergic receptors in peripheral immune cells from RA patients and HC. Quantification of D1–D5 DR expression in CD3+CD56- T cells (a), CD19+ B cells (b), CD3-CD56+ NK cells (c) and CD14+ monocytes (d) from HC (female n = 24, male n = 16) and RA patients (female n = 27, male n = 15). Mixed-effects analysis with Geisser-Greenhouse correction and Sidak multiple comparison test was used for comparing DR expression between groups; **p ≤ 0.01, ***p ≤ 0.001.
No differences in the D1DR expression on B cells compared to the HC were observed in axSpA and PsA patients (Suppl. Figs. 4 and 5). However, D1DR on B cells tended to be higher in diseased women compared to HC and not in men, and expression of D5DR was significantly lower in diseased women compared to HC for T cell, B cells and NK cells (Suppl. Figs. 4 and 5), whereas D4DR expression was significantly lower in T cells of axSpA male patients compared to HC and no differences were observed in women (Suppl. Fig. 4b). Based on these results, a sex-biased effect of dopamine is plausible not only in RA but also in other chronic rheumatic diseases.
D1DR expression on B cells correlates with clinical parameters in RA patients
To investigate a possible clinical relevance of D1DR on B cells in RA, we correlated its expression with clinical parameters (Fig. 3 and Suppl. Fig. 5). D1DR expression on B cells tended to be increased also in female naïve RA patients compared to HC, but the amount of D1DR + B cells is significantly higher in treated RA female patients compared to HC (Fig. 3a). No differences were observed in men (Fig. 3a). D1DR expression on B cells positively correlated with disease duration in female RA patients and tended to negatively correlate with disease duration in men (Fig. 3b). A negative correlation was observed between D1DR expression on B cells and the Hannover functional questionnaire (FFbH) (Fig. 3c), a self-assessment questionnaire encompassing functional physical limitations42, thus displaying a positive correlation between disease disability and D1DR expression. This suggests an involvement of D1DR-positive B cells on disease perpetuation in women. In male, no significant correlation was observed, but the number of samples available was limited (Fig. 3c). No significant correlation was found regarding disease activity score (DAS) 28 (Fig. 3d). To find out if the correlation of D1DR expression with disease duration is due to a physiological increase of D1DR on B cells during ageing, we correlated the expression of D1DR on B cells with age, and found no significant correlation in RA. However, a correlation was found for female HC (Suppl. Fig. 5a), therefore we decided to perform a multiple linear regression for RA patients, which includes both age and disease duration. A statistical significance between D1DR expression and disease duration was obtained for women (**p = 0.0044, β = 0.9339; age: p = 0.5542, β = 0.062) as well as for men (*p = 0.020, β = − 0.9767; age: p = 0.1204, β = − 0.2931). To examine present correlations of age, sex, and disease with D1DR expression, multivariable analyses were performed with pooled data including all diseases and both sexes. Therefore, multiple linear regression with age, sex and disease as independent variables and D1DR expression as the dependent variable was used. It was found that age has no impact (p = 0.2149), whereas sex (***p = 0.0001) and RA (*p = 0.0024) have a statistically significant effect on D1DR expression. This statistical significance was not confirmed for PsA (p = 0.1803) and SpA (p = 0.0744), however the trend towards being an influencing variable is observable as well. This strengthens the hypothesis of a direct involvement of D1DR expression in RA pathophysiology in women.
D1DR expression on peripheral B cells from female RA patients correlates with clinical parameters. Differential expression of D1DR on CD19+ B cells from RA patients was correlated with available patient information at day of blood withdrawal. (a) comparison of D1DR level on CD19+ B cells from HC (female n = 24, male n = 16) as well as naïve RA (female n = 5, male n = 4) and treated RA (female n = 20, male n = 11) patients (b–d) D1DR level on CD19+ B cells from RA patients was analyzed regarding disease duration (b, female n = 27, male n = 14), FFbH (c, female n = 27, male n = 7) and DAS28 score (d, female n = 27, male n = 6). (e, f) Quantification of D1DR expression on CD19+ B cells from HC (female n = 23, male n = 14) and RA patients categorized into rheumatoid factor (RF) positive and negative (e, female n = 14 and 13, male n = 4 and 9 respectively)) or anti-citrullinated protein antibodies (ACPA) positive and negative (f, female n = 16 and 10, male n = 4 and 6 respectively). Simple linear regression with Pearson correlation analysis was used to analyze D1DR expression in relation to clinical parameters; Brown-Forsythe and Welch ANOVA tests with Dunnett T3 multiple comparison test were used to compare D1DR expression between groups; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Of interest, D1DR expression was higher on B cells both from seronegative as well as from seropositive female RA patients (Fig. 3e,f), and seropositive male RA patients tended to have lower D1DR expression on B cells compared to HC and seronegative RA patients (Fig. 3e,f), thus indicating that D1DR activation on B cells might influence non-canonical proinflammatory functions of B cells such as cytokine production13 in female patients rather than antibody production.
D1DR expression increases during B cell maturation
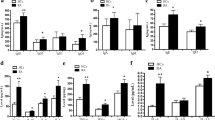
As D1DR expression was found to be significantly increased in total CD19+ B cells of female RA, we next investigated D1DR expression in naive, non-switched memory, switched memory B cells and plasmablasts by flow cytometry. The gating strategy is shown in Fig. 4a. D1DR expression was higher in RA B cells already in the early maturation steps compared to HC in females (Fig. 4b). Moreover, we detected an increased D1DR expression during B cell maturation both in HC as well as in RA both in females and males, with plasmablasts showing the highest D1DR expression (Fig. 4b and Suppl. Fig. 6a). Assuming that an increase in D1DR has an impact on B cell function these results suggest a stronger effect on more mature B cells, not only during RA but also in the physiological conditions. Moreover, stimulation of D1DR in vitro with the specific agonist A6893043, in combination with CpG ODN 2006 (shortly CpG), a TLR9 ligand and thus B cell-stimulus, significantly increased B cell proliferation only in female RA patients but no significant differences were observed in HC (Fig. 4d) and in men (Suppl. Fig. 6c), even if the basal proliferation indices of the two groups were comparable (Fig. 4c and Suppl. Fig. 6b), thus indicating a stronger impact of dopamine on B cell function in female RA.
D1DR expression in different maturation stages of B cells and during B cell proliferation. D1DR expression was analyzed in defined B cell subpopulations from HC and RA patients by flow cytometry. (a) Gating strategy to investigate D1DR expression in naïve (1, CD19+TCRα/β-IgD+CD27-), non-switched memory (2, CD19+TCRα/β-IgD+CD27+), switched memory B cells (3, CD19+TCRα/β-IgD-CD27+) and plasmablasts (4, CD19+TCRα/β-CD27+CD38+), black: stained sample, grey: FMO control. (b) Quantification of D1DR on aforementioned B cell subsets from HC and RA female patients (n = 17 and 21 respectively). (c, d) PBMCs from HC and RA female patients were stimulated with CpG and indicated concentrations of D1-like receptor agonist A68930 for 6 days in vitro. Proliferation of CD19+ B cells was then analyzed by CFSE-dye dilution via flow cytometry. (c) Proliferation index of CD19+ B cells from HC and RA patients (n = 10 and 9 respectively) under pure CpG stimulation are presented as median with SD. (d) Proliferation index of CD19+ B cells of both groups after D1-like stimulation were normalized to CpG controls and are presented as relative changes (n = 10). One-Way ANOVA with Geisser-Greenhouse correction and Tukey multiple comparison test was used to analyze expression between B cell subsets within HC and RA group; Welch’s t test was used to compare CpG stimulated controls from HC and RA group; Raw data were logarithmized and analyzed by mixed-effects analysis with Geisser-Greenhouse correction and Dunnett multiple comparison test to determine the influence of D1-like receptor stimulation within the HC and RA group; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Effects of D1DR stimulation on B cell regulation and activation
To further study effects of D1DR stimulation on B cell regulation and activation, PBMCs were stimulated in vitro with CpG, in combination or not with the D1-like DR agonists A68930 or SKF38393. After 24 h, expression of CD95, which regulates the death of autoreactive B cells44, and HLA-DR, which represents an activation marker being important for antigen presentation to T cells, were analyzed by flow cytometry in different B cell subpopulations (Figs. 5 and 6 and Suppl. Figs. 7 and 8, gating strategy in Suppl. Fig. 2b). We observed an increase of CD95 throughout maturation, with switched memory B cells showing the highest CD95 expression level being comparable between RA and HC. Also, CpG stimulation led to a significant increase of CD95 in naïve B cell, non-switched memory B cells as well as in switched memory B cells in both groups (Fig. 5a,c,e, Suppl. Fig. 7a,c,e). A significant increase in CD95 was observed only after D1DR stimulation in switched memory B cells from female RA (Fig. 5f), whereas expression of CD95 on naïve B cells from female RA patients tended to decrease after D1DR stimulation (Fig. 5b). No effects of D1DR stimulation were observed in men (Suppl. Fig. 7b,d,f).
D1-like receptor stimulation slightly increases CD95 expression on switched memory B cells from female RA patients. PBMCs from HC (n = 13) and RA patients (n = 10–11) were stimulated with CpG and indicated concentrations of D1-like receptor agonists A68930 and SKF38393 for 24 h in vitro. Expression of CD95 was analyzed in naïve B cells (CD19+IgD+CD27-), non-switched memory B cells (CD19+IgD+CD27+) and switched memory B cells (CD19+IgD-CD27+) by flow cytometry and expression data are shown as MFI. (a, c, e) Absolute CD95 expression is shown for naïve B cells (a), non-switched memory B cells (c) and switched memory B cells (e) in unstimulated and CpG-stimulated PBMCs from HC and RA patients. Lines indicate median with SD. (b, d, f) CD95 expression of naïve B cells (b), non-switched memory B cells (d) and switched memory B cells (f) after D1-like stimulation was normalized to CpG controls from HC and RA patients and are presented as relative changes on a logarithmic scale. Two-Way ANOVA or mixed-effects-analysis, depending on missing values, with Sidak multiple comparison test was used to analyze CD95 expression between unstimulated and CpG stimulated samples from HC and RA; Raw data were logarithmized and analyzed by One-Way ANOVA or mixed-effects analysis, depending on missing values, with Geisser-Greenhouse correction and Dunnett multiple comparison test to determine the influence of D1-like receptor stimulation on CD95 expression within HC and RA group; *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
D1-like receptor stimulation increases activation of memory B cells from female RA patients. PBMCs from HC (n = 13–12) and RA patients (n = 11) were stimulated with CpG and indicated concentrations of D1-like receptor agonists A68930 and SKF38393 for 24 h in vitro. Expression of HLA-DR was analyzed in naïve B cells (CD19+IgD+CD27-), non-switched memory B cells (CD19+IgD+CD27+) and switched memory B cells (CD19+IgD-CD27+) by flow cytometry and expression data are shown as MFI. (a, c, e) Absolute HLA-DR expression is shown for naïve B cells (a), non-switched memory B cells (c) and switched memory B cells (e) in unstimulated and CpG-stimulated PBMCs from HC and RA patients. Lines indicate median with SD. (b, d, f) HLA-DR expression of naïve B cells (b), non-switched memory B cells (d) and switched memory B cells (f) after D1-like stimulation was normalized to CpG controls from HC and RA patients and are presented as relative changes on a logarithmic scale. Two-Way ANOVA or mixed-effects-analysis, depending on missing values, with Sidak multiple comparison test was used to analyze HLA-DR expression between unstimulated and CpG stimulated samples from HC and RA; Raw data of HLA-DR expression were logarithmized and analyzed by One-Way ANOVA or mixed-effects analysis, depending on missing values, with Geisser-Greenhouse correction and Dunnett multiple comparison test to determine the influence of D1-like receptor stimulation on HLA-DR expression within HC and RA group; *p ≤ 0.05, **p ≤ 0.01.
Expression of HLA-DR slightly increased with B cell maturation (Fig. 6a,c,e, and Supplementary Fig. 8a,c,e). CpG treatment led to a significant higher expression of HLA-DR in all B cell subpopulations (Fig. 6a,c,e, and Supplementary Fig. 8a,c,e). D1DR stimulation led to significant increase of HLA-DR in non-switched memory B cells and in switched memory B cells in female RA patients and to an increase of HLA-DR expression on switched memory B cells of female HC (Fig. 6d,f). Of interest, an opposite effect was observed for switched memory B cells from male HC (Suppl. Fig. 8f) and D1DR stimulation did not affect HLA-DR expression in B cells from male RA patients (Suppl. Fig. 8b,d,f).
As B cells were shown to contribute to bone loss in RA by supporting osteoclastogenesis via RANKL45 we also aimed to investigate the effect of D1-like stimulation on RANKL expression. Membrane-bound RANKL as well as soluble RANKL secreted by unstimulated and of CpG-treated PBMCs were very low (data not shown), therefore no functional analyses of RANKL after D1DR stimulation were possible.
D1DR stimulation increases IL-8 and CCL3 release in female RA
Besides RANKL secretion, B cells were also shown to promote bone loss in RA by secretion of other cytokines14. Thus, we further analyzed if D1DR stimulation has an influence on the release of proinflammatory cytokines.
After 24 h of B cell stimulation via CpG, IL-8 release was higher in HC and tended to be higher in RA patients as well (Fig. 7a and Suppl. Fig. 9a). However, D1-like stimulation led to a significant decrease of IL-8 in female HC, whereas in RA patients this anti-inflammatory effect was lost (Fig. 7b). In male RA patients, an opposite, inhibitory effect of D1-like stimulation was observed (Suppl. Fig. 9b).
D1-like receptor stimulation alters cytokine secretion of PBMCs from female RA patients compared to HC. PBMCs from HC (n = 13–12) and RA patients (n = 13–12) were stimulated with CpG and indicated concentrations of D1-like receptor agonists A68930 and SKF38393 for 24 h in vitro. Supernatants were stored at -80 °C for subsequent analysis of IL-8 and CCL3 concentrations by ELISA. (a, c) Absolute IL-8 (a) and CCL3 (c) concentrations in supernatants from unstimulated and CpG-stimulated PBMCs from HC and RA patients are shown. Lines indicate median with SD. (b, d) IL-8 (b) and CCL3 (d) concentrations in supernatants after D1-like stimulation were normalized to CpG controls from HC and RA patients and are presented as relative changes on a logarithmic scale. Mixed-effects-analysis with Sidak multiple comparison test was used to analyze cytokine secretion between unstimulated and CpG stimulated samples from HC and RA; Raw data of cytokine concentrations were logarithmized and analyzed by mixed-effects analysis with Geisser-Greenhouse correction and Dunnett multiple comparison test to determine the influence of D1-like receptor stimulation on cytokine secretion within HC and RA group; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Furthermore, CCL3 release was significantly increased in CpG-treated PBMCs from RA patients but not in HC subjects (Fig. 7c and Suppl. Fig. 9c) and the D1-like stimulation to a significant increase of CCL3 release specifically in female RA (Fig. 7d, Suppl. Fig. 9d). Collectively, D1DR stimulation seems to promote cytokine secretion in B cells of RA patients.
Discussion
Accumulating evidences suggest sex-related differences in predisposition and incidence of RA1,4. These findings underline the importance of a sex-specific approach to better understand RA pathophysiology, as well as for a more targeted treatment. Due to the fact that dopamine seems to be involved in RA and estrogens can modulate dopaminergic pathways, we studied here the role of dopamine in RA patients with special focus on sex differences.
Our results show that the dopaminergic receptor D1DR is overexpressed on B cells from female RA patients compared to HC and present its possible functional effects. The D1DR upregulation on RA B cells seems to be sex-specific, as we could not observe any alteration of D1DR expression in B cells of male RA patients compared to HC. However, a tendency to a reduced expression of all DRs in almost all PBMC subsets was found for RA men in comparison to healthy men, which underlines the overall sex bias observed in this study. Of interest, no D1DR alteration was observed in the other investigated rheumatic diseases PsA and axSpA, therefore we assume that a higher D1DR level on B cells from female RA patients is disease-specific. However, we observed some sex-specific differences in DR expression in SpA and PsA as well, therefore a sex-specific effect of dopamine on immune response is plausible also in other chronic rheumatic diseases rather than RA. Further investigations will be required. Of interest, D1DR expression correlated with disease duration and functional disability in RA female, thus suggesting that D1DR expression has a pathogenic role in disease progression and might be used as a diagnostic marker in women.
Whether the increased D1DR expression during RA could also be an effect of the therapy, has not been finally clarified. Indeed, treatments with DMARDs led to an improvement of the clinical features and to a higher expression of D2DR in RA patients in a previous study34. However, due to the multitude of treatment options in RA, a very large number of patients would be required in order to establish treatment-specific effects of D1DR expression. So far, we did not find a correlation between D1DR expression on B cells and current biological treatment (data not shown). We therefore assume that the increased expression of D1DR is probably rather related to inflammation and disease activity, as also supported by the observed positive correlation of D1DR expression and the disease severity parameter FFbH. The absence of a statistically significant increase in D1DR expression for naïve patients can probably be explained less by a lack of treatment and rather by the fact that they were newly diagnosed and have a very low disease duration. This matches the observation that D1DR expression increases with disease duration in treated RA women. Thus, it is unlikely that an overexpressed D1DR pathway is a driver in the development of RA but it might rather contribute to proinflammatory effects during the course of the disease. The reduced D2DR expression in RA previously described by Wei et al.34 could not be confirmed in our study. However, due to the opposite effects described for D1-like and D2-like receptors24, these results are not really in contrast with our findings. Taken together, these data support the hypothesis of an involvement of the dopaminergic pathway in the immune response in RA, suggesting that D1-like DRs exert a pro-inflammatory effect and D2-like DRs play an anti-inflammatory role at least in women. Of interest, for RA men we found a statistically significant negative correlation of D1DR expression with disease duration after adjustment to age and it also tended to correlate negatively with DAS28. So far, these data suggest a rather anti-inflammatory role in RA men. However, for a more expressive investigation of the impact of D1DR pathway in men, further studies are required.
The higher D1DR expression on B cells of female RA patients contributed to increased B cell proliferation. As dopamine content in inflamed RA joints is increased37, proliferation of pathogenic B cells could thus be accelerated locally. Higher HLA-DR expression in response to D1-like stimulation further suggests that dopamine may potentiate the interaction between B cells and T cells promoting inflammation. Additionally, D1DR activation seems to play a role for cytokine secretion after B cell activation. Our results show an increase of CCL3 after combined B cell- and D1-like DR stimulation of female RA PBMCs in vitro. It was previously shown that CCL3 is produced and released by B cells of RA patients and is responsible for osteoblast suppression and thus bone erosion14. Our results suggest therefore a possible involvement of the dopaminergic pathway on bone erosion via its activation on B cells. Also, we could observe an increase of IL-8 after D1DR stimulation in female RA compared to HC. This effect was due to the lack of IL-8 suppression observed in HC after B cell- and D1-like DR stimulation. As IL-8 could also be produced by other immune cells within the cultivated PBMCs, it is not possible so far to state if the here described change in IL-8 secretion is indirect or if B cells release IL-8. Nevertheless, due to the proinflammatory role of IL-8 in RA46, the effects we could observe for women seems to be of clinical relevance. In addition, rather opposite effects in cytokine production after D1-like stimulation were found for RA men in comparison to healthy men. Both lower IL-8 and a tendency towards lower CCL3 levels were observed for diseased men. This anti-inflammatory effect is also consistent with the sex bias we found regarding correlations of D1DR expression with disease parameters.
Previous studies also described TNF-α release by B cells in RA and its role on osteoblast inhibition and thus bone resorption47. We could not observe any significant alteration of TNF-α after D1DR activation (data not shown) probably due to the very strong patient-to-patient variability of TNF-α levels related to the ongoing biological treatments.
Previous studies indicated a role of dopamine in B cell maturation and immunoglobulin production33,48. To assess a possible effect of D1DR on antibody production, we performed ELISA for total IgG, but found no impact of D1-like receptor stimulation (data not shown). These results, together with our findings on elevated D1DR expression in both female seronegative and seropositive patients (Fig. 2D,E), support our hypothesis that D1DR might have a stronger role on cytokine-producing B cells rather than on autoantibody production in RA13. Nevertheless, seronegative RA patients could be positive for autoantibodies not classically included in the diagnostics, therefore a role of the dopaminergic pathway also on antibody release cannot be excluded.
This study revealed strong differences and also opposite effects of dopamine in male and female RA patients as well as in HC. These differences could be due to an effect of sex hormones on the expression of dopamine receptors, which is already suggested by other publications. For instance, Lee et al. identified a half palindromic sequence of the estrogen response element as a binding site for estrogen on the D1DR promoter41. Thus, they suggested that estrogen can act as a transcription factor on D1DR expression. Furthermore, in a study with ovariectomized female rats it was shown that chronic treatment with β-estradiol caused an increase in the striatal D1DR density49 supporting the hypothesis of an upregulation of D1DR expression by female sex hormones. However, they also described that the striatal D1DR density was higher in intact male rats than in female50, which underlines that the D1DR expression can be influenced differently within female and male organisms. Interestingly, this observation is in line with our findings regarding the D1DR level on B cells in healthy women and men. However, to the best of our knowledge nothing more is described about the connection between the hormonal and dopaminergic system in humans. Besides the production of sex hormones, also other genetic or epigenetic regulations could be involved in the observed sex bias after D1-like stimulation in our study. Thus, these aspects need to be further investigated.
One pitfall of our study was the cell culture of mixed PBMCs instead of isolated B cells. This was due to the very small amount of RA PBMCs (and thus B cells) available. However, due to the B cell stimulation with CpG and the short-term treatment with D1DR agonists, a direct involvement of B cells in the cytokine release observed is plausible.
Taken together, our results reveal a strong increase of D1DR expression on B cells, as well as a significant increase of dopamine in PBMCs from female RA patients, thus suggesting an involvement of the dopaminergic pathway in the immune response in these patients. The correlation between the frequency of D1DR + B cells and clinical parameters indicates a contribution in RA pathogenesis in women, thus suggesting the proinflammatory D1DR pathway as a new therapeutic target for future treatment approaches in RA women. The observed dichotomy of the dopaminergic pathway between male and female RA patients still needs to be further elucidated, but it once again underlines the importance of sex-specific studies.
Methods
Study cohort
The study was approved by the ethics committee of IfADo (IfADo2017/125/2019-02-04). All experiments were performed in accordance with the Declaration of Helsinki. All subjects signed informed consent for study participation.
Patients with confirmed diagnosis of RA as well as healthy controls (HC) and two cohorts of patients with other chronic inflammatory musculoskeletal rheumatic diseases (psoriasis arthritis (PsA) and axial spondyloarthritis, (axSpA, including both radiographic-SpA as well as non-radiographic-axSpA)) were recruited. Peripheral venous blood was collected in Li-Heparin tubes (Sarstedt) and processed within 6 h upon blood collection. Clinical parameters (medication, disease duration, disease activity score etc.) were provided by the clinicians after data pseudonymization.
PBMC isolation and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (density: 1.077 g/ml; PanBiotech) and washed twice with DPBS (Gibco). Cells were resuspended in FBS (Gibco) containing 10% DMSO (Merck) and gradually frozen to -180 °C. For cell culture experiments, PBMCs were thawed, washed twice with DPBS, resuspended in B cell medium consisting of IMDM with L-Glutamine and 25 mM HEPES supplemented with 10% FBS, 1% Penicillin/Streptomycin, 1 mM Sodium Pyruvate, 1% MEM non-essential aminoacids (all from Gibco) and 0.055 mM β-Mercaptoethanol (Carl Roth) and seeded at a concentration of 0.25 × 106 cells/well into 96-well round bottom plates. Cells were rested for 2 h at 37 °C and 5% CO2 in a humified incubation chamber, if not differently described.
To analyze the effect of D1-like receptor stimulation on cytokine release, 0.25 × 106 PBMCs were seeded per well of a 96-well round bottom plate and stimulated with CpG ODN 2006 (0.35 μM, InvivoGen) with or without indicated concentrations of the agonists A68930 (Tocris) and SKF38393 (Tocris) for 24 h. Afterwards, cells were centrifuged and supernatants were frozen at − 80 °C until analysis.
Flow cytometry
After thawing, PBMCs were washed twice in PBS and then stained with Zombie NIR Fixably Viability dye (BioLegend) in PBS. Unspecific binding was then blocked by incubation with 2% BSA (Carl Roth). Cells were stained extracellularly with optimally diluted antibodies in FACS buffer (2% FBS in PBS). For intracellular stainings samples were fixed with 2% Formaldehyde (Carl Roth) in FACS buffer. Afterwards cells were permeabilized and then stained intracellularly for D2DR, D4DR or TH. Unconjugated antibodies were labeled with a secondary PE-labeled donkey anti rabbit antibody. All antibodies used and the staining panels are listed in Supplementary Table 1, and a detailed description of the method is included in “Supplementary material”.
Cells were analyzed on a BD LSR Fortessa. Doublets and dead cells were already excluded at acquisition so at least 100,000 events were recorded in the live gate, whenever possible. Data were analyzed with FlowJo Version 10.3. FlowJo Version 8.87 was used for analysis of B cell proliferation.
For these multi-color panels, gates were set based on appropriate fluorescence minus one (FMO) controls, which include all antibodies of interest except one. The gating strategies are shown in Supplementary Fig. 1.
Dopamine quantification via ELISA
For quantification of catecholamine content, 1 × 106 freshly isolated PBMCs were centrifuged at 400×g, 4 °C for 10 min, supernatant was discarded and pellet was frozen at − 80 °C. TriCat ELISA was performed as indicated by the manufacturer (IBL). This kit allowed also to measure norepinephrine and epinephrine, which are also active catecholamines synthesized from dopamine. Briefly, cell pellets were lysed in 100 μl 0.1 M HClO4 with 100 μM ascorbic acid. Thereafter, catecholamines were extracted. Limits of detection were 4, 8 and 20 pg for dopamine, norepinephrine and epinephrine, respectively.
Cell proliferation
Proliferation was analyzed by CFSE-dye dilution. PBMCs were thawed, washed once with DPBS, and stained with the Vybrant CFDA-SE Cell Tracer Dye. 1 × 106 cells were resuspended in 1 ml DPBS/0.5 μM CFDA-SE and stained for 30 min at 37 °C, 5% CO2. Excess staining was blocked with medium containing 32.5% FBS. After centrifugation CFSE-stained cells were resuspended in pre-warmed B cell medium. 0.25 × 106 cells were seeded per well of a 96-well plate and stimulated with 0.35 μM CpG ODN 2006 (InvivoGen) and indicated concentrations of D1-like receptor agonist A68930 (Tocris). Cells were cultured for 6 days at 37 °C, 5% CO2. Afterwards B cell proliferation was analyzed by flow cytometry. Protocol was adapted from Marasco et al.51.
Cytokine quantification via ELISA
For quantification of secreted IL-8 and CCL3, human IL-8 ELISA MAX Standard Sets (BioLegend) and human CCL3 uncoated ELISA Kit (Invitrogen) were used, respectively. Assays were performed as described by the manufacturers. Samples were measured in duplicates or in single detection depending on sample availability. Optimal dilutions of supernatants were determined in preliminary assays.
Statistical analysis
Statistical analysis was performed with Prism 8 software (GraphPad, v 8.3.0, www.graphpad.com). Numbers of investigated subjects are indicated in figures for each experiment. Outliers were identified and removed by ROUT method (Q = 0.01). Non-equal variability of differences regarding SDs and sphericity were computed for each comparison of two (t-test) or more groups (ANOVA or mixed-effects analysis). Pearson analysis was used for correlations. A detailed description of statistical analysis is included in “Supplementary material”. Single dots represent individual values. Box plots show mean value, 25th percentile, 50th percentile (median) 75th percentile, and maximum value.
Ethics approval and consent to participate
The study was approved by the ethics committee of IfADo (IfADo2017/125/2019-02-04). All subjects signed informed consent for study participation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- HC:
-
Healthy controls
- PsA:
-
Psoriasis arthritis
- axSpA:
-
Axial spondyloarthritis
- DR:
-
Dopaminergic receptor
- PBMCs:
-
Peripheral blood mononuclear cells
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- CCL:
-
C–C motif chemokine ligand
- DMARD:
-
Disease-modifying anti-rheumatic drug
- RANKL:
-
Receptor activator of nuclear factor kappa B ligand
- HLA:
-
Human leukocyte antigen
- CD:
-
Cluster of differentiation
- TH:
-
Tyrosine hydroxylase
- FFbH:
-
Hannover functional questionnaire
- DAS:
-
Disease activity score
References
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001 (2018).
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M. & Gabriel, S. E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 62(6), 1576–1582 (2010).
Tobon, G. J., Youinou, P. & Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 35(1), 10–14 (2010).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35(3), 347–369 (2014).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28(5), 521–574 (2007).
Tedeschi, S. K., Bermas, B. & Costenbader, K. H. Sexual disparities in the incidence and course of SLE and RA. Clin. Immunol. 149(2), 211–218 (2013).
Krasselt, M. & Baerwald, C. Sex, symptom severity, and quality of life in rheumatology. Clin. Rev. Allergy Immunol. 56(3), 346–361 (2019).
Rizzetto, L., Fava, F., Tuohy, K. M. & Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 92, 12–34 (2018).
Wu, C. Y., Yang, H. Y., Luo, S. F., Lai, J. H. From rheumatoid factor to anti-citrullinated protein antibodies and anti-carbamylated protein antibodies for diagnosis and prognosis prediction in patients with rheumatoid arthritis. Int. J. Mol. Sci. 22(2), 686. https://doi.org/10.3390/ijms22020686 (2021).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365(23), 2205–2219 (2011).
Lal, P. et al. Inflammation and autoantibody markers identify rheumatoid arthritis patients with enhanced clinical benefit following rituximab treatment. Arthritis Rheum. 63(12), 3681–3691 (2011).
van Vollenhoven, R. F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63(7), 1782–1792 (2011).
Fillatreau, S. B cells and their cytokine activities implications in human diseases. Clin. Immunol. 186, 26–31 (2018).
Sun, W. et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 9(1), 5127 (2018).
Weitzmann, M. N. Bone and the immune system. Toxicol. Pathol. 45(7), 911–924 (2017).
Basu, S. & Dasgupta, P. S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 102(2), 113–124 (2000).
Sarkar, C., Basu, B., Chakroborty, D., Dasgupta, P. S. & Basu, S. The immunoregulatory role of dopamine: An update. Brain Behav. Immun. 24(4), 525–528 (2010).
Matt, S. M. & Gaskill, P. J. Where is dopamine and how do immune cells see it? Dopamine-mediated immune cell function in health and disease. J. Neuroimmune Pharmacol. 15(1), 114–164 (2020).
Vidal, P. M. & Pacheco, R. Targeting the dopaminergic system in autoimmunity. J. Neuroimmune Pharmacol. 15(1), 57–73 (2020).
Marino, F. et al. Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp. Hematol. 27(3), 489–495 (1999).
Cosentino, M. et al. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 64(11), 975–981 (1999).
Cosentino, M. et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109(2), 632–642 (2007).
Sibley, D. R. et al. Molecular neurobiology of dopamine receptor subtypes. Neurochem. Int. 20(Suppl), 17S-22S (1992).
Beaulieu, J. M. & Gainetdinov, R. R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63(1), 182–217 (2011).
Sibley, D. R., Monsma, F. J. Jr. & Shen, Y. Molecular neurobiology of dopaminergic receptors. Int. Rev. Neurobiol. 35, 391–415 (1993).
Sellgren, C., Frisell, T., Lichtenstein, P., Landen, M. & Askling, J. The association between schizophrenia and rheumatoid arthritis: A nationwide population-based Swedish study on intraindividual and familial risks. Schizophr. Bull. 40(6), 1552–1559 (2014).
Rugbjerg, K. et al. Autoimmune disease and risk for Parkinson disease: A population-based case-control study. Neurology 73(18), 1462–1468 (2009).
Bes, C., Altunrende, B., Yilmaz Turkoglu, S., Yildiz, N. & Soy, M. Parkinsonism in elderly rheumatoid arthritis patients. Clin. Ter. 165(1), 19–21 (2014).
Sung, Y. F. et al. Reduced risk of parkinson disease in patients with rheumatoid arthritis: A nationwide population-based study. Mayo Clin. Proc. 91(10), 1346–1353 (2016).
Gjevre, J. A. & Taylor Gjevre, R. M. Restless legs syndrome as a comorbidity in rheumatoid arthritis. Autoimmune Dis. 2013, 352782 (2013).
Mitchell, U. H. et al. Peripheral dopamine in restless legs syndrome. Front. Neurol. 9, 155 (2018).
Vignon, E., Broquet, P., Mathieu, P., Louisot, P. & Richard, M. Histaminergic H1, serotoninergic, beta adrenergic and dopaminergic receptors in human osteoarthritic cartilage. Biochem. Int. 20(2), 251–255 (1990).
Papa, I. et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547(7663), 318–323 (2017).
Wei, L. et al. Dopamine receptor DR2 expression in B cells is negatively correlated with disease activity in rheumatoid arthritis patients. Immunobiology 220(3), 323–330 (2015).
van Nie, L. et al. Dopamine induces in vitro migration of synovial fibroblast from patients with rheumatoid arthritis. Sci. Rep. 10(1), 11928 (2020).
Lu, J. H., Liu, Y. Q., Deng, Q. W., Peng, Y. P. & Qiu, Y. H. Dopamine D2 receptor is involved in alleviation of type II collagen-induced arthritis in mice. Biomed. Res. Int. 2015, 496759 (2015).
Nakano, K. et al. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J. Immunol. 186(6), 3745–3752 (2011).
Nakashioya, H. et al. Therapeutic effect of D1-like dopamine receptor antagonist on collagen-induced arthritis of mice. Mod. Rheumatol. 21(3), 260–266 (2011).
Zhu, H. et al. Carbidopa, a drug in use for management of Parkinson disease inhibits T cell activation and autoimmunity. PLoS ONE 12(9), e0183484 (2017).
Capellino, S. Dopaminergic agents in rheumatoid arthritis. J. Neuroimmune Pharmacol. 15(1), 48–56. https://doi.org/10.1007/s11481-019-09850-5 (2019).
Lee, S. H. & Mouradian, M. M. Up-regulation of D1A dopamine receptor gene transcription by estrogen. Mol. Cell Endocrinol. 156(1–2), 151–157 (1999).
Lautenschlager, J. et al. Comparative evaluation of a German version of the Health Assessment Questionnaire and the Hannover Functional Capacity Questionnaire. Z. Rheumatol. 56(3), 144–155 (1997).
Gilmore, J. H. et al. “Full” dopamine D1 agonists in human caudate: Biochemical properties and therapeutic implications. Neuropharmacology 34(5), 481–488 (1995).
Koncz, G. & Hueber, A. O. The Fas/CD95 receptor regulates the death of autoreactive B cells and the selection of antigen-specific B cells. Front. Immunol. 3, 207 (2012).
Meednu, N. et al. Production of RANKL by memory B cells: A link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. 68(4), 805–816 (2016).
Chen, D. Y. et al. Proinflammatory cytokine profiles of patients with elderly-onset rheumatoid arthritis: A comparison with younger-onset disease. Gerontology 55(3), 250–258 (2009).
Wei, L. et al. The effects of dopamine receptor 2 expression on B cells on bone metabolism and TNF-alpha levels in rheumatoid arthritis. BMC Musculoskelet. Disord. 17, 352 (2016).
Ponsford, M. et al. Clozapine is associated with secondary antibody deficiency. Br. J. Psychiatry. 214(2), 1–7. https://doi.org/10.1192/bjp.2018.152 (2018).
Levesque, D. & Di Paolo, T. Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Sci. 45(19), 1813–1820 (1989).
Levesque, D., Gagnon, S. & Di Paolo, T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci. Lett. 98(3), 345–350 (1989).
Marasco, E. et al. B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur. J. Immunol. 47(1), 131–143 (2017).
Acknowledgements
The authors would like to thank Mrs. Gabi Baumhoer and Mrs. Marion Page for analysis of dopamine levels in PBMCs, Dr. Peter Bröde for support with statistical issues, the whole staff of the Rheumazentrum Ruhrgebiet for patients’ recruitment, and all patients who participated in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was entirely funded by the IfADo-Leibniz Research Centre for Working Environment and Human Factors.
Author information
Authors and Affiliations
Contributions
Study conception and design: S.C., K.W., L.F., J.B., X.B. Analysis and interpretation of data: S.C., K.W., L.F., S.T., J.R., J.B., X.B. Manuscript drafting: S.C., K.W., L.F., S.T., J.B., X.B. All authors were involved in revising the article critically for important intellectual content, and all authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wieber, K., Fleige, L., Tsiami, S. et al. Dopamine receptor 1 expressing B cells exert a proinflammatory role in female patients with rheumatoid arthritis. Sci Rep 12, 5985 (2022). https://doi.org/10.1038/s41598-022-09891-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09891-6
This article is cited by
-
Impaired cerebral microvascular endothelial cells integrity due to elevated dopamine in myasthenic model
Journal of Neuroinflammation (2024)
-
Dopamine D2 receptor on CD4+ T cells is protective against inflammatory responses and signs in a mouse model of rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
Role of neurotransmitters in immune-mediated inflammatory disorders: a crosstalk between the nervous and immune systems
Neurological Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.