Abstract
There is a lack of epidemiological information concerning intestinal parasitic infections, and especially in soil-transmitted helminths, occurring in some departments of Nicaragua. Up to now, this is the first study involving two nearby areas (Puerto Cabezas and Siuna municipalities) of the Región Autónoma Atlántico Norte (R.A.A.N.). One stool sample was analyzed by Kato-Katz, formaldehyde-ethyl acetate concentration method and modified Ziehl–Neelsen technique, and a simple questionnaire concerning demographic, sanitary and behavioral data was distributed among 735 children and evaluated. Overall prevalence of infection reached 97.0%, being the highest prevalences detected in all Nicaragua. The higher protozoan prevalence appears in Siuna (94.5%), a rural interior municipality, with a typical tropical monsoon climate, while the higher helminths rates were reached in Puerto Cabezas (92.8%), the urbanized coastal capital, with a typical tropical rainforest climate. No statistical differences were found with regard to sex. However, the 6–11-year age-group children presented the highest prevalences. Most T. trichiura infections (59.4%) were of light intensity, while 51.7% of Ascaris lumbricoides were of moderate intensity. Multivariable logistic regression analysis indicated that those who drink rainwater and walk barefoot were 2.9 and 2.5 times more likely to have helminth infections, respectively. Results from one geographical setting might not be applied to other nearby with different climatic conditions. The use of anthelmintic drugs only will not be sufficient to bring prevalence to low levels. It is necessary to design geographically more specific intervention, with communication and interaction between different disciplines (e.g. parasitology, biochemistry, molecular biology, epidemiology, public health, etc.) being imperative to reduce STH infection.
Similar content being viewed by others
Introduction
World Health Organization (WHO) list of Neglected Tropical Diseases (NTD) include several intestinal parasitic infections (IPI) that are most prevalent in developing countries where high burdens of morbidity and mortality appear1,2.
According to WHO data, a large part of the world population, basically school-age children are infected with a broad spectrum of parasitic protozoa and helminths3.
Poor socio-economic and sanitation situations are related to protozoan infections, that even cause morbidity problems when they reach mild intensity and/or concomitant infections4,5,6,7.
Among helminths, soil-transmitted helminths (STH) are among the most common infections worldwide, with a large proportion occurring in developing countries of the Americas, in China and East Asia, as well as Sub-Saharan Africa8.
IPI in children can lead to severe complications such as malabsorption, malnutrition, growth- and development disorders, anemia, diarrhea as well as physical and mental consequences constituting important health and social problems9.
Differences in the prevalence rates of intestinal parasites can be associated with environmental factors, such as vegetation, temperature, humidity and precipitation, as well as geographical site, and a variety of cultural, economic, and social characteristics10,11. All of this is linked to the lack of access to safe water and sanitation, poor hygiene practices and unsafe human waste disposal12.
Nicaragua is the largest country of Central America, with approximately 6 million inhabitants, occupying a landmass of 130,967 km2, and being the second poorest country in the Latin American and Caribbean (LAC) region. This country is made up of three regions: the Pacific region, with seven departments; the Center region, with eight departments; and the Atlantic/Caribbean region including the Región Autónoma Atlántico Norte (R.A.A.N.) and the Región Autónoma Atlántico Sur (R.A.A.S.). In the 1990s, Nicaragua established massive deworming programs, with a joint effort of various actors (ministries, NGOs, companies, etc.) in such a way that an 87% coverage of the child population was reported in 2011, and nowadays deworming is included as a new category on child vaccination cards13.
Several studies have been conducted on the prevalence of intestinal parasitic infections in Nicaragua14,15,16,17,18,19,20,21, although this is the first study published in SCI journals involving R.A.A.N. The goal of this epidemiological study was to determine the prevalence of intestinal parasite infections and the intensity of STH, assessing which variables (demographic/sanitary/behavioral) among schoolchildren were related to the infection, providing the baseline for appropriate strategies against STH infections.
Methods
Study area and climatic conditions
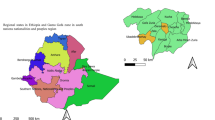
R.A.A.N. covers the largest area (33,105 km2) of Nicaragua, covering 26.3% of the national territory (Fig. 1A). It borders Honduras to the north and has a long coastline along the Caribbean Sea. R.A.A.N., with an estimated population of around 490,390 inhabitants, is divided into eight municipalities (Fig. 1B). The administrative capital is the municipality of Puerto Cabezas (lat. 14°03′N, long. 83°22′W) with around 113,534 inhabitants and 63% from urban areas. The second largest municipality is Siuna (lat. 13°44′N, long. 84°46′W), with around 103,139 inhabitants and 84% belonging to rural areas. In general, homes in rural areas of both municipalities tend to be more dispersed, being surrounded by grassland, without any urbanization and without sanitation.
(A) Map of the study area Región Autónoma Atlántico Norte (R.A.A.N.) in Nicaragua; (B) Detail of municipalities of R.A.A.N.; (C) Different climatology conditions: Tropical rainforest climate (Af) in Puerto Cabezas; Tropical monsoon climate (Am) in Siuna; and tropical savanna climate with dry-winter characteristics (Aw) (according to Köppen-Geiger climate classification). We use administrative area spatial data from the Global Administrative Area Database (GADM) v3.6 and the Köppen-Geiger climate classification system at 1 km resolution for the present (1980-2016) (Beck et al. 2018: https://doi.org/10.1038/sdata.2018.214) using QGIS software v3.22.5 (https://www.qgis.org/en/site/) to represent the study area and its climate classification.
According to the Köppen-Geiger climate classification22, the Pacific region in Nicaragua shows a tropical savanna climate (with a pronounced dry season while the Atlantic region have: a) a tropical monsoon climate (Am), with 2400 to 3000 mm annual rainfall, temperature oscillating between 24 °C and 29 °C, with a winter or rainy season (from May to January) and a summer or dry season (from February to April) with only sporadic rains, typical conditions of the municipality of Siuna; and b) a tropical rainforest climate (Af), with 2000 to 6000 mm annual rainfall, temperature oscillating between 27 °C and 39 °C, 80%-90% relative humidity, with a rainy season only (Fig. 1C), typical conditions of the municipality of Puerto Cabezas.
Population and sample collection
The survey was carried out in both municipalities, Puerto Cabezas and Siuna, involving randomly selected schools. The school teachers and the students’ parents were informed about the objective of the study, allowing schoolchildren and their little siblings to participate voluntarily. The sample size (about 25,000 children, based on a level of confidence of 95%, an expected prevalence of 50%, and a marginal error of 5%) was estimated in 378 children.
The survey was carried out from February to April 2015 and finally involved a total of 735 children (342 boys and 393 girls) aged 2–15 years (mean ± S.D. = 10 ± 1.2) coming from both municipalities: 318 from Puerto Cabezas (185 from an urban school and 133 from a rural school), and 417 from Siuna (140 from an urban school and 277 from a rural school). In both municipalities, urban schools were determined with paved ground and cobbled streets while rural schools by no urbanization and surrounded by grassland. A clean, plastic, numbered container with a snap-on lid was handed out to each child. With the aid of parents/teachers, if necessary, one stool sample per child was collected and a simple questionnaire concerning various data of the children (sex, age and area; water supply; sanitation; habits) was filled out.
Laboratory methods
A Kato-Katz slide was made from each stool sample and examined, within 30–60 min after preparation, to count the number of eggs of STHs. The number of eggs per gram of feces (EPG) was calculated by multiplying by 24 the total egg counts in all fields. WHO criteria (reference range values for each STH species) were used to classify each infection as light, moderate or heavy intensity infections23.
The rest of the fecal material of each sample was fixed in 10% formalin solution (1:3) and shipped to the Institute Politécnico de la Salud (IPS) laboratory in Managua where all samples were concentrated using the formaldehyde-ethyl acetate concentration technique (FECT). Finally, each fecal sample was microscopically examined at Department of Parasitology, Universidad de Valencia (Spain), and one aliquot of sediment obtained with FECT was stained using a modified Ziehl–Neelsen technique (MZNT). The FECT and the Kato-Katz slides were used to obtain prevalence data of STHs.
Data analysis
Statistical analyses were carried out using the SPSS version 19 software package for Windows (SPSS Inc, Chicago, IL, USA). Statistical comparison of categorical variables was carried out with the Chi-squared test. Univariable analysis, OR (95%b CI) and significance levels of all independent variables were assessed. Factors identified as statistically significant at the 5% level in univariable analysis were entered into a multiple logistic regression model. All results were considered significant if the p value was < 0.05.
Ethical approval and consent to participate
Universidad de Valencia. Estudi General granted the ethical approval of the study (H1477378643204) in accordance with the Declaration of Helsinki. Verbal informed consent was obtained from the parents/guardians of children enrolled. Diagnostic results were sent to IPS which informed the Nicaraguan Ministry of Health, being in charge of appropriate treatments.
Consent for publication
Verbal consent for children publication was obtained from the parents/guardians.
Results
Total infections
Table 1 summarizes the total infection results obtained in R.A.A.N., stratified by municipalities. The entire spectrum was made up to 10 protozoa and 6 helminth species, although not all parasite species were found in both municipalities. The isolated cases of Balantidium coli detected in Puerto Cabezas municipality and of Cryptosporidium sp., Taenia sp. and Strongyloides stercoralis detected in Siuna municipality are noteworthy.
Overall prevalence of infection, with at least one parasite species, reached 97.0% in the children surveyed, either being protozoa (90.7%) or helminths (61.6%). Among the R.A.A.N. children surveyed, protozoan infections were more prevalent than helminths, with statistical differences (p < 0.0001).
Blastocystis spp. (75.0%) was the most common intestinal protozoan diagnosed, followed by Giardia intestinalis (45.3%). Among STH, Trichuris trichiura, Ascaris lumbricoides and Hookworm were the most prevalent ones (56.3%, 36.9% and 9.9%, respectively).
The overall prevalence of infection presents statistical significant differences (p = 0.002) among both municipalities studied (99.4% in Puerto Cabezas vs 95.2% in Siuna), with a higher protozoan prevalence in Siuna municipality (94.5%) (p < 0.0001), whereas a higher helminth prevalence was reached in Puerto Cabezas (92.8%) (p < 0.0001). Among STH, T. trichiura and A. lumbricoides prevalences were higher in Puerto Cabezas than in Siuna, with statistically significant differences (p < 0.0001). In Hookworm, although higher prevalence rates occur in Siuna (11.0%), no significant differences were detected (p = 0.188).
Gender, age groups and urban/rural area infections
Prevalence results according to gender, age groups and urban/rural school area are shown in Table 2. No statistical differences were found with regard to gender neither in the total prevalence rates nor within each municipality.
The distribution of total infection prevalence according to age groups revealed significant differences. Total prevalence increased, always being highest in the group of 6–11 years-old children, both in Puerto Cabezas (p = 0.006) and Siuna (p = 0.036) municipalities. However, protozoa infection in Siuna municipality was higher in the adolescent group with statistical differences (p = 0.019) and it was higher in the municipality of Puerto Cabezas in the youngest age group with statistical differences (p = 0.049).
The total infection rate of helminths in urban schools was significantly higher than in rural schools (p = 0.0003). In particular, A. lumbricoides prevalence rates obtained in urban schools were higher than those obtained in rural schools (p < 0.0001), while Hookworm prevalence rates were higher in rural schools (p = 0.005). No differences between the two areas were detected in the case of T. trichiura infections (p = 0.367), with similar rates in urban (49.7%) as well as rural (46.3%) schools.
Polyparasitism
Most infected children presented polyparasitism (86.9%) with statistical differences (p < 0.0001) to monoinfection. This monoinfection resulted more frequent in Siuna (11.6%) than in Puerto Cabezas, with statistical differences (p = 0.007).
The highest percentages correspond to co-infection with three (21.3%), two (19.2%) and four (18.5%) different species, although children harboring five to ten species were also detected (from 13.9 to 0.1%, respectively). Only one 8-year-old boy from Siuna municipality harbored 10 species: E. coli, E. hartmanni, E. nana, I. buetschlii, G. intestinalis, Blastocystis spp., T. trichiura, A. lumbricoides, hookworm and S. stercoralis.
The extent of polyparasitism in the entire study according to gender, age groups and urban/rural school area is shown in Fig. 2. There were no statistical differences between females and males or between urban and rural schools. However, polyparasitism was lower (57.3%), with statistical differences (p < 0.0001), among the youngest children studied.
STH infection intensity and grouping
T. trichiura, A. lumbricoides and Hookworm median (range) infection intensity in R.A.A.N. was 960 (24–88.752), 18.096 (24–149.544) and 24 (24–264) eggs per gram (epg) of feces, respectively (Table 3).
Table 4 shows the prevalence of each class of infection intensity obtained in the two municipalities, according to gender, age group and urban/rural schools studied. Among those infected, most T. trichiura infections were light intensity (59.4%), while 51.7% of A. lumbricoides were moderate-intensity infections. No differences in infection intensity classes appeared among the two municipalities studied in any STH species.
Hookworm infection intensity classes were of light infection intensity (100%) independent of municipality, sex, age group and urban/rural schools studied. In the case of T. trichiura, light-infection intensity in girls and in the urban schools was higher with statistical differences than in boys (p = 0.003) and in the rural schools (p = 0.0007). In the case of A. lumbricoides, moderate and heavy infection intensities were higher, with statistical differences (p < 0.0001 and p = 0.003, respectively) in the 6–11-year-old age group.
Table 5 shows the frequency of STH infection grouping. Infection with two STH species is the most frequent association found in R.A.A.N., with the association of T. trichiura and A. lumbricoides standing out.
Only single Hookworm infections appear more frequently in Siuna municipality, with significant differences (p = 0.001). However, double and triple STH infections appear more frequently in Puerto Cabezas than in Siuna municipality, with significant differences (p < 0.0001 and p = 0.023, respectively). Significant positive associations were reached in the co-infection analysis of T. trichiura with respect to other STH detected in this study, but no associations were observed between A. lumbricoides and Hookworm co-infections.
Univariable and multivariable analyses of factors related to infections
We assessed the association between infection and several variables to determine their impact on the intestinal parasite infection. During univariable analysis, 3 variables were identified with p value < 0.05 in relation to any infection status, such as children’s age, source of drinking water and habit of wearing shoes (Table 6).
The odds of helminth infection were highest among 6–11-year-old children, in those drinking rain water (p < 0.0001) and in those walking barefoot (p = 0.025). On the other hand, the odds of helminth infection were lowest among children of the urban schools (p < 0.0001).
The variables with p < 0.05 in the univariable analysis were analyzed using multivariable logistic regression model using the backward stepwise method to assess the helminth infection (Table 7). The variable urban/rural school area was left out with p value = 0.06. The model identified that the risk of helminth infection is multiplied by 2.6 if the child is 6–11 years old, by 2.9 if the child does not drink safe water, and by 2.5 if the child walks barefoot.
Discussion
The very high infection prevalence of intestinal parasites detected in children from R.A.A.N. is worth mentioning. In fact, it is the highest detected in Nicaragua, with the municipality of Puerto Cabezas standing out. The results obtained are likely to be the consequence of drinking and eating contaminated water and food, habits of playing or handling infested soil, eating with soiled hands, and unhygienic toilet practices. All of this is another clear example of the typical scenario of intestinal parasites present in LAC countries considered climatically suitable for parasite transmission. Besides, the presence of commensal protozoan species is important since they are indicators of poor hygienic-sanitary conditions and that have transmission mechanisms identical to the pathogenic species.
Blastocystis spp. and T. trichiura were detected as the predominant enteropathogens among children from R.A.A.N., just as in previous Nicaraguan studies18,19,20,21 and other countries24,25,26. However, in other regions of Nicaragua, G. intestinalis and A. lumbricoides were the most common parasites found1,27.
Focusing on the two studied municipalities, significant differences appear with helminth infection mainly in Puerto Cabezas, while in Siuna protozoan infection is higher. In addition to the scarce knowledge and awareness of parasitic infections24,28,29 and the similar hygienic-sanitary conditions in both municipalities, it could be the climatic conditions that mark the differences. Although these two municipalities are separated by only about 210 km, the climate is different between them: Puerto Cabezas presents a tropical rainforest climate, while Siuna is characterized by a tropical monsoon climate. Besides, Puerto Cabezas is a coastal, more urbanized, municipality than Siuna, which is located in the interior and basically rural. This fact indicates that results from one geographical setting might not be applied to other nearby, and thus, climatic conditions, surface temperature and humidity could be more favorable for the survival of one parasite or another.
Each of the infective stages of STH (eggs of T. trichuris and A. lumbricoides; infective larvae of hookworm) has its climatic thresholds for growth and development. In fact, altered climatic parameters due to climate change are bound to influence the biological development of STH.
The high relative humidity of Puerto Cabezas throughout the entire year could be one of the causes of the higher viability of STH eggs30 showing the dominance of A. lumbricoides and T. trichiura. Both species are more susceptible to increasing temperature, while in the case of Hookworm the trend is contrary and the higher prevalence rates are found in Siuna, more related to the fact that the prevalence of infection is apparently more susceptible to increasing desiccation besides a decreasing level of urbanization26,31.
Due to the climatic variability, we found that human helminthiases present a heterogenic distribution in children depending on where exactly they live, even within the same region of Nicaragua (R.A.A.N.). Likewise, significant regional differences in the prevalence and intensity of STH infections have been found in other LAC countries such as Ecuador38 and Paraguay32.
Similar to other LAC countries25,32, no differences of infection between boys and girls were found. However, prevalence of infection was directly related to age, being highest in the 6–11-year-old group, related to starting playing outdoors and getting exposed to infections26. Moreover, polyparasitism, the most common form of infection, was also age-related. These results agree with previous work in Nicaragua27,28 and other countries11,34.
Disease caused by STH is directly related to infection intensity. Although prevalence is used in STH epidemiological surveys, intensity of infection is more important as a determinant of morbidity35. The light intensity of T. trichiura is normal in endemic areas where most students harbor low or moderate burdens, while only a few individuals harbor a heavy burden19,33,36. Moreover, the moderate intensity of A. lumbricoides appears mostly in children of 6–11 years of age. In agreement with our results, surveys from other parts of the world also found light intensities of T. trichiura and hookworm infections, while values for A. lumbricoides were of light and moderate intensities35.
Adequate sanitation facilities may be important when explaining the spatial variation of helminth infection prevalence11. The odds of STH infection among children of R.A.A.N. who had safe water sources at their disposal were lower compared to those who had to rely on unsafe water sources (rain water). This finding corroborates previous LAC study37 and it is in line with the result of a systematic review and meta-analysis which stated that "access to piped water was associated with lower odds of A. lumbricoides and T. trichiura infection"38, as having a water tap means easy access to water for hygienic practices.
The odds of STH infection increase with age since there is an age-related change in exposure to STHs, considering that as they get older they are able to play on their own in outdoor activities. This trend was also observed in other studies conducted elsewhere39.
There has been an increase in attention to wearing shoes for preventing soil-related disease conditions as well as STH infection. Previous research estimates that shoe-wearing lowers the odds of Hookworm infection40,41. The prevalence of Hookworm infection in our work is not the most commonly STH infection detected, but the lack of shoes is actually being a surrogate marker of lower socioeconomic status, which therefore increases the odds of total STH infection.
Effective strategies for controlling STH infection would have to center on reducing risk factors together with regular deworming, as anthelminthic drugs kill the parasite within the host but do not prevent reinfections and will not be sufficient to bring prevalence to low levels35,42. In accordance with WHO guidelines8, the children from R.A.A.N. are exposed to a high risk of STH infection (prevalence of any STH > 50%) and should thus continue to be treated with anthelmintics twice a year. Moreover, there is a need to increase people’s knowledge on proper and environmental hygiene, mainly in those communities with lack of water sanitation and hygiene (WASH) such as Puerto Cabezas and Siuna. Results obtained in our work can be used by policy makers in Nicaragua to design geographically more specific intervention programs to reduce STH infections.
Collaborations between research disciplines and between researchers and workers in the field responsible for individual and community health (e.g. parasitology, biochemistry, molecular biology, epidemiology, public health, etc.) is imperative to improve outcomes for people at risk from these STH infections.
The results of our study should be interpreted in the light of some limitations, mainly: only two municipilaties have been studied out of the eight that comprise R.A.A.N.; the used of Kato-Katz technique, which can miss some egg count in an area with light-intensity infections; the prevalence obtained for S. stercoralis larvae may not be considered definitive because the specific etiological technique for the detection of this nematode was not applied; and also, the low number of completed questionnaires, as well as the veracity of their responses.
Data availability
All the material analyzed as well as the database is available in the Laboratory of Parasitology of the University of Valencia from the corresponding author on reasonable request.
References
Karan, A., Chapman, G. B. & Galvani, A. the influence of poverty and culture on the transmission of parasitic infections in rural Nicaraguan villages. J Parasitol. Res. 2012, 478292. https://doi.org/10.1155/2012/478292 (2012).
Houweling, T. A. J. et al. Socioeconomic inequalities in neglected tropical diseases: A systematic review. PloS Negl. Trop. Dis. 10, e0004546. https://doi.org/10.1371/journal.pntd.0004546 (2016).
Chelkeba, L., Mekonnen, Z., Alemu, Y. & Emana, D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: A systematic review and meta-analysis. BMC Pub. Health 20, 117. https://doi.org/10.1186/s12889-020-8222-y (2020).
Fletcher, S. M., Stark, D., Harkness, J. & Ellis, J. Enteric protozoa in the developed world: A public health perspective. Clin. Microbiol. Rev. 25, 420–449. https://doi.org/10.1128/CMR.05038-11 (2012).
Osman, M. et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Negl. Trop. Dis. 10, e0004496. https://doi.org/10.1371/journal.pntd.0004496 (2016).
Burgess, S. L., Gilchrist, C. A., Lynn, T. C. & Petri, W. A. Parasitic protozoa and interactions with the host intestinal microbiota. Infect. Immun. 85, e00101-e117. https://doi.org/10.1128/IAI.00101-17 (2017).
Ngui, R., Lim, Y. A., Chong Kin, L., Sek Chuen, C. & Jaffar, S. Association between anemia, iron deficiency anemia, neglected parasitic infections and socioeconomic factors in rural children of west Malaysia. PLoS Negl. Trop. Dis. 6, e1550 (2012).
World Health Organization. Helminth Control in School-Age Children: A Guide for Managers of Control Programs 2nd edn. (WHO, 2011).
Gutierrez-Jimenez, J. et al. Malnutrition and the presence of intestinal parasites in children from the poorest municipalities of Mexico. JIDC 7, 741–747 (2013).
Okulewicz, A. The impact of global climate change on the spread of parasitic nematodes. Ann. Parasitol. 63, 15–20. https://doi.org/10.17420/ap6301.79 (2017).
Owada, K. et al. Spatial distribution and populations at risk of A. lumbricoides and T. trichiura co-infections and infection intensity classes: An ecological study. Parasit. Vectors 11, 535. https://doi.org/10.1186/s13071-018-3107-y (2018).
Boithias, L. et al. Hydrological regime and water shortage as drivers of the seasonal incidence of diarrheal diseases in a tropical Montane environment. PLoS Negl. Trop. Dis. 10, e0005195. https://doi.org/10.1371/journal.pntd.0005195 (2016).
PAHO. Pautas operativas para la puesta en marcha de actividades integradas de desparasitación: contribución al control de las geohelmintiasis en América Latina y el Caribe. Washington, DC. OPS/OMS (2015). ISBN: 978-92-75-31861-4.
Cavuoti, D. & Lancaster, K. R. Intestinal parasitism of children on Corn Island, Nicaragua. Ped. Inf. Dis. J. 11, 775–776 (1992).
Téllez, A. et al. Prevalence of intestinal parasites in the human population of Leon, Nicaragua. Acta Trop. 66, 119–125 (1997).
Oberhelman, R. A. et al. Correlations between intestinal parasitosis, phisical growth, and physchomotor development among infants and children from rural Nicaragua. Am. J. Trop. Med. Hyg. 58, 470–475 (1998).
Rosewell, A. et al. Soil-transmitted helminth infection and urbanization in 880 primary school children in Nicaragua, 2005. Trop. Doct. 40, 141–143. https://doi.org/10.1258/td.2010.090425 (2010).
Muñoz-Antoli, C., Pavón, A., Marcilla, A., Toledo, R. & Esteban, J. G. Prevalence and risk factors related to intestinal parasites among children in Department of Rio San Juan, Nicaragua. Trans. R. Soc. Trop. Med. Hyg. 108, 774–782. https://doi.org/10.1093/trstmh/tru160 (2014).
Muñoz-Antoli, C., Pavón, A., Pérez, P., Toledo, R. & Esteban, J. G. Soil-transmitted Helminth Infections in Schoolchildren of Laguna de Perlas (Nicaragua). J. Trop. Pediatr. 63, 124–134. https://doi.org/10.1093/tropej/fmw061 (2017).
Muñoz-Antoli, C. et al. Enteroparasites in preschool children on the pacific region of Nicaragua. Am. J. Trop. Med. Hyg. 98, 570–575. https://doi.org/10.4269/ajtmh.17-0551 (2018).
Muñoz-Antoli, C., Pérez, P., Pavón, A., Toledo, R. & Esteban, J. G. Soil-Transmitted Helminth infections and anemia in schoolchildren from Corn Island Archipelago (RAAS, Nicaragua). Am. J. Trop. Med. Hyg. 99, 1591–1597. https://doi.org/10.4269/ajtmh.18-0195 (2018).
Peel, M. C., Finlayson, B. L. & McMahon, T. A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644. https://doi.org/10.5194/hess-11-1633-2007 (2007).
Montresor, A., Crompton, D. W. T., Bundy, D. A. P., Hall, A. & Savioli, L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level (WHO/CDS/SIP/98.1) (WHO, 1998).
Ojja, S. et al. Prevalence, intensity and factors associated with soil-transmitted helminths infections among preschool-age children in Hoima district, rural western Uganda. BMC Infect. Dis. 18, 408. https://doi.org/10.1186/s12879-018-3289-0 (2018).
Moncayo, A. L., Lovato, R. & Cooper, P. J. Soil-transmitted helminth infections and nutritional status in Ecuador: Findings from a national survey and implications for control strategies. BMJ Open 8, e021319. https://doi.org/10.1136/bmjopen-2017-021319 (2018).
Punsawad, C. et al. Prevalence of intestinal parasitic infections and associated risk factors for hookworm infections among primary schoolchildren in rural areas of Nakhon Si Thammarat, southern Thailand. BMC Pub. Health. 18, 1118. https://doi.org/10.1186/s12889-018-6023-3 (2018).
Chammartin, F. et al. Soil-transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 13, 507–518. https://doi.org/10.1016/S1473-3099(13)70071-9 (2013).
Shivairo, R., Muleke, C., Mukabane, D. & Kumba, J. Soil transmitted helminthes prevalence among pre-school age children in Elburgon municipality, Kenya. J. Biol. Agric. Health. 4, 36–41 (2014).
Kabatereine, N. B. et al. Integrated prevalence mapping of schistosomiasis, soil-transmitted helminthiasis and malaria in lakeside and island communities in Lake Victoria, Uganda. Parasit. Vectors 4, 232 (2011).
Navone, G. T. et al. Estudio transversal de las parasitosis intestinales en poblaciones infantiles de Argentina. Rev. Panam. Salud. Pub. 41, e24. https://doi.org/10.26633/RPSP.2017.24 (2017).
Tabi, E. S. B., Eyong, E. M., Akum, E. A., Löve, J. & Cumber, S. N. Soil-transmitted Helminth infection in the Tiko Health District, South West Region of Cameroon: A post-intervention survey on prevalence and intensity of infection among primary school children. Pan. Afr. Med. J. 30, 74. https://doi.org/10.11604/pamj.2018.30.74.15676 (2018).
Vázquez, F. A. et al. Prevalencia e intensidad de infección por geohelmintos, caracterizando los factores socio culturales y ambientales que inciden en la infección de escolares, Paraguay, 2015. Rev Chilena Infectol. 35, 501–508 (2018).
Imam, A., Farouk, Z. L., Hassan-Hanga, F. & Ihesiulor, U. G. A comparative cross-sectional study of prevalence and intensity of soil-transmitted helminthic infection between healthy and severe acutely malnourished pre-school aged children in Kano, Northern Nigeria. BMC Inf. Dis. 19, 121. https://doi.org/10.1186/s12879-019-3755-3 (2019).
Moser, W. et al. Efficacy and safety of oxantel pamoate in school-aged children infected with Trichuris trichiura on Pemba Island, Tanzania: A parallel, randomised, controlled, dose-ranging study. Lancet Infect. Dis. 16, 53–60 (2016).
Dunn, J. C. et al. Soil-transmitted helminth reinfection four and six months after mass drug administration: results from the delta region of Myanmar. PLoS Negl. Trop. Dis. 13, e0006591. https://doi.org/10.1371/journal.pntd.0006591 (2019).
Blackwell, A. D., Martin, M., Kaplan, H. & Gurven, M. Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc. Biol. Sci. 280, 20131671. https://doi.org/10.1098/rspb.2013.1671 (2013).
Echazu, A. et al. Effect of poor access to water and sanitation as risk factors for soil-transmitted helminth infection: Selectiveness by the infective route. PLoS Negl. Trop. Dis. 9, e0004111. https://doi.org/10.1371/journal.pntd.0004111 (2015).
Strunz, E. C. et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: A systematic review and meta-analysis. PLoS Med. 11(3), e1001620. https://doi.org/10.1371/journal.pmed.1001620 (2014).
Masaku, J. et al. Soil-transmitted helminths and schistosomiasis among pre-school age children in a rural setting of Busia County, Western Kenya: a cross-sectional study of prevalence, and associated exposures. BMC Pub. Health 20, 356 (2020).
Tomczyk, S. et al. Association between footwear use and neglected tropical diseases: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 8, e3285 (2014).
Paige, S. B. et al. Combining footwear with public health iconography to prevent soil-transmitted helminth infections. Am. J. Trop. Med. Hyg. 96, 205–213. https://doi.org/10.4269/ajtmh.15-0910 (2017).
Ziegelbauer, K. et al. Effect of sanitation on soil- transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 9, e1001162. https://doi.org/10.1371/journal.pmed.1001162 (2012).
Acknowledgements
Collaboration by teachers of each school and parents/guardians of the children participating in this study is acknowledged.
Funding
This work was supported by Ministerio de Economía y Competitividad (Madrid, Spain) (No. BFU2016-75639-P) and by Ministerio de Sanidad y Consumo (Madrid, Spain) (No. RD12/0018/0013, Red de Investigación Cooperativa en Enfermedades Tropicales- RICET, IV National Program of I+D+I 2008–2011, ISCIII – Subdirección General de Redes y Centros de Investigación Cooperativa and FEDER).
Author information
Authors and Affiliations
Contributions
C.M.A. participate in the conceptualization, formal analysis, investigation, methodology, writing the original draft and review and editing it; P.P. participated in the formal analysis, investigation and methodology; A.P. participated in the formal analysis, investigation and supervision; R.T. participated in the funding acquisition, resources, supervision and validation; J.G.E. participated in the conceptualization, funding acquisition, resources, supervision, validation and review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muñoz-Antoli, C., Pérez, P., Pavón, A. et al. High intestinal parasite infection detected in children from Región Autónoma Atlántico Norte (R.A.A.N.) of Nicaragua. Sci Rep 12, 5872 (2022). https://doi.org/10.1038/s41598-022-09756-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09756-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.