Abstract
Little is known about the role of diet in the risk of invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) of the breast, the most common histological subtypes of breast cancer (BC). This is because, the majority of studies on the association of diet and the risk of BC are focused on single food items, and studies considering the overall diet in terms of dietary patterns are limited. Also, the potential heterogeneity in the impact of Western diet (WD) on histological subtypes of BC is not established. This, the age-frequency-matched case–control study included 1009 incident BC cases and 1009 healthy controls. The required data was obtained from the patients’ medical files and interviews using a previously validated researcher-designed questionnaire for collecting data on socio-economic and anthropometric statuses and a valid food frequency questionnaire (FFQ) to measure the participants’ dietary intake. We used multinomial logistic regression, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A positive and significant association was observed between higher adherence to a WD and risk of IDC (OR comparing highest with the lowest tertile: 2.45, 95% CI 1.88, 3.17; p-trend < 0.001), whereas no significant association was observed between adherence to the WD and the risk of ILC (OR comparing highest with the lowest tertile: 1.63, 95% CI 0.63, 3.25) (p for heterogeneity = 0.03). The results of an analysis stratified by menopausal status suggested a similar pattern. We provided evidence that adherence to a WD raises the risk of IDC, but not ILC, suggesting different etiological mechanisms for IDC and ILC.
Similar content being viewed by others

Introduction
Accounting for about 25% of all female malignancies and 1 million new cases worldwide annually, breast cancer (BC) is a common neoplasm among women. Concerning developed countries, BC is even more common as it is accounted for 27% of all cancers among women from these countries1. However, the rate of age-related mortality from BC is decreasing in high-income countries, whereas the mortality is increasing in lower-income countries2. BC is also the most common cancer among Iranian women, and the mean age at diagnosis is significantly lower compared with the western countries3,4. As a result, the vast majority of BC cases among Iranian women are premenopausal3.
Breast malignancies may occur in the mammary glands with a wide variety of morphological, immunohistochemical and histopathological subtypes and different clinical presentations and outcomes5. For example, clinical and epidemiologic studies suggested that the histopathological subtypes of BC differ in terms of behaviour, risk factors and even response to treatment6,7. Among different types of BC, invasive ductal (IDC) and lobular carcinomas (ILC) are the first and second most common breast carcinomas (75% and 15% of all breast malignant tumours, respectively). Also, the locations of these tumours are different as the IDC mostly starts in the cells that line a milk duct in the breast and ILC starts in the milk-producing glands (lobules). It is also reported that these two subtypes have distinguishing clinical, molecular and pathologic features7,8. For example, it has been suggested that, prognosis of ILC was significantly worse compared to IDC9. Also, compared to IDC, ILC patients tend to be at risk for distant recurrence for longer than 5–10 years period10 and ILC is considered to be less chemo-sensitive for either adjuvant or neoadjuvant chemotherapy compared to IDC9. However, limited evidence is available on the risk factors of ILC and IDC, and very few epidemiological studies have examined the heterogeneity in the factors associated with these subtypes of BC, with no attention to these two BC subtypes7,11,12. Also, evidence on this topic from less-developed countries is limited13.
With regard to the effect of diet on the risk of BC, numerous studies are conducted measuring the association between single food items and risk of BC in general14,15,16,17. Knowing that individuals do not consume nutrients separately, it is important to investigate the effect of our diet on health with a holistic dietary approach rather than individual nutrients especially when evaluating the association of diet and BC risk18. As a consequence of the Neolithic and industrial revolutions, the staple foods of the Western diet (WD), such as processed meats, sugar, alcohol, and refined grains, became the main component of the diet of a people19. We know for years that the WD is potentially detrimental to our health. For example, the advent of the WD has been associated to an increase in the occurrence of obesity, mortality from heart diseases, type 2 diabetes, hypertension, cancer and other diet-related diseases20. It has been suggested that the Iranian population is different from those in the developed world with respect to genetics, lifestyle, diet, and environment21,22. However, the rapid demographic change, urbanization, and social development are causing several important health-related changes among the Iranian population, including nutritional transition to a westernised diet and sedentary lifestyle17.
The importance of dietary pattern in our health has only recently received attention and evidence on the association of dietary patterns and risk of IDC and ILC is still scarce. Accordingly, a meta-analysis of six studies on the association between a WD and the risk of IDC reported a significant and positive association between WD and risk of IDC13. Moreover, combined the results of studies showed a positive association between higher adherence to a WD and risk of ILC13. This meta-analysis also highlighted the lack of well-designed large epidemiological studies on this topic and suggested that well-designed studies are required to approve the association of WD and histopathological subtypes of BC.
Given the importance of WD in the aetiology of majority of diseases including cancer, the main body of evidence on diet and BC comes from epidemiologic studies conducted in developed countries. The studies also have mainly focused on single food items approach with less attention to BC subtypes. In the current study, our aim was to assess the potential associations between a WD and the risk of developing IDC and ILC. We further examined if the pattern of association differs by subtype with regard to menopausal status. The observed difference in the impact of WD on BC subtypes might provide new insight in the aetiology of BC.
Results
General characteristics
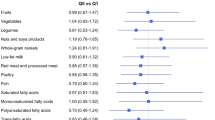
General characteristics, dietary information, and adherence to the WD between controls and IDC cases, and between controls and ILC cases are reported in Table 1. The mean (± standard deviation [SD]) age of controls was 48.74 (± 10.48) and for IDC cases, and ILC patients it was 47.2 ± 9.4 and 50.5 ± 10.3 respectively (p < 0.001). Family history of BC was reported among 14% of control participants, whereas 27% of IDC and 16% of ILC cases reported BC among their family members (p = 0.001). Overall, 36% of control participants, 39% of IDC cases and 41% of ILC cases were post-menopausal (p > 0.05). Also, compared to the control participants (7%), IDC and ILC cases were more likely to be smoker (14% and 15%, respectively) (p < 0.05). The mean (± SD) of the WDS was 24.3 (± 2.5), 23.1 (± 4.1) and 22.4 (± 4.4) for IDC, ILC cases and controls, respectively (p = 0.01) (Table 1). Compared to the control group, the average of consumption of all the WD components was higher among IDC and ILC cases. However, there is no difference in fruit consumption between cases and control group.
General characteristics and dietary information based on tertiles of adherence to the Western dietary pattern are presented in Table 2. As shown in Table 2, those in the highest vs. lowest tertile of adherence to WD were more likely to have IDC (52%) and less likely to be control (38%) and ILC (9%) (p < 0.001). Dietary information based on tertiles of adherence to the components of a WD pattern among the control participants and IDC and ILC of the breast are presented in Supplementary Table 1.
Associations between the WD pattern and risk of IDC
The estimated ORs for adherence to the WD pattern in IDC patients are presented in Table 3. Since there were no significant differences between the two models, (model 1: not adjusted for fruit and vegetable and model 2: adjusted for fruit and vegetable), we reported the results of the fully adjusted model 2. Overall, more adherence to the WD pattern was associated with an increased risk of IDC in both model 1 and model 2 (Model 2: OR highest vs. lowest tertile: 2.45, 95% CI 1.88, 3.17). Also, test for a linear trend across the tertiles of WD adherence was significant (p‐trend < 0.001).
Associations between the WD pattern and ILC
As Table 3 shows the estimated ORs for adherence to the WD pattern and ILC breast cancer risk, we found that higher adherence to the WD pattern was not associated with the risk of ILC in both model 1 and model 2 (Model 2: OR highest vs. lowest tertile: 1.63, 95% CI 0.63, 3.25, p-trend = 0.06). Test for heterogeneity comparing the association of adherence to WD and risk of IDC and ILC was also significant (p-heterogeneity = 0.03).
Stratification analysis
Stratification results based on menopausal status are shown in Table 3. As such, among pre-menopause women more adherence to a WD pattern was associated with an increased risk of IDC (Model 2: OR highest vs. lowest tertile: 2.95, 95% CI 1.91, 4.56; p-trend < 0.001). However, results for ILC are similar to ILD but not statistically significant (p-het = 0.02). Among menopausal women, greater adherence to the WD pattern was associated with an increased risk of IDC (Model 2: OR highest vs. lowest tertile: 2.16, 95% CI 1.39, 3.37) but the results were not statistically significant for ILC (Model 2: OR highest vs. lowest tertile: 1.35, 95% CI 0.64, 2.85) (p-heterogeneity = 0.04).
The findings were also in line with the overall results when we stratified the results by smoking status or BMI, suggesting that apart from smoking status or BMI, higher adherence to a WD is a risk factor for IDC, but not for ILC (data are not shown). No significant interaction between the study variables (including smoking status) and menopausal status was found (p for interaction ≥ 0.05 for all). Besides, there was no significant collinearity between the WD score and any other factors.
Discussion
Results of the present study indicated a significant heterogeneity in the association of adherence to a WD and risk of IDC and ILC as a positive association was observed between WD and risk of IDC. Overall, WD was associated with 2.45 times increase in the risk of IDC. Results among both pre- and post-menopausal women were in line with the overall findings.
In accordance with our findings, in the pooled result of the only available meta-analysis13 on IDC and ILC, found a significant and positive association between the WD and the risk of IDC (RR 1.36, 95% CI 1.18, 1.53). Also, in line with our findings, Ronco et al. based on a case control study reported a more than twofold increase in the risk of IDC among women with a higher adherence to the WD, and the association was linear (p-trend = 0.002)23. The results of several other previously published case control studies are also in accordance with our findings, suggesting those with a higher adherence to the WD were more prone to IDC24,25,26,27. However, in contrast with our findings, the results of a cohort study by Cottet et al. found no significant association between higher adherence to a WD and IDC risk (RR 1.17, 95% CI 0.98–1.40)28. Nevertheless, the possibility that the positive association observed in case–control studies might be due to recall bias in case–control studies and/or small sample size of the included case control studies, should be taken in to consideration.
Regarding ILC, there are only two published studies (one cohorts and one case–control) that examined the associations between WD and ILC risk and the results were inconsistent as although the case–control study reported a positive association between WD and risk of ILC (OR 1.36)25, the cohort study also found a positive association but this did not reach statistical significance (RR = 1.65)28. The results of the only available meta-analysis were based on these two above-mentioned studies on dietary patterns and risk of ILC, revealed a marginally positive association between adherence to the WD and risk of ILC (RR 1.45, 95% CI 1.04, 1.86)13. To address the inconsistencies between the results of previous studies, the impacts of some important confounders that might affect the findings of the included studies should take in to consideration. As such, although most previous studies adjusted a large number of confounding factors (i.e.; BMI and smoking) which may potentially confound the association between dietary patterns and BC29, their results were not fully adjusted for some potentially important confounders, such as physical activity and reproductive factors. In our study, we addressed these issues, as our analysis included reproductive variables, health behaviours, smoking and physical activity. However, another plausible explanation of the non-significant finding in our study is the much lower sample size for ILC (compare with the other two studies) and therefore insufficient power to detect a significant association.
Regarding the subtypes of BC, the MCC-Spanish (multicase-control study on common tumours in Spain) study suggested that higher adherence to the WD seems to increase BC risk in both premenopausal and postmenopausal women with no difference by subtypes18. The results of another meta-analysis showed a possible increase in the risk of BC with a higher adherence to WD29. The study also reported that among postmenopausal women a significant association is observed between hormone receptor-positive tumours in the subgroup analyses. However, in contract with our study, the authors did not assess the associations of dietary patterns in histological subtypes of BC29. Although a previous study claimed that WD is associated with an increased risk of BC among premenopausal women30, we found no significant difference in the association of the WD and BC subtypes between pre- and post-menopausal women. However, it should be taken into account that the present study contained limited participants on both menopausal status and breast subtypes, which could have led to a power issue, and the detection of small size effects.
In our study, Western diet, or unhealthy diet, which is notably characterized by higher intakes of red and processed meat, sugar, and sugar-sweetened products, animal fats, and vegetable-processed fats; including margarine, dressings, and dips, was associated with a higher risk of BC. It is suggested that the N-nitroso components of meat might increase the risk of breast carcinoma31,32. These detrimental components of the WD, might accelerate the initiation, promotion and progression of cancers through several potential biologic mechanisms, including upsurge cellular oxidative stress and potential increase in DNA damage in different tissues of the breast33. It has been suggested that adherence to a WD might alter the composition of the gut microbiota, and in turn, the presence and composition of short-chain fatty acids34. The short-chain fatty acids generated by gut microbiota seem to have crucial roles in breast cancer occurrence and progression35,36.
During the recent years, researchers are becoming more interested in the effect of dietary patterns and risk of BC, as diet can accommodate the complex interplay of nutrients within our food and body. The available evidence, however, on the associations between dietary patterns and BC risk are inconsistent, which might be related to the fact that dietary patterns have distinct impacts on various BC subtypes. Although previous epidemiological investigations on the relationship between BC and food patterns, hormone status, and menopausal state have shown heterogeneous results28,37, to date very few studies have examined the association between WD and risk of different subtypes of BC13,29,38,39, and the literature on the association of WD and risk of IDC and ILC is still inconclusive. Hence, taking into account the differences in the risk factors, response to treatment and histopathological differences of BC subtypes6,7 and also the impacts of components of the WD on BC subtypes, we conclude that, so far, there is still limited robust evidence available on the effect of WD and its components on the risk of IDC and ILC40, hence, further research on the specific relation between WD (and its components) and BC subtypes, and the mechanism behind these associations is warranted.
Strengths and limitations
To the best of our knowledge, this is one of the first case–control studies from a developing country that compares the associations between adherence to a WD and the risk of ILC and IDC in pre- and postmenopausal women. The results are not prone to survival bias because we only considered newly diagnosed cases (incident cases). Anticipating the presence of recall bias, in order to minimize even more the effect of this possible bias, only cases that responded to the questionnaire within the 6 months following the diagnosis were included. Also, factors of interest in this study are considered to be among those that are generally well remembered, regardless of the status of the participants. Likewise, we adjusted the associations for a wide range of established risk factors of BC. Although, the information bias, as a consequence of self-reporting information on food consumption is a common bias in nutritional studies41, the strength and direction of this bias should not be significantly different between cases and controls, suggesting that the impact of information bias on our findings might be minimal. The recruitment of women to the control sample only on the basis of an oral declaration of no breast cancer could also be considered as a limitation to this study. As a result, the confirmatory exams or tests might not be necessary7. Likewise, another limitation is the relatively small subgroup of ILC. Another limitation of our study was the lack of information on the status of oestrogen (ER) and progesterone (PR) hormone receptor and human epidermal growth factor receptor 2 (HER2) to perform a stratification analysis by hormone receptor status. Thus, a larger study on the heterogeneity of associations of a WD by BC subtypes, hormone receptor status and menopausal status is recommended.
Conclusions
In conclusion, our findings showed that WD was significantly associated with an increased risk of IDC but this association was not significant but in the same direction for ILC in premenopausal women. In the present study, the results stratified by the menopausal status were in line with the overall findings. Given the significance and strength of the associations found with the WD pattern, in order to determine which dietary habits should be recommended and which should be avoided to reduce BC risk, it is critical to focus not only on the potential protective effects of the healthy dietary patterns, but also on the harmful components of the Western diet.
Methods
The method section is in accordance with STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) statement for reporting observational studies (including case–control studies)42.
Ethics statement
The ethical committee of Shiraz University of Medical Sciences (no.13748) approved the study. The study subjects were informed about the study process and confidentiality of data and provided oral informed consent.
Informed consent
Literate individuals read and signed informed consent forms, and verbal consent was gathered from illiterate participants. Also, written informed consent obtained from a legal guardian of illiterate participants.
Study population
Details of the study participants and methodology (including case and control selection criteria) of this study have been described elsewhere7. In the previous paper, we assessed the associations of some well-recognized risk factors of BC between IDC and ILC7. This case–control study included women who were newly diagnosed (within the 6 months following the diagnosis) with invasive BC and were referred to Motahari breast clinic located in Namazi Hospital (affiliated with the Shiraz University of Medical Sciences). This medical centre is referral in Shiraz (the capital of Fars province), and above 80% of newly diagnosed BC women within the Fars province are registered with this centre for BC cares3. All women whose diagnosis of primary invasive BC (IDC or ILC) was histologically confirmed during the research period were invited to participate. Those in the control group were considered cancer-free if they verbally verified that they had no current or previous cancer history (no confirmatory exam or test was mandatory).
Inclusion and exclusion criteria
Female patients with newly diagnosed breast cancer, having positive histopathology report on IDC and/or ILC, and also, those who did not followed a prescribed diet regimen by nutritionist were include in the analysis. Patients who experienced a recent significant change in their weight or size from the last 6 months were not included. The latter was done to eliminate the possibility of reverse causation of the association between weight or diet and BC17. The case participants were excluded if they had relapsed or recurrent cancer after treatment. Likewise, those with mental disorders or with impaired hearing were excluded4. Additionally, participants reporting extreme energy intakes, < 3200 or > 18,000 kJ/day, were excluded in the analysis. A total of 1073 patients (response rate 94%) agreed to participate, of whom, patients were excluded for several reasons including recent significant change in weight (n = 2), relapsed or recurrent cancer after treatment (n = 9), extreme energy intakes (n = 6), lack of information on tumour subtype (n = 29), and incomplete pathological reports (n = 18). After applying the above-mentioned inclusion and exclusion criteria, the study population consisted of 1009 BC cases (849 IDC and 160 ILC) and 1009 healthy controls7. The study flow diagram is presented in Fig. 1.
Data collection
Again, more detail about the method of collecting data on socio-demographic, reproductive and health behaviour of newly diagnosed cases and controls has been reported previously7. In addition to the information on dietary consumption, several potential risk factors or confounders including family history of BC, smoking, oral contraceptive pill (OCP), chest X-ray history, history of benign breast disease (BBD), BMI, physical activity, age at first delivery, breastfeeding, history of miscarriage, menarche age, and menopause status were collected7.
In this study, premenopausal women were classified as those who had regular menstrual cycles 12 months before to the interview, while postmenopausal women were defined as those who had no menstrual periods in the previous 12 months. Those with no information on menopausal status (nine in the case group and seven in the control group) were classified as premenopausal if they were 47 or under, and postmenopausal if they were 47 or older (the median age of menopause among Iranian women)43.
Dietary intake assessment
Dietary data were collected using a semi-quantitative food frequency questionnaire (FFQ) containing 168 food items with standard serving sizes. The validity and reliability of this questionnaire in an Iranian population has been published previously44. Participants were asked to report their consumption frequency of a given serving of each food item daily, weekly, monthly or in a year through an in-person interview with experienced nutritionists. Then, the consumption of frequencies were converted to the daily grams of intake for each food item by using the manual for household measures specialized for Iranians45. The nutrient as well as energy intake of participants were then calculated in a daily manner by entering the daily grams of intake of each food item into the Nutritionist-IV software (First Databank; Hearst, San Bruno, CA, USA). This nutrient database is based on the USDA food composition table which is modified for Iranian foods46. Then, participants reporting extreme energy intakes, < 3200 or > 18,000 kJ/day, were excluded in the analysis. All food items were categorized into 20 food groups based on the similarity of nutrients or culinary usage of foods47.
Western diet score (WDS)
To test our hypothesis on the association of WD with breast cancer in Iranian population we applied the definition of WD and methods used by the previously published studies on cancer research47,48,49,50. Briefly, a priori Western dietary pattern was defined based on 8 food groups (i.e., red and processed meats, eggs, animal fat, butter, sugar and sugar products, margarine, dressings, and dips). Based on quintiles of total consumption, a score of 1 to 5 was given to each food item. Those in the lowest quintiles received a score of “1”, while those in the highest quintiles received a score of “5”. The total score for each participant was derived by adding the scores for each dietary item. As a result, the score varied from 8 (the lowest level of adherence) to 40 (the greatest level of adherence) (highest adherence). According to their score, participants were divided into tertiles (low, medium, and high adherence to a Western food pattern). Tertiles were based on distribution in total study population.
Statistical analysis
The baseline characteristics of the study participants were compared between the WDS tertiles using analysis of variance (ANCOVA) or T-test, for continues variables. Chi-square test was used for categorical variables. Multinomial logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) of the association between WD and risk of IDC and ILC separately. Based on the multiple logistic models, the p-value for heterogeneity was calculated using Wald test. In the multivariable multinomial logistic regression, models were adjusted for energy intake (kcal per day), fruit and vegetable intake (gram per day), family history of BC, smoking, OCP use, history of chest X-ray, history of BBD, BMI, physical activity, age at first delivery, breastfeeding, history of miscarriage, menarche age, and menopause status. We tested whether associations differed according to subtype by adding an interaction term to the model for WD and subtype. We further performed the stratified analyses based on menopausal status. A post-hoc power analysis suggested that our study had statistical power of 80% to detect associations with OR > 1.65 for ILC and OR > 1.24 for IDC. Based on the adjusted model 2, the p for heterogeneity was calculated using the Wald test. P values for trend were estimated by assigning medians to each category of consumption as a continuous variable. All p-values were two-sided and results were considered to be statistically significant at less than 0.05. All analyses were performed using Stata/SE version 14.2 (Stata Corporation, College Station, TX, USA)51.
Ethics approval and consent to participate
The study was approved by the ethic committee of Shiraz University of Medical Sciences. Informed consent was obtained from all individual participants included in the study. Also, we confirm that all methods were performed in accordance with the declaration of Helsinki.
Data availability
Datasets that are minimally required to replicate the outcomes of the study will be made available upon reasonable request.
Abbreviations
- DPs:
-
Dietary patterns
- WD:
-
Western diet
- ORs:
-
Odds ratios
- CIs:
-
95% Confidence intervals
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- WDS:
-
Western diet score
- FFQ:
-
Food frequency questionnaire
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- BMI:
-
Body mass index
- OCP:
-
Oral contraceptive pills
- ER:
-
Oestrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
References
Miller, K. D. et al. Cancer statistics for hispanics/latinos, 2018. CA Cancer J. Clin. 68, 425–445 (2018).
Hashim, D. et al. The global decrease in cancer mortality: Trends and disparities. Ann. Oncol. 27, 926–933 (2016).
Dianatinasab, M. et al. Socioeconomic factors, health behavior, and late-stage diagnosis of breast cancer: Considering the impact of delay in diagnosis. Clin. Breast Cancer 18, 239–245 (2018).
Foroozani, E. et al. Determinants of delay in diagnosis and end stage at presentation among breast cancer patients in Iran: A multi-center study. Sci. Rep. 10, 21477. https://doi.org/10.1038/s41598-020-78517-6 (2020).
Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 8, S31563 (2015).
Najafi, B., Anvari, S. & Roshan, Z. A. Disease free survival among molecular subtypes of early stage breast cancer between 2001 and 2010 in Iran. Asian Pac. J. Cancer Prev. 14, 5811–5816 (2013).
Dianatinasab, M. et al. Heterogeneity in risk factors for ductal and lobular breast carcinomas: A case–control study. Int. J. Cancer 145, 2917–2925 (2019).
Kotsopoulos, J. et al. Risk factors for ductal and lobular breast cancer: Results from the nurses’ health study. Breast Cancer Res. 12, R106 (2010).
Yang, C. et al. Comparison of overall survival between invasive lobular breast carcinoma and invasive ductal breast carcinoma: A propensity score matching study based on SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2020.590643 (2020).
Mathew, A. et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 77, 660–666 (2017).
Williams, L. A. et al. Reproductive risk factor associations with lobular and ductal carcinoma in the Carolina Breast Cancer Study. Cancer Causes Control 29, 25–32 (2018).
Ursin, G. et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br. J. Cancer 93, 364–371 (2005).
Dianatinasab, M. et al. Dietary patterns and risk of invasive ductal and lobular breast carcinomas: A systematic review and meta-analysis. Clin. Breast Cancer 20, e516–e528. https://doi.org/10.1016/j.clbc.2020.03.007 (2020).
Linos, E., Willett, W. C., Cho, E. & Frazier, L. Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol. Prev. Biomark. 19, 689–696 (2010).
Gonzalez, C. A. & Riboli, E. Diet and cancer prevention: Contributions from the European prospective investigation into cancer and nutrition (EPIC) study. Eur. J. Cancer 46, 2555–2562 (2010).
Mandal, C. C., Ghosh-Choudhury, T., Yoneda, T., Choudhury, G. G. & Ghosh-Choudhury, N. Fish oil prevents breast cancer cell metastasis to bone. Biochem. Biophys. Res. Commun. 402, 602–607 (2010).
Fararouei, M. et al. Dietary habits and physical activity are associated with the risk of breast cancer among young Iranian women: A case-control study on 1010 premenopausal women. Clin. Breast Cancer 19, e127–e134 (2019).
Castello, A. et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 103, 8–15. https://doi.org/10.1016/j.maturitas.2017.06.020 (2017).
Cordain, L. et al. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 81, 341–354. https://doi.org/10.1093/ajcn.81.2.341 (2005).
Drake, I., Sonestedt, E., Ericson, U., Wallström, P. & Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 119, 1168–1176. https://doi.org/10.1017/s000711451800079x (2018).
Naderimagham, S. et al. National and sub-national burden of breast cancer in Iran; 1990–2013. Arch. Iran. Med. 17, 794–799 (2014).
Rakhra, V., Galappaththy, S. L., Bulchandani, S. & Cabandugama, P. K. Obesity and the western diet: How we got here. Mo Med. 117, 536–538 (2020).
Ronco, A. L., De Stefani, E., Mendoza, B., Abbona, E. & Deneo-Pellegrini, H. Dietary patterns and risk of breast cancer: A factorial analysis of food and nutrients. Rev. Med. Del Urug. 32, 242–253 (2016).
Ronco, A. L. et al. Food patterns and risk of breast cancer: A factor analysis study in Uruguay. Int. J. Cancer 119, 1672–1678. https://doi.org/10.1002/ijc.22021 (2006).
Ronco, A. L. et al. Nutrient patterns and risk of breast cancer in Uruguay. Asian Pac. J. Cancer Prev. 11, 519–524 (2010).
Zhang, C. X. et al. Dietary patterns and breast cancer risk among Chinese women. Cancer Causes Control 22, 115–124. https://doi.org/10.1007/s10552-010-9681-8 (2011).
Ronco, A. L. et al. Dietary patterns and risk of ductal carcinoma of the breast: A factor analysis in Uruguay. Asian Pac. J. Cancer Prev. 11, 1187–1193 (2010).
Cottet, V. et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am. J. Epidemiol. 170, 1257–1267. https://doi.org/10.1093/aje/kwp257 (2009).
Xiao, Y. et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 21, 16. https://doi.org/10.1186/s13058-019-1096-1 (2019).
Rossi, R. E., Pericleous, M., Mandair, D., Whyand, T. & Caplin, M. E. The role of dietary factors in prevention and progression of breast cancer. Anticancer Res. 34, 6861–6875 (2014).
Turesky, R. J. Mechanistic evidence for red meat and processed meat intake and cancer risk: A follow-up on the International Agency for Research on Cancer evaluation of 2015. CHIMIA Int. J. Chem. 72, 718–724 (2018).
Inoue-Choi, M., Sinha, R., Gierach, G. L. & Ward, M. H. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP Diet and Health Study. Int. J. Cancer 138, 1609–1618 (2016).
Lopez-Miranda, J. et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 20, 284–294. https://doi.org/10.1016/j.numecd.2009.12.007 (2010).
Agus, A. et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci. Rep. 6, 19032. https://doi.org/10.1038/srep19032 (2016).
Mirzaei, R. et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 139, 111619. https://doi.org/10.1016/j.biopha.2021.111619 (2021).
Thirunavukkarasan, M. et al. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells. PLoS ONE 12, e0186334. https://doi.org/10.1371/journal.pone.0186334 (2017).
Castello, A. et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br. J. Cancer 111, 1454–1462 (2014).
Brennan, S. F., Cantwell, M. M., Cardwell, C. R., Velentzis, L. S. & Woodside, J. V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 91, 1294–1302. https://doi.org/10.3945/ajcn.2009.28796 (2010).
Hou, R. et al. Healthy dietary patterns and risk and survival of breast cancer: A meta-analysis of cohort studies. Cancer Causes Control 30, 835–846. https://doi.org/10.1007/s10552-019-01193-z (2019).
Catsburg, C. et al. Dietary patterns and breast cancer risk: A study in 2 cohorts. Am. J. Clin. Nutr. 101, 817–823. https://doi.org/10.3945/ajcn.114.097659 (2015).
Margetts, B., Vorster, H. & Venter, C. Evidence-based nutrition—The impact of information and selection bias on the interpretation of individual studies. S. Afr. J. Clin. Nutr. 16, 3 (2003).
Von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 147, 573–577 (2007).
Fallahzadeh, H. Age at natural menopause in Yazd, Islamic Republic of Iran. Menopause 14, 900–904 (2007).
Esfahani, F. H., Asghari, G., Mirmiran, P. & Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J. Epidemiol. 20, 150–158 (2010).
Ghaffarpour, M., Houshiar-Rad, A. & Kianfar, H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy 7, 42–58 (1999).
Kohlmeier, L. The Eurocode 2 food coding system. Eur. J. Clin. Nutr. 46, S25-34 (1992).
Jalilpiran, Y. et al. Western dietary pattern, but not mediterranean dietary pattern, increases the risk of prostate cancer. Nutr. Cancer 70, 851–859 (2018).
Dianatinasab, M. et al. Adherence to a Western dietary pattern and risk of bladder cancer: A pooled analysis of 13 cohort studies of the Bladder Cancer Epidemiology and Nutritional Determinants international study. Int. J. Cancer 147, 3394–3403 (2020).
Pala, V. et al. Meat, eggs, dairy products, and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am. J. Clin. Nutr. 90, 602–612. https://doi.org/10.3945/ajcn.2008.27173 (2009).
Sieri, S. et al. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol. Biomark. Prev. 13, 567–572 (2004).
Stata-Corp LP. Stata User’s Guide (Stata Press, 1985).
Acknowledgements
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
M.D. contributed to the design and implementation of the study, analysis and interpretation of data and was involved in drafting the manuscript. H.S., D.B. and E.F. contributed to the design and implementation of the study, interpretation of data and was involved in drafting and revising the manuscript. N.R., M.F., S.A. and A.A. contributed to the conception and design of data and drafting the manuscript. S.A. and E.F. contributed to the design and implementation of the study and was involved in drafting and revising the paper. D.B. contributed to the conception and design of study and drafting the manuscript. M.D. contributed to the analysis and interpretation of data and was involved in revising the manuscript. All authors reviewed and approved the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foroozani, E., Akbari, A., Amanat, S. et al. Adherence to a western dietary pattern and risk of invasive ductal and lobular breast carcinomas: a case–control study. Sci Rep 12, 5859 (2022). https://doi.org/10.1038/s41598-022-09725-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09725-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.