Abstract
Although giant snakes are abundant in some tropical forests, their ecology is far less well-known than for smaller species of snakes in cooler climates. Information on spatial ecology can clarify management issues such as the sizes and types of habitats needed for conservation. We radio-tracked 27 scrub pythons (Simalia amethistina; snout-vent lengths 2.02 to 3.70 m) in Cape York, near the northeastern tip of Australia, for a mean period of 426 days (up to 1001 days) per snake. Home ranges were larger in males than females (means 0.60 vs. 0.28 km2) and overlapped considerably among individuals. All snakes used rainforest habitat, but seasonal shifts into open woodland were common. Snakes were active primarily by night, with larger snakes hunting less of the time overall, and more often by day. Hunting behaviour was seen more often during the wet season than the dry season. Average daily displacement was < 10 m, typically involving a shift from diurnal refuge to nocturnal ambush-site. A reliance on sit-and-wait predation results in small home ranges and limited movements, despite the large body size of this species.
Similar content being viewed by others
Introduction
All diverse biological radiations include some species that are more difficult to study than are others. Inevitably, then, our understanding of any speciose group will be based disproportionately on taxa that occur in abundance, in places where they are accessible to study1. For example, field-based research is more feasible if a species occurs in relatively open habitats; lives on the ground rather than above or below it; is easy to observe and capture; and occurs in sites that are close to scientific infrastructure. Such biases cannot be avoided but they mean that, wherever possible, we should also try to “fill in the gaps” by studying species that do not possess those logistically-convenient attributes. Thus, for example, we need more information on species from the tropics, because these highly biodiverse areas are less intensively-studied than are habitats in cool-temperature Eurasia and North America2.
Ecological research on snakes has grown rapidly over the last four decades, largely spawned by changing community attitudes and by the increasing availability of miniature radio-transmitters3. However, most of the species that have been studied are medium-sized terrestrial (rather than arboreal, fossorial or aquatic) colubrids, natricids, and viperids4,5. Most studies on giant snakes (a group restricted to tropical regions) have involved examination of field-collected specimens6,7 or short-term monitoring of the movements and habitat use of small numbers of individuals8,9. Long-term monitoring of large numbers of individuals has rarely been accomplished, because of logistical problems such as equipment malfunction and the scarcity of longterm funding for field-based research.
The scarcity of detailed ecological data on giant snakes in tropical forests poses a problem for conservation and management of these animals. Some taxa are exploited by the commercial trade in leather, meat, and/or pets, and a lack of basic information about the biology of such species has spawned disagreements about harvest sustainability7. The large size of these snakes suggests that they may move over large distances, an issue with strong ramifications for questions such as optimal reserve sizes10. Similarly, the size of area required to conserve a viable population depends upon the degree to which individuals maintain separate territories, versus overlap in space use within the same area11. Patterns of habitat use are important also; if a species depends upon some specific habitat type (even if that dependence is seasonal, such as for reproduction), managers need to retain sufficient areas of that habitat; or (even more challenging) ensure that a mosaic of multiple critical habitat types is maintained12. In the present study, we radio-tracked 27 adult scrub pythons (Simalia amethistina; previously Morelia amethistina) in a rainforest-woodland region in extreme northern Queensland, Australia, to quantify distances moved, habitats used, and seasonal and diel cycles of activity.
Methods
Study species
Scrub pythons are among the longest snake species in the world, with adults sometimes exceeding 5.5 m total length and 20 kg13. At around 700 mm total length and 50 g14, hatchlings of this species are larger than the adults of most other snake species15. Authorities disagree about the taxonomic distinctiveness of several regionally-restricted forms across northeastern Queensland and southern New Guinea, with Australian specimens identified as either S. amethistina or S. kinghorni16.
Reflecting frequent arboreality, scrub pythons are slender-bodied (Fig. 1a); a milky iridescent sheen on their scales gives them the alternative common name of amethystine python. The dorsal scales are a mosaic of brown and tan, creating a dappled colour that renders the snake inconspicuous among vegetation. Males engage in combat bouts during the mating season (in cooler months of the year) and females lay a clutch of around 12 eggs in the late dry-season, and remain with those eggs until hatching14,17. Scrub pythons consume a wide variety of avian and mammalian prey, and congregate under trees containing colonies of metallic starlings, feeding on nestlings that fall from the trees18. These pythons sometimes consume relatively large prey, including macropodid marsupials19,20.
(a) Scrub python (Simalia amethistina) in hunting posture (photograph by Terri Shine); and (b) map of the Lockerbie Scrub in Cape York Peninsula, showing home ranges of scrub pythons (map created using the Zoatrack online platform;29). Dark green vegetation depicts closed rainforest habitat while brown areas depict adjacent woodlands, swamps, sand plains, and heathlands. Inset map shows the location of the study area within Cape York Peninsula, northern Queensland.
Study site
The Lockerbie Scrub is a 130 km2 area of semi-deciduous notophyll vine forest interspersed by tropical woodlands at the northern tip of Cape York Peninsula, Australia (10.78 S, 142.50 E)21 (Fig. 1b). The area is hot year-round (mean monthly minimum and maximum air temperatures from 23 to 32 °C22) but most rain falls during the wet season from December to April (mean rainfall 1543 mm). The remainder of the year is relatively dry (mean of 202 mm22).
Protocol for radiotelemetry
We hand-captured scrub pythons from throughout the study area during nocturnal surveys of snake habitat and returned them to the laboratory for measurement and transmitter implantation. We anesthetized the pythons using vaporized isoflurane and recorded snout-vent length (SVL) using a steel ruler, body mass using PESOLA® spring scales, and sex by probing the cloacal bursae and recording depth. Each snake was then fitted with a radio-transmitter (AI-2, Holohil Systems Limited, Ontario, Canada) that was surgically implanted into the body cavity following the procedure described by Whitaker & Shine23. Transmitters weighed between 17 and 28 g (with smaller snakes receiving smaller transmitters) and ranged from 0.19 to 1.9% of snake body mass. This is a small burden compared to the size of meals often consumed by this species (prey items sometimes weigh more than the predator20). We released all snakes at their original point of capture within one day of surgery. During subsequent monitoring, no snakes exhibited overt negative responses (slow wound-healing, lethargy) to capture, surgery, or transmitter implantation.
We radio-tracked pythons between 24 November 2013 and 30 May 2016, locating the animals by using a Regal 2000 handheld receiver and 3-element Yagi antenna (Titley Scientific, Brendale, Australia). We located individual pythons at a wide range of times (both by day and at night), and frequently located snakes at multiple times on the same day to clarify short-term movement patterns. On average, we located each scrub python at least once every 1–2 weeks. The order in which we tracked animals both within and among days was changed frequently, to avoid temporal autocorrelation in the data24.
When a snake was located, we recorded the broad habitat type, the time of day, and the snake’s position to 5 m using a handheld GPS unit (Garmin GPSMAP® 62 s). When possible, we also recorded the straight-line distance to the snake’s previous position using a flexible measuring tape, or by pacing the distance, to later check the accuracy of GPS estimates of distance. On many occasions snakes could not be visually sighted when located, due to being concealed within hollow logs, tree hollows, or in the tops of tall trees. When snakes could be sighted, we recorded their posture as resting, moving, or hunting following Natusch et al.18. Briefly, we considered pythons to be in ambush posture (hunting) if they were motionless with the head and neck in an “S” position (see also25); to be resting if they were tightly coiled; and to be moving if they were fully outstretched when encountered. To evaluate spatial ecology of snakes we separated telemetry data into two seasons: (1) the wet season (from 1 December to 30 April), and (2) the dry season (1 May to 30 November).
Analyses of home range and use area
We initially tested several home range estimators on our data for scrub python spatial ecology, including minimum convex polygons (MCPs), kernel densities, and dynamic brownian bridge movements models (dBBMMs). All models have their limitations and assumptions, and their use depends on the tracking regime, species’ biology, and the questions being asked26,27. After testing several methods, we chose to calculate scrub python total home ranges using 100% MCPs. We considered MCPs to best represent total potential scrub python home range based on our experience tracking this species. Scrub pythons are capable of making large daily displacements and can utilize all of the contiguous habitat types in our remote study area. Therefore, although MCPs have been criticized because they sometimes include areas not actually used by the animal (and thus can overestimate range size e.g.20 and references therein), we considered those sites to be relevant even if use was not detected during the current study.
To complement estimates of total home range size, we also calculated 50% kernel densities to understand core use areas within this broader range. We chose the kernel smoothing factor (h) by first calculating 95% kernel densities and varying h until kernel area approximated the home range sizes calculated using the 100% MCP method. We then used the calculated h-values as the smoothing factor in our 50% kernel density estimates28. Although we report estimated range sizes for all snakes, we excluded individuals with < 20 fixes from our comparative analyses, as well as one individual where fixes occurred over a short period of time when the specimen was utilizing a spatially discrete resource hotspot (trees housing starling colonies18; Table 1). In addition, individuals were excluded from our analysis of core use areas if the animals restricted their movements to small areas for long periods of time, due to reproduction (clutch-brooding) or to use of resource hotspots (bird-colony trees). In these cases, overrepresentation of fixes at these sites had a disproportionate influence on the analysis (Table 1).
We analyzed the data in R version 3.6.3 using the adehabitatHR package (R Core Team, Vienna, Austria, 2021), and used the ZoaTrack online program29 to visualize the data against satellite imagery to verify the accuracy of our estimates. We examined variation in home range and core use area sizes separately using analysis of covariance (ANCOVA) with snake sex as the factor, ln SVL as the covariate, and ln MCP and ln 50% kernel density estimates as the dependent variables. For snakes with sufficient fixes, we repeated the above analysis but included season (and its interaction) to examine seasonal difference in home range sizes. We performed these analyses in JMP Pro 14 (SAS Institute, Cary, NC).
Analysis of habitat use
The only available habitat maps and shapefiles describing the vegetation of Cape York Peninsula are 25 years old and did not provide enough resolution to accurately examine habitat use by scrub pythons. Therefore, we manually recreated vegetation maps to improve habitat resolution based on the Queensland regional ecosystem guide and shapefiles provided by Neldner & Clarkson21. We overlaid MCPs from each snake onto these maps in QGIS (Quantum GIS Development Team, v. 3.2.1) and calculated the proportion of each habitat type within the polygon relative to the total home range area. We considered all habitats within those MCPs to be available for use. We then calculated the number of python fixes in each habitat type as a proportion of total fixes. To test if pythons used certain habitats disproportionately to their availability, we performed a chi-squared goodness of fit test with the proportion of available habitat within each snake’s MCP home range as the expected frequency and the proportion of fixes in each habitat as the observed frequency. We repeated this analysis to test for shifts in habitat use between wet and dry seasons by including the proportion of fixes in each habitat type in each season as the expected and observed frequencies.
Analysis of activity and movement
We used nominal logistic regression to test the influence of season, time of day, sex, ln SVL, and their interactions on snake activity (resting, moving, hunting). We sequentially deleted non-significant interaction terms until we were left with main effects. To quantify movement patterns, we calculated mean daily movement distance for each telemetered snake as the total straight-line distance moved between successive fixes divided by the number of days between fixes. We analyzed movement distance using a linear mixed model (LMM) with season and sex as factors, ln SVL as a covariate, and ln movement distance as the dependent variable. Because we obtained multiple movement records per snake, we included snake ID as a random effect in the model to address pseudoreplication. We performed all analyses using JMP Pro 14 (SAS Institute, Cary, NC). Finally, because our calculation of mean daily movement distance relies on dividing total movement distance by the number of fixes obtained for each snake, and thus fails to include back-and-forth movements (net zero displacements), we also examined movement distances of a smaller sample of snakes (15) located on successive days and between night and day. We do not examine the resulting small number of records (58; i.e., 116 individual fixes) statistically, but we present them because they provide a more detailed understanding of individual movements.
Ethical note
This research was carried out under Queensland Department of Environment and Heritage Protection permits (WISP12944313) and University of Sydney animal ethics committee guidelines (approval number: L04/3-2013/ 3/5969). All procedures involving animals were carried out in accordance with relevant guidelines and regulations (including ARRIVE guidelines).
Results
We obtained 1406 fixes of 27 snakes tracked for a mean of 426 days (range 50–1001) over the course of this study (Table 1). Several snakes were tracked for only short durations due to expulsion of transmitters30 or natural mortality. Fixes were approximately evenly distributed between wet and dry seasons, but sample sizes varied among individual pythons. Of these fixes, 467 were made at night while 939 were made during the day.
Home range and core use areas
Overall, home range sizes of scrub pythons averaged 0.38 ± 0.05 km2 (range: 0.17–1.77 km2; Table 1). Core use areas were fourfold smaller, averaging 0.09 ± 0.013 km2 (range = 0.1–0.34 km2; Table 1). Male scrub pythons had larger home ranges than did females (F1,20 = 6.58, P = 0.019), but used similar-sized core areas (F1,15 = 4.25, P = 0.06; Fig. 2). A snake’s body size did not significantly influence the size of its home range (F1,20 = 1.28, P = 0.273) or core use area (F1,15 = 0.46, P = 0.509).
For snakes with sufficient detections to examine seasonal effects, the mean home range was 143% (range: −6.7 to 533%) larger during the dry season than the wet season for males but only 6% (range: −40 to 118%) larger for females. However, due to small sample sizes this change was not statistically significant for either sex (males N = 5; F1,10 = 2.53, P = 0.15; females N = 6; F1,11 = 0.05, P = 0.82). There was considerable overlap in the home ranges and core use areas of multiple snakes in our study. At one site, eight telemetered pythons occupied part of another individual’s home range, along with numerous non-telemetered individuals located in this same area over the course of the study.
Habitat use
Scrub pythons used five broad habitats, comprising six regional ecosystems (Table 2). The proportion of each habitat type within a snake’s home range varied among individuals, with several snakes using four different habitat types whereas others restricted their movements to rainforest throughout the study. Rainforest was the only habitat type used by all individuals, with snakes showing a clear preference for this habitat type. Our chi-squared analyses revealed that 41% (7/17) of snakes used closed forest habitat at a higher rate than expected from its availability, whereas the remaining animals either showed no significant preference (41%) or preferred woodland, swamp, or sand plains (18%; Table 2). Several (6/11) individuals with sufficient fixes in each season showed significant seasonal shifts in habitat use. Four snakes used rainforest in the wet season but moved to open habitats (woodland, swamp and sand plains) in the dry season, whereas the other two individuals showed the opposite pattern (Table 2).
Activity patterns
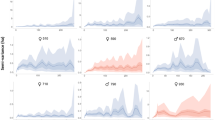
After deletion of non-significant three- and two-way interactions, our nominal logistic regression on main effects revealed that python activity was influenced by the time of day that snakes were located (χ2 = 252, df = 2, P < 0.0001), by their body size (χ2 = 17.5, df = 2, P = 0.0002), and by the season in which they were located (χ2 = 6.1, df = 2, P = 0.048). Scrub pythons were primarily nocturnal at our study site. When we located telemetered snakes by day, they were typically resting whereas at night they were often moving or hunting (Fig. 3). Overall, larger snakes spent less time hunting than small snakes, but hunted more often during the day (Fig. 3). Pythons made most movements around dusk (1800 to 2000 h) and retreated to their diurnal resting sites around dawn (0600 to 0800 h). The snakes were most often found hunting during the wet season and resting during the dry season, with no significant influence of sex (χ2 = 0.05, df = 2, P = 0.97) on activity patterns.
Movements
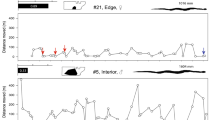
Our radio-tracked scrub pythons moved an average of 9.8 ± 1.1 m/day (range: 1.7–36 m/day). After deletion of non-significant interactions, our linear mixed model revealed no significant difference in daily displacements between sexes (F1,38 = 0.19, P = 0.67), seasons (F1,38 = 2.83, P = 0.11) or snakes of different body sizes (F1,38 = 0.29, P = 0.60). However, the overall mean displacement of less than 10 m per day is somewhat misleading, in that most snakes moved back-and-forth between refuges and ambush sites. Examination of night-day and day-day movements over known time periods (and thus, incorporating back-and-forth movements) saw an increase in mean daily movement distance to 107 m per day and approximately half that for movement between diurnal and nocturnal resting sites (Fig. 4). The longest single daily movement recorded was 364 m (Fig. 4).
Movements from diurnal resting sites to nocturnal ambush sites were typically small, and sometimes only a few metres (Fig. 4). Rather than moving from their daytime resting position, snakes often simply uncoiled the anterior portion of the body and set up ambush at their resting location. On other occasions, snakes resting in tree hollows during the day emerged to hunt in the same tree, either in the branches or near the hollow itself. Movement distances from nocturnal hunting sites back to resting positions were equally small (Fig. 4). By far the most common pattern was for snakes not to move at all from one day to the next.
Discussion
Our results are consistent with previous work on this species8,18, and with telemetry-based studies on other large sit-and-wait predatory snakes (e.g., viperids31,32,33,34). In strong contrast to snakes from the cool-temperate zone, which often migrate long distances from winter hibernacula to summer feeding ranges35,36, warm conditions year-round in the Lockerbie Scrub mean that seasonal shifts by pythons were minor, often involving movements between adjacent habitat types. In tropical systems where pronounced wet-dry seasonality generates flooding that redistributes prey across the landscape, predatory snakes also may move considerable distances37. In the Lockerbie Scrub, in contrast, wet-season rains do not cause major flooding over most areas; and hence, the seasonally-shifting variables that affect prey availability for scrub pythons likely involve issues such as vegetation density (and hence, the effectiveness of camouflage and ambush predation) and seasonal reproduction of mammalian and avian prey (generating offspring accessible to pythons18). As a result, heterogeneity in prey availability likely varies over a small spatial scale in this system, favouring only minor movements between adjacent habitat types rather than broadscale migration across the landscape. Regular movements over long distances may be more important for active-searching predators; once they have checked all available prey refuges in an area, the optimal tactic may be to move further afield23. In contrast, a generalist ambush predator such as a scrub python is unlikely to consume enough prey items to substantially deplete overall feeding opportunities in a local area.
Requirements for reproduction can also drive changes in habitat use; for example, gravid females of many viviparous snake species in cool climates accelerate developmental rates of their embryos by congregating in sun-exposed (and thus warm) microhabitats38,39,40. The movement of scrub pythons from rainforest to woodlands in our study, during the dry season when reproduction occurs, may also be for this reason. Nevertheless, we recorded mating in both open woodland and rainforest habitats during the course of our study. Three radio-tracked female pythons built nests and attended their broods until hatching, all within rainforest habitat. However, the juxtaposition of woodland and rainforest within our study area, with most pythons utilizing interface habitats (Fig. 1), meant that a snake could select a rainforest nesting site without moving far from any location within its home range. In essence, the Lockerbie Scrub contains a mosaic of habitat types, which may differ slightly in their suitability for feeding and breeding, but variation occurs over too small a spatial scale to necessitate long-distance movements on either a diel or seasonal basis.
The scarcity of long-distance displacements in our radio-tracked snakes hints that movements may be costly and/or dangerous. Intuition suggests that such large animals would be invulnerable to predation, but there are several reports of relatively large Australian pythons being killed and consumed by canids such as dingos41,42. Additionally, moving is incompatible with ambush predation. Not only does movement render a snake conspicuous to potential prey, but it also may leave scent trails that are detectable by prey animals43; and ambush predators rely upon being undetected44.
The primary effects of season were to increase home range sizes of our snakes (although not statistically significant), to increase hunting effort (proportion of time spent in ambush-foraging pose), and to alter the habitat of some (but not all) snakes. The heightened effort at hunting in the wet season suggests that the expansion in home range sizes of male snakes in the dry season reflects mate-searching, as recorded in many other snake species23,45. Diel cycles in behaviour were pronounced, with snakes (especially smaller individuals) usually inactive by day, often hidden within refuges (Fig. 3). Most of the mammals consumed by scrub pythons are primarily nocturnal18, such that night offers the best opportunity for an ambush predator to seize a moving prey item. Larger species such as agile wallabies (Macropus agilis) sometimes are active by day as well46, such that a large python (the only size class capable of consuming such a large item) may benefit by extending its foraging effort into daylight hours (Fig. 3). Interestingly, the trend for increased diurnal hunting by larger snakes is opposite to that seen in a closely-related python species from cooler climates: around Sydney, juvenile diamond pythons (Morelia spilota) ambush by day (unlike conspecific adults), apparently because suitably small prey (lizards) are diurnally active, and because nights are too cool for a small python to retard heat loss for long enough to remain an effective predator47. Those constraints are not applicable to scrub pythons: even hatchlings are large enough to feed on endothermic prey, and the tropical climate of the Lockerbie Scrub allows snakes to retain high body temperatures throughout the night.
Perhaps the most important facet of our results is the flexibility of python ecology, as exemplified by strong variation among individual snakes. Thus, for example, some snakes moved into the rainforest in the dry season, whereas other individuals went the other way. As reported in other studies of snakes (e.g.,48), minor differences in habitat attributes and/or behavioural traits of individuals generate substantial inter-individual variation in spatial ecology. Those differences are amplified by flexible responses to local conditions. In the case of scrub pythons, such flexibility is exemplified by facultative use of resource hotspots, whereby snakes remain within small areas and shift to active searching rather than ambush foraging under trees containing bird colonies18. The same flexibility is evident in the success of this species in highly disturbed suburban and urban environments, where the snakes take refuge within buildings and feed on commensal mammals and birds49.
Our results are encouraging for the conservation of these giant pythons. Even large individuals required only small areas, and tolerated high levels of overlap with conspecifics. Flexible use of habitats—as exemplified by urban pythons as well as by free-ranging snakes utilizing both dense and open forests—renders this species resilient to habitat change. As long as suitable endothermic prey are common, a scrub python can thrive even in a small habitat patch, because it can adjust its behaviour (habitat selection, activity pattern, diet choice, foraging tactics) to exploit the opportunities available (e.g.,18). This is exemplified by the persistence of scrub pythons on small sand islands in Cape York (Milman Islet; 0.22 km2—the size of a single python home range49). Such flexibility is common in many snakes, but is especially evident in giant tropical species because the large range of body sizes within a single species creates wide variation in traits such as dietary composition and reproductive output (e.g.,6), and reduced thermal constraints in tropical habitats provide a greater opportunity for flexible adjustment of behaviour to resources.
Although the scientific literature on snake ecology remains strongly focused on small-to-medium-sized species in cool-temperate habitats, recent years have seen a welcome expansion of study systems in terms of body sizes, phylogenetic affiliations, and geographic locations. Thus, for example, recent work has documented the spatial ecology of large venomous snakes both on land (Ophiophagus hannah50,51) and underwater (Hydrophis curtus52) as well as pythons and boids both in their native ranges (Eunectes marinus53; Malayopython reticulatus54; Python natalensis55) and in areas that they have invaded (Python bivittatus56). This renaissance suggests that we may soon be able to interpret the ecology and conservation needs of giant tropical snakes from actual field data on those animals, rather than by sometimes-unreliable extrapolation from studies based on smaller species from different climatic zones.
Data availability
Data will be deposited in the Dryad repository upon manuscript acceptance.
References
Seigel, R. A. & Ford, N. B. Reproductive ecology in Snakes: Ecology and Evolutionary Biology (eds. Seigel, R. A., Collins, J. T. &. Novak, S. S.). 210–252. (MacMillan Publishing, 1987).
Kremen, C., Merenlender, A. M. & Murphy, D. D. Ecological monitoring: A vital need for integrated conservation and development programs in the tropics. Conserv. Biol. 8, 388–397 (1994).
Shine, R. & Bonnet, X. Snakes: A new ‘model organism’ in ecological research?. Trends Ecol. Evol. 15, 221–222 (2000).
Vilela, B., Villalobos, F., Rodríguez, M. Á. & Terribile, L. C. Body size, extinction risk and knowledge bias in New World snakes. PLoS ONE 9, e113429 (2014).
Mathies, T. Reproductive cycles of tropical snakes. in Reproductive Biology and Phylogeny of Snakes (eds. Sever, D. & Aldridge, R.). 523–562. (CRC Press, 2016).
Shine, R., Harlow, P. S. & Keogh, J. S. The allometry of life-history traits: Insights from a study of giant snakes (Python reticulatus). J. Zool. 244, 405–414 (1998).
Natusch, D. J., Lyons, J. A., Riyanto, A., Khadiejah, S. & Shine, R. Detailed biological data are informative, but robust trends are needed for informing sustainability of wildlife harvesting: A case study of reptile offtake in Southeast Asia. Biol. Conserv. 233, 83–92 (2019).
Freeman, A. & Freeman, A. Habitat use in a large rainforest python (Morelia kinghorni) in the wet tropics of north Queensland, Australia. Herpetol. Conserv. Biol. 4, 252–260 (2009).
Smith, S. N., Jones, M. D., Marshall, B. M. & Strine, C. T. Native Burmese pythons exhibit site fidelity and preference for aquatic habitats in an agricultural mosaic. Sci. Rep. 11, 7014 (2021).
Kramer, D. L. & Chapman, M. R. Implications of fish home range size and relocation for marine reserve function. Environ. Biol. Fishes 55, 65–79 (1999).
Spong, G. Space use in lions, Panthera leo, in the Selous Game Reserve: Social and ecological factors. Behav. Ecol. Sociobiol. 52, 303–307 (2002).
Webb, J. K. & Shine, R. A field study of spatial ecology and movements of a threatened snake species, Hoplocephalus bungaroides. Biol. Conserv. 82, 203–217 (1997).
Fearn, S. & Sambono, J. A reliable size record for the scrub python Morelia amethistina (Serpentes: Pythonidae) in north east Queensland. Herpetofauna 30, 2–6 (2000).
Grow, D., Wheeler, S. & Clark, B. Reproduction of the Amethystine python Python amethystinus kinghorni at the Oklahoma City Zoo. Int. Zoo Year. 27, 241–244 (1988).
Feldman, A. & Meiri, S. Length–mass allometry in snakes. Biol. J. Linn. Soc. 108, 161–172 (2013).
Harvey, M. B., Barker, D. G., Ammerman, L. K. & Chippindale, P. T. Systematics of pythons of the Morelia amethistina complex (Serpentes: Boidae) with the description of three new species. Herpetol. Monogr. 14, 139–185 (2000).
Fearn, S., Schwarzkopf, L. & Shine, R. Giant snakes in tropical forests: A field study of the Australian scrub python, Morelia kinghorni. Wildl. Res. 32, 193–201 (2005).
Natusch, D. J. D., Lyons, J. A. & Shine, R. Rainforest pythons flexibly adjust foraging ecology to exploit seasonal concentrations of prey. J. Zool. 313, 114–123 (2021).
Martin, R. W. Field observation of predation on Bennett’s tree-kangaroo (Dendrolagus bennettianus) by an amethystine python (Morelia amethistina). Herpetol. Rev. 26, 74–75 (1995).
Natusch, D., Lyons, J., Mears, L. A. & Shine, R. Biting off more than you can chew: Attempted predation on a human by a giant snake (Simalia amethistina). Austral. Ecol. 46, 159–162 (2021).
Neldner, V. J. & Clarkson, J. R. Vegetation of Cape York Peninsula. (Department of Environment and Heritage, 1995).
Bureau of Meteorology. Climate Data Online. http://www.bom.gov.au/climate/data/. Accessed 17 July 2020 (2020).
Whitaker, P. B. & Shine, R. A radiotelemetric study of movements and shelter-site selection by free-ranging brownsnakes (Pseudonaja textilis, Elapidae). Herpetol. Monogr. 17, 130–144 (2003).
Harris, S. et al. Home-range analysis using radio-tracking data–A review of problems and techniques particularly as applied to the study of mammals. Mamm. Rev. 20, 97–123 (1990).
Fearn, S. & Sambono, J. Some ambush predation postures of the Scrub Python Morelia amethistina (Serpentes: Pythonidae) in north east Queensland. Herpetofauna 30, 39–44 (2000).
Caswell, H. Theory and models in ecology: A different perspective. Ecol. Model. 43, 33–44 (1988).
Silva, I., Crane, M., Marshall, B. M. & Strine, C. T. Reptiles on the wrong track? Moving beyond traditional estimators with dynamic Brownian bridge movement models. Move. Ecol. 8, 43 (2020).
Row, J. R. & Blouin-Demers, G. Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 2006, 797–802 (2006).
Newman, P., Dwyer, R. G., Belbin, L. & Campbell, H. A. ZoaTrack—An online tool to analyse and share animal location data: User engagement and future perspectives. Aust. Zool. 41, 12–18. https://zoatrack.org/toolkit/doi (2020).
Pearson, D. J. & Shine, R. Expulsion of interperitoneally-implanted radiotransmitters by Australian pythons. Herpetol. Rev. 33, 261–263 (2002).
Hale, V. L. et al. Radio transmitter implantation and movement in the wild timber rattlesnake (Crotalus horridus). J. Wildl. Dis. 53, 591–595 (2017).
Martin, A. E., Jørgensen, D. & Gates, C. C. Costs and benefits of straight versus tortuous migration paths for Prairie Rattlesnakes (Crotalus viridis viridis) in seminatural and human-dominated landscapes. Can. J. Zool. 95, 921–928 (2017).
Glaudas, X., Rice, S. E., Clark, R. W. & Alexander, G. J. Male energy reserves, mate-searching activities, and reproductive success: Alternative resource use strategies in a presumed capital breeder. Oecologia 194, 415–425 (2020).
Glaudas, X., Rice, S. E., Clark, R. W. & Alexander, G. J. The intensity of sexual selection, body size and reproductive success in a mating system with male–male combat: is bigger better?. Oikos 129, 998–1011 (2020).
Gannon, V. P. J. & Secoy, D. M. Seasonal and daily activity patterns in a Canadian population of the prairie rattlesnake, Crotalus viridus viridis. Can. J. Zool. 63, 86–91 (1985).
Heard, G. W., Black, D. & Robertson, P. Habitat use by the inland carpet python (Morelia spilota metcalfei: Pythonidae): Seasonal relationships with habitat structure and prey distribution in a rural landscape. Austral. Ecol. 29, 446–460 (2004).
Madsen, T. & Shine, R. Seasonal migration of predators and prey—A study of pythons and rats in tropical Australia. Ecology 77, 149–156 (1996).
Graves, B. M. & Duvall, D. Reproduction, rookery use, and thermoregulation in free-ranging, pregnant Crotalus v. viridis. J. Herpetol. 27, 33–41 (1993).
Chiaraviglio, M. The effects of reproductive condition on thermoregulation in the Argentina boa constrictor (Boa constrictor occidentalis) (Boidae). Herpetol. Monogr. 20, 172–177 (2006).
Smith, C. F., Schuett, G. W., Earley, R. L. & Schwenk, K. The spatial and reproductive ecology of the copperhead (Agkistrodon contortrix) at the northeastern extreme of its range. Herpetol. Monogr. 23, 45–73 (2009).
Shine, R. & Fitzgerald, M. Large snakes in a mosaic rural landscape: The ecology of carpet pythons Morelia spilota (Serpentes: Pythonidae) in coastal eastern Australia. Biol. Conserv. 76, 113–122 (1996).
Heard, G. W. et al. Canid predation: A potentially significant threat to relic populations of the Inland Carpet Python 'Morelia spilota metcalfei’ (Pythonidae) in Victoria. Vic. Nat. 123, 68–74 (2006).
Downes, S. & Shine, R. Sedentary snakes and gullible geckos: Predator–prey coevolution in nocturnal rock-dwelling reptiles. Anim. Behav. 55, 1373–1385 (1998).
Miller, A. K., Maritz, B., McKay, S., Glaudas, X. & Alexander, G. J. An ambusher’s arsenal: chemical crypsis in the puff adder (Bitis arietans). Proc. R. Soc. B 282, 20152182 (2015).
Maritz, B. & Alexander, G. J. Dwarfs on the move: Spatial ecology of the world’s smallest viper, Bitis schneideri. Copeia 2012, 115–120 (2012).
Stirrat, S. C. Seasonal changes in home-range area and habitat use by the agile wallaby (Macropus agilis). Wildl. Res. 30, 593–600 (2003).
Ayers, D. Y. & Shine, R. Thermal influences on foraging ability: Body size, posture and cooling rate of an ambush predator, the python Morelia spilota. Funct. Ecol. 11, 342–347 (1997).
Pearson, D., Shine, R. & Williams, A. Spatial ecology of a threatened python (Morelia spilota imbricata) and the effects of anthropogenic habitat change. Austral. Ecol. 30, 261–274 (2005).
Freeman, A. A study in power and grace: The amethystine python. Wildl. Aust. 53, 27–29 (2016).
Silva, I., Crane, M., Suwanwaree, P., Strine, C. & Goode, M. Using dynamic Brownian bridge movement models to identify home range size and movement patterns in king cobras. PLoS ONE 13, e0203449 (2018).
Marshall, B. M. et al. Space fit for a king: Spatial ecology of king cobras (Ophiophagus hannah) in Sakaerat Biosphere Reserve, Northeastern Thailand. Amphibia-Reptilia 40, 163–178 (2019).
Udyawer, V., Simpfendorfer, C. A., Heupel, M. R. & Clark, T. D. Temporal and spatial activity-associated energy partitioning in free-swimming sea snakes. Funct. Ecol. 31, 1739–1749 (2017).
Smaniotto, N. P., Moreira, L. F., Rivas, J. A. & Strüssmann, C. Home range size, movement, and habitat use of yellow anacondas (Eunectes notaeus). Salamandra 56, 159–167 (2020).
Low, M. R. Rescue, rehabilitation and release of reticulated pythons in Singapore. in Global Reintroduction Perspectives: 2018. Case Studies from Around the Globe (ed. Soorae, P. S.) 78–81 (IUCN/SSC Reintroduction Specialist Group, 2018).
Alexander, G. J. & Maritz, B. Sampling interval affects the estimation of movement parameters in four species of African snakes. J. Zool. 297, 309–318 (2015).
Smith, B. J. et al. Betrayal: Radio-tagged Burmese pythons reveal locations of conspecifics in Everglades National Park. Biol. Invasions 18, 3239–3250 (2016).
Acknowledgements
We thank the Apudthama Land Trust and Gudang Traditional Owners for allowing us to conduct this research on their country. Thanks to John Mulholland for logistical support, and Damien Lettoof and Martin Mayer for field assistance. Funding was provided by the Australian Research Council, the Holsworth Wildlife Research Trust and the Skyrail Rainforest Foundation.
Author information
Authors and Affiliations
Contributions
All three authors helped to frame the study; D.N. and J.L. collected data; D.N. conducted statistical analyses; D.N. and R.S. drafted the manuscript; all three authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Natusch, D., Lyons, J. & Shine, R. Spatial ecology, activity patterns, and habitat use by giant pythons (Simalia amethistina) in tropical Australia. Sci Rep 12, 5274 (2022). https://doi.org/10.1038/s41598-022-09369-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09369-5
This article is cited by
-
Analysing spatiotemporal patterns of snake occurrence in an Australian city to help manage human-wildlife conflict
Biodiversity and Conservation (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.