Abstract
Ceramides and phosphatidylcholines (PCs) are bioactive lipids and lipid bilayer membrane components. Distinct ceramides/PCs (ratios) predict cardiovascular outcome in patients with coronary artery disease. Extracellular vesicles (EVs) are proposed biomarkers for cardiovascular disease and contain ceramides/PCs. Ceramides/PCs have not been studied in patients undergoing carotid endarterectomy (CEA) nor in EVs. We therefore investigated whether levels of ceramides/PCs in plasma and EVs are associated with postoperative risk of major adverse cardiovascular events (MACE) following CEA. In 873 patients undergoing CEA of the Athero-Express biobank, we quantitatively measured seven ceramides/PCs in preoperative blood samples: Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:0), Cer(d18:1/24:1), PC(14:0/22:6), PC(16:0/16:0) and PC(16:0/22:5) in plasma and two plasma EV-subfractions (LDL and TEX). We analyzed the association of ceramides, PCs and their predefined ratios with the three-year postoperative risk of MACE (including stroke, myocardial infarction and cardiovascular death). A total of 138 patients (16%) developed MACE during the three-year follow-up. In the LDL-EV subfraction, higher levels of Cer(d18:1/24:1) and Cer(d18:1/16:0)/PC(16:0/22:5) ratio were significantly associated with an increased risk of MACE (adjusted HR per SD [95% CI] 1.24 [1.01–1.53] and 1.26 [1.04–1.52], respectively). In the TEX-EV subfraction, three ratios Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0) and Cer(d18:1/24:1)/Cer(d18:1/24:0) were positively associated with MACE (adjusted HR per SD 1.34 [1.06–1.70], 1.24 [1.01–1.51] and 1.31 [1.08–1.58], respectively). In conclusion, distinct ceramides and PCs in plasma EVs determined in preoperative blood were independently associated with an increased 3-year risk of MACE after CEA. These lipids are therefore potential markers to identify high-risk CEA patients qualifying for secondary preventive add-on therapy.
Similar content being viewed by others
Introduction
Carotid endarterectomy (CEA) is a common effective treatment to lower the risk of future ipsilateral stroke in patients with a high degree asymptomatic or symptomatic extracranial carotid artery stenosis. Despite CEA, the residual risk for future cardiovascular (CV) events after CEA is still markedly high with approximately 20% in the three years after CEA1. CEA patients with high risk for secondary CV events qualify for more intensive medical treatment2, for instance by increasing the dose of statins or addition of PCSK-9 inhibitors. Also, anti-inflammatory drug therapies (such as colchicine), addition of anticoagulants or sodium-glucose cotransporter 2 (SGLT2) inhibitors are potential options that are currently under investigation3,4,5,6,7,8. These intensified treatments are often accompanied by high costs or detrimental side effects. Considering the varying risk of MACE across patients9, risk stratification tools that assist individualized secondary prevention are warranted. However, clinical prediction models including CV risk factors have poor predictive ability to discriminate between high and low risk CEA patients for future CV events9,10. Biomarkers may improve risk stratification models to identify patients at high risk for future CV events.
Previous lipidomic studies have identified ceramides and phosphatidylcholines (PCs) as promising biomarkers for cardiovascular disease11,12,13,14,15,16,17. Ceramides belong to sphingolipids consisting of a sphingosine backbone with an attached fatty acid. PCs are phospholipids that are characterized by a choline headgroup and two fatty acyl side chains. Both are main components of lipid bilayer membranes but also act as key signaling molecules18. Ceramides and PCs are suggested to affect atherogenic processes and CV risk factors such as diabetes and obesity18,19. In patients with established coronary artery disease (CAD), particular ceramides and PCs and their distinct ratios were predictive for CV death and future CV events11,12,13,14,15,16,17. Until date, no studies have evaluated the role of ceramides and PCs for future CV events in patients following CEA.
Plasma extracellular vesicles (EVs) are a novel source of biomarkers for cardiovascular disease20. EVs are lipid bilayer membrane microstructures that transfer biological information including lipids (e.g. ceramides and PCs), proteins and RNA over distance from cell to cell. EVs originate either from budding of the outer cell membrane or subcellular compartments hereby including parent-cell membrane lipids and enclosing cytosolic material that could affect remote cells and influence pathophysiological processes20. The biological information in EVs is derived from the cell of origin and can reflect its status. It is known that EV subsets vary in size and in their biological content20,21. Protein content in EVs have been associated with recurrent CV events22. However, the content of ceramides and PCs in plasma EVs in relation with CV events have not yet been studied.
We therefore aimed to investigate whether circulating levels of ceramides and PCs in plasma and in two plasma EV subsets (the LDL- and TEX subfraction) are associated with the postoperative risk of MACE in patients undergoing CEA.
Methods
Study participants
Study participants originated from the Athero-Express Biobank. This ongoing prospective biobank study includes consecutive patients undergoing CEA in two tertiary referral hospitals (University Medical Center Utrecht and St. Antonius Hospital Nieuwegein, The Netherlands). A comprehensive description of the study design has been published earlier1,23. All patients undergoing CEA were asked for study participation. Indications for CEA were adjudicated by a multidisciplinary vascular team and based on recommendation from the European Carotid Surgery Trial24 and the North American Symptomatic Carotid Endarterectomy Trial25,26 for symptomatic patients and Asymptomatic Carotid Surgery Trial 27,28 for asymptomatic patients. Patients included in the Athero-Express Biobank from April 1st, 2002 until December 31, 2016 were eligible for the current study. Inclusion criteria were availability of citrate blood plasma sample and follow-up data. Exclusion criteria were patients undergoing CEA for restenosis. All patients provided written informed consent. Ethical approval for study conduction was provided by the Medical Research Ethics Committee United (MEC-U) of St. Antonius Hospital Nieuwegein, The Netherlands on April 10, 2002 (TME/C01.18). Study conduction complied with the Declaration of Helsinki.
Data collection
Baseline characteristics were collected by standardized questionnaires that were verified against medical records including medical history, medication use, cardiovascular risk factors and basic laboratory parameters. A preoperative blood sample was taken and stored in − 80 °C freezer until further use. The atherosclerotic plaque was freshly obtained during CEA and immediately transferred to the laboratory for further histological analyses.
Follow-up
Patients underwent yearly follow-up for a total duration of 3 years after CEA through standardized questionnaires send by post inquiring whether a patient had experienced any CV event or had been admitted to a hospital in the past year. Follow-up questionnaires were cross-checked with hospital medical records. In case of no response to questionnaires or when additional information regarding a CV event was necessary, the general practitioner was consulted to provide additional follow-up information consisting of medical records and hospital discharge letters from institutions where the event had occurred. All information regarding potential CV events were reviewed by two independent researchers. In case of disagreement, a third expert (GJdB) was consulted.
Study outcomes
The primary outcome of the current study was defined as the 3-year postoperative risk of major adverse CV events (MACE) including fatal- or nonfatal ischemic or hemorrhagic stroke, fatal- or nonfatal myocardial infarction (MI) and any CV death also including sudden cardiac death, fatal aneurysm rupture and fatal cardiac failure. The definition of MACE was in concordance with a previous study in coronary patients and the European Perioperative Clinical Outcome (EPCO) definition12,29. Secondary outcomes were histological atherosclerotic carotid plaque characteristics: content of macrophages, smooth muscle cells (SMCs), collagen, calcification, intraplaque hemorrhage (IPH), intraplaque vessels and lipid core size.
Biomarker selection
Selection of ceramides and phosphatidylcholines (PCs) was based on previous biomarker discovery and validation studies in CAD patients11,12,13,14. Ratios of ceramide/ceramide or ceramide/PC were predefined based on proven associations with CV outcomes in CAD cohorts11,12,13,14,16.
Measurement of ceramides/PCs in plasma and EV-subfractions
Levels of ceramides and PCs were measured in unfractionated plasma and in two subpopulations of plasma extracellular vesicles (EV) called the LDL-EV subfraction and TEX-EV subfraction. A detailed overview of the EV isolation procedure has been reported previously21 and is described in the “Supplemental Materials”. In brief, the LDL-EV subfraction was precipitated using Dextran Sulphate (DS, 0.05%, MP Biomedicals) and Manganese II Chloride (MnCl2, 0.05 M, Sigma-Aldrich), the TEX-EV fraction with Xtractt buffer (1:4, Cavadis BV). Magnetic dextran nanoparticles (nanomag®-D plain for the LDL-EV fraction and Nano-mag®-D PEG-OH for the TEX-EV fraction) were added and EVs were isolated with use of a bio-plex handheld magnet. The pellet, containing the EVs, was lysed with lysis buffer to free its content. Magnetic nanoparticles and debris were separated and removed from the pellet by centrifugation. Ceramides and PC concentrations were quantified in plasma and in the LDL-EV and TEX-EV subfractions using liquid chromatography-mass spectrometry (LC–MS, Sciex TripleQuad 5500 mass spectrometer coupled to Sciex MPX LC system). Final concentrations of ceramides and PCs were expressed in µM. Details of the LC–MS quantification analyses of ceramides and PCs are stated in the “Supplemental Materials”. EV characterization in plasma EV subfractions have been described in previous studies21,30,31. Previous Nanoparticle Tracking Analyzer (NTA) experiments showed the smallest EVs in the TEX-fraction (mean 84 nm) and relatively larger EVs in LDL-fraction (mean 101 nm)21. Additional density gradient analyses of EV subfractions confirmed the presence of ceramides and PCs in EVs (see “Supplemental Materials” for details; Figs. S2, S3 and Table S1).
Histological atherosclerotic plaque characterization
Histological examination of the carotid atherosclerotic plaque was performed according to the standardized Athero-Express biobank protocol1,23. Details are described in the “Supplemental Materials”1,23. The carotid plaque was cross-sectionally cut into segments of 5 mm. The segment with the largest plaque volume was considered the culprit lesion was allocated to immunohistochemical analysis of collagen, smooth muscle cells (SMCs), macrophages, calcifications, intraplaque hemorrhage (IPH) and lipid core. Plaque characteristics were scored semi quantitatively as no/minor or moderate/heavy staining, except for IPH (scored as absent or present) and lipid core (size was visually estimated relative to the total plaque area and expressed as < 10%, 10–40%, > 40% of the total plaque area). In addition, SMCs, macrophages and intraplaque vessels were quantified by computerized analysis software (AnalySIS 3.2, Soft Imaging Systems GmbH, Munster, Germany). SMCs and macrophage infiltration were expressed as the percentage of positive staining of the total plaque area. CD34 positive intraplaque vessels were counted in three hotspots with highest vessel density and the average number per square millimeter was calculated, as described previously32.
Statistical analyses
Associations with MACE
Continuous baseline characteristics and categorical baseline characteristics were respectively compared by Students t-test or Mann Whitney U test and Pearson’s Chi-squared test. Cholesterol and creatinine levels were logarithmically transformed because of skewness. The associations of ceramides and PCs (ratio) levels with the 3-year postoperative risk of MACE were analyzed by univariable, adjusted for LDL-C and HDL-C, and multivariable Cox proportional hazards models, similar to the previous CAD study11. To facilitate easy comparison with existing literature ceramides and PCs (ratios) concentrations were standardized to Z-scores11,12. Hazard ratios (HR) were expressed per one SD increase. Potential confounders for multivariable analyses were a priori selected based on available literature10,11,14,16,33,34; age, history of coronary artery disease (CAD) and/or peripheral artery disease (PAD), preprocedural cerebrovascular symptoms, current smoking, hypertension, diabetes, lipid-lowering drug use, triglycerides, total cholesterol, creatinine and contralateral carotid artery stenosis of 50–100%. Model reduction using Akaike information criterion in a stepwise backward regression resulted in the final model including age, history of CAD and/or PAOD, cerebrovascular symptoms, current smoking, LDL-C and HDL-C. Percentage of missing covariates was low (range 0.0–4.2%), see Supplemental Table S2. To gain further insight in the association of ceramides and PCs (ratios) and the occurrence of MACE during three-year follow-up, multivariable significant ceramides/PCs were visualized in quartiles by Kaplan–Meier graphs.
Predictive value for MACE
To explore the added predictive value of ceramide/PCs (ratios) for MACE on top of clinical risk factors, model performance was assessed. Bootstrapping techniques were used for internal validation in order to control for optimism and overfitting. Calibration was assessed by visual inspection of the calibration plots. Discriminative performance was assessed by calculating the C-index and the Integrated Discrimination Improvement (IDI)35.
Association with plaque characteristics
To unravel potential underlying biological mechanisms, the association of multivariable significant ceramides and PCs (ratios) with histological atherosclerotic plaque characteristics were investigated by univariable and multivariable logistic or linear regression. Age, sex, LDL-C, HDL-C, triglycerides and total cholesterol were added as potential confounders for multivariable analyses of plaque characteristics34,36. All p-values resulted from two-tailed hypothesis testing. A p-value < 0.05 indicated statistically significance. Analyses were performed in R statistical software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
Results
Study population
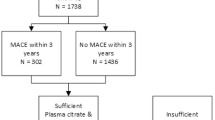
A total of 887 patients were included in the current study (Fig. 1). Measurement of ceramides and PCs failed in 14 patients leaving 873 patients for analyses. Patient characteristics are summarized in Table 1. The overall mean age was 69 years, 70% were men and most were operated for high degree (70–99%) symptomatic carotid stenosis. The prevalence of CV risk factors, history of CAD and PAD were high, exemplified by the high frequency of antiplatelet and lipid lowering drug use at study inclusion. Patients that experienced MACE after three years of follow up were significantly older, were more often diabetic, more often had a history of coronary or peripheral artery disease, had higher creatinine levels and had lower HDL levels at baseline than patients that remain free from MACE.
During a median postoperative follow-up duration of 3.0 years [IQR 2.2–3.0], 138 patients (15.8%) experienced MACE consisting of 74 (8.5%) fatal and non-fatal stroke, 44 (5%) fatal and non-fatal MI and 20 (2.3%) CV death due to other causes. Patients that experienced MACE were at inclusion significantly older, more often had diabetes, had lower HDL-C levels, higher creatinine levels and were more likely to have a history of CAD and/or PAD compared to patients who remained free from MACE (Table 1). Availability of ceramide and PC measurements, as well as absolute concentrations compared between patients that experienced MACE and those that did not, are shown in Supplemental Table S3.
Associations with the 3-year postoperative risk of MACE
Univariable and multivariable associations of ceramides and PCs, measured in plasma and EV subfractions, with the 3-year risk of MACE are depicted in Table 2. After correction for all confounders (LDL-C, HDL-C, age, history of CAD and/or PAD, cerebrovascular symptoms and current smoking), one ceramide and four ceramide ratios remained significantly associated with MACE in the LDL-EV subfraction as well as in the TEX-EV subfraction (Table 2).
In the LDL-EV subfraction, higher levels of Cer(d18:1/24:1) were independently associated with a higher 3-year postoperative risk of MACE with adjusted HR 1.24 per SD, 95% CI 1.01–1.53 (p = 0.040). A higher ratio of Cer(d18:1/16:0)/PC(16:0/22:5) in the LDL-EV subfraction was independently associated with a higher 3-year risk of MACE with adjusted HR of 1.26 per SD, 95% CI 1.04–1.52 (p = 0.016).
In the TEX-EV subfraction, three ceramide ratios were positively associated with the 3-year risk of MACE, namely Cer(d18:1/16:0)/Cer(d18:1/24:0) with adjusted HR 1.34 per SD, 95% CI 1.06–1.70 (p = 0.016), Cer(d18:1/18:0)/Cer(d18:1/24:0) with adjusted HR 1.24 per SD, 95% CI, 1.01–1.51 (p = 0.042) and Cer(d18:1/24:1)/Cer(d18:1/24:0) with adjusted HR 1.31 per SD, 95% CI 1.08–1.58 (p = 0.005).
In plasma, multiple ceramide ratios were significantly associated with MACE in univariable analyses, however, these became insignificant after correction for confounders.
As statins are known to modify ceramide/PC levels14, sub analyses with addition of statin use to multivariable models were performed (Supplemental Table S4). All the lipids or lipid ratios in the LDL-EV and two in the TEX-EV subfractions remained significantly associated with MACE after CEA, but Cer(d18:1/18:0)/Cer(d18:1/24:0) in TEX-EV subfraction became borderline non-significant (Supplemental Table S4).
In order to gain further insight in the association of ceramide/PC ratios with MACE, we analyzed quartile levels by Kaplan–Meier plots and Cox-regression analyses (Fig. 2A–E). Non-linearity was found for the ratios of Cer(d18:1/16:0)/Cer(d18:1/24:0) and Cer(d18:1/18:0)/Cer(d18:1/24:0) in the TEX-EV fraction where patients in the 4th quartile were at relatively high risk (respective HR for patients in the 4th quartile versus those in the 1st quartile were 2.15, 95% CI 1.33–3.49 and 1.66, 95% CI 1.05–2.63) (Fig. 2A,B). The association of Cer(d18:1/24:1) in the LDL-EV fraction with MACE appeared to be dichotomous (Fig. 2C). Patients with levels above the median had a HR of 1.44, 95% CI 1.01–2.06, compared to patients with levels below the median. The 3rd and 4th quartiles of the Cer(d18:1/24:1)/Cer(d18:1/24:0) ratio in the TEX-EV subfraction and Cer(d18:1/16:0)/PC(16:0/22:5) ratio in the LDL-EV subfraction seemed to be more linearly associated with increased risk of MACE (Fig. 2D,E).
(A)–(E). Kaplan–Meier estimates of major adverse cardiovascular event (MACE) for quartiles of multivariable significant associated ceramides/PC ratios. The p-value indicates the overall comparison of the MACE-free survival across quartile levels by the log-rank test. The hazard ratios in the legends indicate the univariable quartile-specific hazard relative to the first quartile.
Predictive value on top of clinical risk factors
Possible added predictive value of multivariable significant ceramides/PCs on top of clinical risk factors was evaluated using stepwise backward regression modelling. Based on likelihood estimates, addition of Cer(d18:1/24:1)/Cer(d18:1/24:0) ratio in the TEX-EV fraction improved the clinical model (including age, history of CAD and/or PAD, cerebrovascular symptoms, current smoking, LDL-C and HDL-C). Calibration plots showed improved calibration with the ceramide ratio (Supplemental Fig. S4). The C-statistic did not improve significantly (C-statistic of the clinical model was 0.67 and for the biomarker model with the ceramide ratio 0.68, p = 0.445 for comparison). Addition of the ceramide ratio to the clinical model resulted in a significant IDI of 0.03 (0.008–0.053), p = 0.008.
Associations with histological atherosclerotic plaque characteristics
Having established an association of ceramide/PC EV-levels with MACE and considering that EVs probably reflect the status of the cell of origin, we investigated the association of the multivariable significant ceramides and PCs with histological plaque characteristics that are considered as key markers for plaque vulnerability. This might unravel potential underlying mechanisms how these lipid ratios contribute to cardiovascular event risk. Increased ratios of Cer(d18:1/18:0)/Cer(d18:1/24:0) and Cer(d18:1/24:1)/Cer(d18:1/24:0) in the TEX-EV fraction were inversely associated with the amount of SMCs in the plaque (respective adjusted odds ratio (OR) 0.76 for moderate/heavy SMC staining, 95% CI 0.61–0.95, p = 0.015) and adjusted beta of -0.26 for the percentage of SMCs staining, 95% CI − 0.47 to − 0.04, p = 0.020) (Table 3). An increased ratio of Cer(d18:1/16:0)/ PC(16:0/22:5) in the LDL-EV subfraction was positively associated with more macrophage infiltration (beta 0.16, 0.05–0.28, p = 0.007). Neither Cer(d18:1/24:1) in the LDL-EV fraction nor the ratio Cer(d18:1/16:0)/Cer(d18:1/24:0) in TEX-EV subfraction were associated with histological plaque characteristics.
Discussion
Despite CEA, there remains a high residual risk for future CV events in these patients after surgery. Early identification of these high-risk CEA patients would allow early initiation of add-on therapy to reduce cardiovascular event risk. In this study, we examined the association of circulating molecular lipids, previously associated with CV outcome in CAD patients, with future CV events in patients undergoing CEA. In plasma EVs, elevated levels of Cer(d18:1/24:1) and the ratios Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0), Cer(d18:1/24:1)/Cer(d18:1/24:0) and Cer(d18:1/16:0)/PC(16:0/22:5) were associated with elevated risk of MACE during the 3 years follow-up independently of conventional cardiovascular risk factors. EV-derived ceramides/PCs ratios may be considered as biomarkers for high residual CV risk.
Previous studies investigated ceramides/PCs only in plasma and did show associations of particular ceramides/PCs (ratios) with an increased risk of CV death and future CV events in patients with acute coronary syndrome (ACS) or stable CAD11,12,13,17. In the general population, elevated plasma levels of these specific ceramides were associated with both primary as well as recurrent MACE16. Although we only found associations of these lipid ratios in plasma EVs subsets in a CEA population, our results further contribute to the concept of ceramides and PCs as markers for future CV events. Specifically, Cer(d18:1/24:1)/Cer(d18:1/24:0) in TEX-EVs may be a promising biomarker to improve risk stratification for MACE. If confirmed in external validation studies as well as impact studies, this biomarker may be valuable in selecting patients at high risk for MACE that may benefit from intensified medical treatment.
This is the first study that examined levels of ceramides and PCs in plasma EVs whereas previous studies only investigated unfractionated plasma11,12,13,17. Although univariable analysis showed significant associations of ceramides and PCs in both unfractionated plasma and plasma EVs, in multivariable analyses only the associations in plasma EVs remained significant. EV subpopulations differ in vesicle size and it is known that the biological composition and clearance mechanisms vary across subpopulations20. Since unfractionated plasma contains all EV subpopulations next to other particles and complexes containing ceramides and PCs, differences in biological information between subpopulations might be masked and remain unnoticed. Therefore, the signal to noise ratio will probably be better for lipid levels in EV subpopulations than in unfractionated plasma. Our results suggest that lipid ratios in EVs may therefore be more potent biomarkers for MACE than in unfractionated plasma.
In general, the ratios of ceramides or ceramides/PCs species were more strongly associated with MACE than the individual lipid species, which is in line with prior studies in CAD patients11,12,13. Very long chain ceramides are produced by the Ceramide Synthase enzyme isotype 2 (CerS2; C24:0 or 24:1), while long chain (C16:0) are produced by other isoforms, CerS6 and CerS537. CerS2 haplo-insufficiency, however leads to compensatory increase of C16:0 ceramides showing that, despite they are produced by different enzymes, changes in ceramide ratios are more pronounced when ceramide pathways are changing37,38. For this, ratios of different ceramides and PCs species may serve as a better reflection of the complex ongoing metabolic pathways and CV disease risk than individual ceramide and PC species.
Ceramides and PCs are related to CV events and modification of lipid profiles by medical or dietary interventions aiming to reduce cardiovascular event risk has increasingly gaining attention34,39,40,41,42. In patients, statins and PCSK-9 inhibitors reduced high-risk ceramide and PC levels14,34. Interestingly, PCSK-9 inhibition by loss-of-function mutation lowered relatively more high-risk ceramide levels than LDL-C levels, suggesting that PCSK-9 inhibitors not solely act via reducing LDL-C but also through profound changes in the lipid metabolism14. In patients with metabolic syndrome, treatment with pioglitazone (an antidiabetic drug) reduced high-risk ceramide levels and concomitantly enhanced insulin sensitivity39. A post-hoc analyses indicated that in patients with elevated levels of high-risk ceramides, a Mediterranean diet may be favorable to reduce the cardiovascular event risk40. Interestingly, higher levels of PC(16:0/22:5) seemed to be protective for cardiovascular death in patients with CAD which is in line with our results15. PC(16:0/22:5) belongs to omega-3 polyunsaturated fatty acids (PUFA) and are abundantly present in fatty fish. They are suggested to reduce the risk of cardiac death potentially by lowering resting heart rate, blood pressure, plasma triglycerides and improving endothelial function43. Previous studies investigating omega-3 supplementation were inconsistent but two recent studies have shown a positive effect in reducing future CV event risk41,42. An interesting topic for future studies may be to examine whether ceramide/PC ratios could be useful in selecting patients that benefit most from dietary interventions or additional medical treatments in reducing cardiovascular risk.
Recently, randomized controlled trials showed that SGLT-2 inhibitors significantly improved cardiovascular outcome in patients with heart failure in those with and without diabetes8,44. An experimental study showed that empagliflozin (an SGLT-2 inhibitor) significantly reduced the ceramide content of cardiac cells of diabetic rats45. Moreover, empagliflozin was associated with the level of ceramidase (an enzyme in the ceramide metabolism) in a proteomics study46. Until date, no clinical studies have been performed on the effect of ceramide levels and SGLT-2 inhibitors. Therefore, it may be interesting for future studies to examine whether SGLT-2 inhibitors alter ceramide levels of patients with established cardiovascular disease and mediate cardiovascular risk reduction.
Due to the observational study design, our findings cannot prove causality between ceramides/PCs and CV events. Based on evidence from experimental studies we could speculate, however, that these lipids contribute to atherosclerotic plaque vulnerability. We found that lipid ratios associated with MACE Cer(d18:1/24:1)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0) and Cer(d18:1/16:0)/PC(16:0/22:5) were also positively associated with a more vulnerable carotid atherosclerotic plaque phenotype suggesting putative underlying pathobiological mechanisms. Experimental studies have implied that ceramides drive main atherosclerotic processes such as LDL aggregation, LDL uptake across the endothelium, foam cell formation, production of reactive oxygen species, apoptosis, endothelial dysfunction and inflammatory processes18. Studies on the relation of ceramides/PCs and atherosclerotic plaque composition in patients are scarce. Two studies in CAD patients have revealed that increased levels of Cer(d18:1/16:0), Cer(d18:1/18:0) and Cer(d18:1/24:1) were associated with higher coronary plaque vulnerability, characterized with a higher lipid volume, necrotic core and a thinner fibrous cap using intravascular ultrasound and optical coherence tomography17,41. In our study, high-risk ceramides/PCs ratios were also associated with a more vulnerable atherosclerotic plaque composed of fewer SMCs and more macrophage infiltration. Experimental data has shown that ceramides induce the activity of matrix metalloproteinases (MMPs) and proinflammatory cytokines18. In vitro, ceramides induced apoptosis of vascular smooth muscle cells (VSMCs)42. It is known that increase of MMPs and loss of VSMCs contributes to thinning of the fibrous cap and subsequent plaque disruption. Additionally, pharmacological inhibition of ceramide synthetic pathway reduced macrophage content and increased SMCs content in mice43. Our results further contribute to evidence of the potential role of ceramides in plaque instability.
Another possible explanation for the association between EV-derived ceramides and MACE may be that high levels of ceramides reflect a general pathological state of the endothelium. The endothelium is an important source of plasma ceramides and are implicated in NO mediated vasodilatation44. Moreover, it has been shown that the endothelium also generates EVs and, when activated by TNFα, EVs are generated with increased ceramide content45. Exact underlying mechanisms how EV-derived ceramides/PCs relate to increased risk of CV events remains to be elucidated. As the biological function of distinct ceramides and PCs in atherogenesis is still poorly understood, future studies should determine whether ceramides and PCs are real effectors or solely markers of progression of atherosclerosis and subsequent CV events. Understanding the role of ceramides/PCs and related synthesizing or degrading biosynthetic enzymes in atherosclerotic diseases may lead to new potential therapeutic targets46.
Reported correlations of ceramides/PCs in plasma EVs with plaque characteristics in our study do not establish the origin of EVs. Determination of the cellular origin of EVs is challenging since expression of cellular surface markers on EVs are not specific to the parent cell types47. It is known that EVs can be released by almost all cell types such as endothelial cells, cardiomyocytes, platelets, red- or white blood cells20. The role of EVs in atherothrombotic processes has been well acknowledged, although exact mechanisms in vivo are unclear20. Future studies should explore the cellular origin of EVs because this would provide more insight in the origin of high-risk lipids and underlying processes related to atherosclerotic events.
Potential study limitations need to be addressed. First, although previous CAD cohorts found stronger associations of ceramide ratios with CV death compared to MACE11,12,15,17, we were not able to perform analyses on separate outcomes due to a limited number of events. Second, during follow-up no data regarding dietary patterns, medication use or adherence were available. This may have modified the observed associations between molecular lipids and MACE. Third, although the IDI may suggest potential incremental prognostic value of Cer(d18:1/24:1)/Cer(d18:1/24:0) in TEX-EVs on top of clinical risk factors, this result needs to be interpreted with caution since the improvement was minor (3%) and no improvement in C-statistic was observed. Predictive performance of ceramides could not be compared to the performance of known biomarkers for MACE48, such as troponin and NT-proBNP, because these data was not available. Future studies should determine the added value of ceramides for MACE prediction in comparison with these existing biomarkers. Validation studies should use clinically relevant cut points that are useful in determining treatment strategies. Last, subgroup analyses in patients undergoing CEA for asymptomatic carotid stenosis could not be performed due to low patient numbers. Ceramides and PCs may be relevant for risk stratification in this particular subgroup in light of the ongoing debate whether or not to perform CEA, but this should be further explored in appropriately designed future studies.
Major strengths include that this is the first study investigating ceramides and PCs in CEA patients and investigating these lipids in both plasma EVs and plasma. Another strength is the prospective design with validated CV outcomes. Due to the unique design of the Athero-Express biobank we were able to concomitantly investigate CV outcomes and histological atherosclerotic plaque characteristics to generate hypotheses regarding possible biological mechanisms.
To conclude, increased levels of ceramide and PCs ratios in plasma EVs, but not in unfractionated plasma, are independently associated with increased risk of MACE after CEA. These EV-derived ceramide- and ceramide/PC ratio are therefore potential biomarkers for MACE. Ceramides/PCs ratios in EVs may be useful biomarkers for selecting high-risk patients in need for intensified secondary preventive therapy, such as add-on therapy.
Abbreviations
- ACS:
-
Acute coronary syndrome (STEMI, Non-STEMI, unstable angina)
- CAD:
-
Coronary artery disease
- CEA:
-
Carotid endarterectomy
- CV:
-
Cardiovascular
- EVs:
-
Extracellular vesicles
- IPH:
-
Intraplaque haemorrhage
- LDL-EV subfraction:
-
Plasma EV-subfraction that co-precipitates with LDL
- MACE:
-
Major adverse cardiovascular events
- MI:
-
Myocardial infarction
- PAD:
-
Peripheral artery disease
- PC:
-
Phosphatidylcholine
- SMCs:
-
Smooth muscle cells
- TEX-EV subfraction:
-
Total extracellular vesicle subfraction
References
Hellings, W. E. et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome. Circulation 121, 1941–1950 (2010).
Mach, F. et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Anand, S. S. et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J. Am. Coll. Cardiol. 73, 3271–3280 (2019).
Bonaca, M. P. et al. Rivaroxaban in peripheral artery disease after revascularization. N. Engl. J. Med. 382, 1994–2004 (2020).
Petrie, M. C. et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 323, 1353–1368 (2020).
Kaasenbrood, L. et al. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation 134, 1419–1429 (2016).
Volkers, E. J., Algra, A., Kappelle, L. J. & Greving, J. P. Prediction models for clinical outcome after a carotid revascularisation procedure: A systematic review. Eur. Stroke J. 3, 57–65 (2018).
Laaksonen, R. et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016).
Hilvo, M. et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 41, 371–380 (2019).
Mundra, P. A. et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 3, e121326 (2018).
Tarasov, K. et al. Molecular Lipids identify cardiovascular risk and are efficiently lowered by Simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99, E45–E52 (2014).
Sigruener, A. et al. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. PLoS ONE 9, e85724 (2014).
Havulinna, A. S. et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430 (2016).
Cheng, J. M. et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 (2015).
Bismuth, J., Lin, P., Yao, Q. & Chen, C. Ceramide: A common pathway for atherosclerosis?. Atherosclerosis 196, 497–504 (2008).
Meikle, P. J. & Summers, S. A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13, 79–91 (2017).
Boulanger, C. M., Loyer, X., Rautou, P.-E. & Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 14, 259–272 (2017).
Dekker, M. et al. Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci. Rep. 10, 12257 (2020).
Kanhai, D. A. et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int. J. Cardiol. 168, 2358–2363 (2013).
Verhoeven, B. A. N. et al. Athero-express: Differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur. J. Epidemiol. 19, 1127–1133 (2004).
Warlow, C., Farrell, B., Fraser, A. & Sandercock, P. S. J. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351, 1379–1387 (1998).
North American Symptomatic Carotid Endarterectomy Trial Collaborators et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 325, 445–453 (1991).
Barnett, H. J. et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 339, 1415–1425 (1998).
Halliday, A. et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 376, 1074–1084 (2010).
Halliday, A. et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet 363, 1491–1502 (2004).
Jammer, I. et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 32, 88–105 (2015).
Zhang, Y.-N. et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: An observational study in a dyspnoea cohort. PLoS ONE 11, e0148073 (2016).
Wang, J.-W. et al. Lowering low-density lipoprotein particles in plasma using dextran sulphate co-precipitates procoagulant extracellular vesicles. Int. J. Mol. Sci. 19, 94 (2017).
Derksen, W. J. M. et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: A large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis 218, 369–377 (2011).
van Lammeren, G. W. et al. Clinical prediction rule to estimate the absolute 3-year risk of major cardiovascular events after carotid endarterectomy. Stroke 43, 1273–1278 (2012).
Hilvo, M. et al. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 269, 159–165 (2018).
Moons, K. G. M., de Groot, J. A. H., Linnet, K., Reitsma, J. B. R. & Bossuyt, P. M. M. Quantifying the added value of a diagnostic test or marker. Clin. Chem. 58, 1408–1417 (2012).
Hellings, W. E. et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J. Vasc. Surg. 45, 289–296 (2007).
Petrache, I. et al. Ceramide synthases expression and role of ceramide synthase-2 in the lung: Insight from human lung cells and mouse models. PLoS ONE 8, e62968 (2013).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Warshauer, J. T. et al. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab. Res. Rev. 31, 734–744 (2015).
Wang, D. D. et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 135, 2028–2040 (2017).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Manson, J. E. et al. Vitamin D, marine n-3 Fatty acids, and primary prevention of cardiovascular disease current evidence. Circ. Res. 126, 112–128 (2020).
Mozaffarian, D. & Wu, J. H. Y. Omega-3 fatty acids and cardiovascular disease. J. Am. Coll. Cardiol. 58, 2047–2067 (2011).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Aragón-Herrera, A. et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem. Pharmacol. 170, (2019).
Ferrannini, E. et al. Mechanisms of sodium-glucose cotransporter 2 inhibition: Insights from large-scale proteomics. Diabetes Care 43, 2183–2189 (2020).
Pan, W. et al. Plasma ceramides in relation to coronary plaque characterization determined by optical coherence tomography. J. Cardiovasc. Transl. Res. 1, 140–149. https://doi.org/10.1007/s12265-020-09978-3 (2020).
Vernooij, L. M. et al. The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all‐cause mortality in patients who undergo noncardiac surgery. Cochrane Database Syst. Rev. 2021, (2021).
Acknowledgements
We thank Qiu Ying van de Pol, Sara van Laar and Evelyn Velema for their technical support in the Athero-Express Biobank.
Funding
This work was supported by the European Union [EU 755320 Taxinomisis Grant to Dr. De Borst, dr. De Kleijn, dr. Pasterkamp and dr. Laaksonen]; and the Dutch Heart Foundation [CVON 2017-05 pERSUASIVE to Dr. de Kleijn and dr. De Winter].
Author information
Authors and Affiliations
Contributions
Study design: N.T., F.W., D.K., R.L. Data collection: N.T., F.W., R.L., A.J., J.B. Sample measurements: A.J., J.B. Data analysis and interpretation: N.T., F.W., M.D., D.K., R.L. Drafting the article and figures: N.T., F.W., M.H., D.K., R.L. Critical revision and final approval: N.T., F.W., M.D., G.B., J.B., R.W., M.H., A.J., G.P., D.K., R.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timmerman, N., Waissi, F., Dekker, M. et al. Ceramides and phospholipids in plasma extracellular vesicles are associated with high risk of major cardiovascular events after carotid endarterectomy. Sci Rep 12, 5521 (2022). https://doi.org/10.1038/s41598-022-09225-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09225-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.