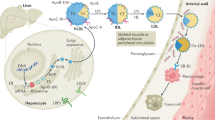
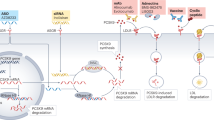
Abstract
Prolonged or excessive exposure to oxidized phospholipids (OxPLs) generates chronic inflammation. OxPLs are present in atherosclerotic lesions and can be detected in plasma on apolipoprotein B (apoB)-containing lipoproteins. When initially conceptualized, OxPL–apoB measurement in plasma was expected to reflect the concentration of minimally oxidized LDL, but, surprisingly, it correlated more strongly with plasma lipoprotein(a) (Lp(a)) levels. Indeed, experimental and clinical studies show that Lp(a) particles carry the largest fraction of OxPLs among apoB-containing lipoproteins. Plasma OxPL–apoB levels provide diagnostic information on the presence and extent of atherosclerosis and improve the prognostication of peripheral artery disease and first and recurrent myocardial infarction and stroke. The addition of OxPL–apoB measurements to traditional cardiovascular risk factors improves risk reclassification, particularly in patients in intermediate risk categories, for whom improving decision-making is most impactful. Moreover, plasma OxPL–apoB levels predict cardiovascular events with similar or greater accuracy than plasma Lp(a) levels, probably because this measurement reflects both the genetics of elevated Lp(a) levels and the generalized or localized oxidation that modifies apoB-containing lipoproteins and leads to inflammation. Plasma OxPL–apoB levels are reduced by Lp(a)-lowering therapy with antisense oligonucleotides and by lipoprotein apheresis, niacin therapy and bariatric surgery. In this Review, we discuss the role of role OxPLs in the pathophysiology of atherosclerosis and Lp(a) atherogenicity, and the use of OxPL–apoB measurement for improving prognosis, risk reclassification and therapeutic interventions.
Key points
-
Phosphocholine-containing oxidized phospholipids (OxPLs) induce chronic inflammation, including in atherosclerotic lesions, and can be detected in plasma on apolipoprotein B-100 (apoB-100)-containing lipoproteins.
-
A method has been developed to quantify OxPLs on a normalized amount of apoB-100 (OxPL–apoB), so that the measurement is independent of plasma apoB-100 and LDL cholesterol levels.
-
Lipoprotein(a) (Lp(a)) particles carry the largest fraction of OxPLs among apoB-containing lipoproteins; the OxPLs are bound covalently to apolipoprotein(a) and are free in the lipid phase of the associated LDL-like particle.
-
Plasma OxPL–apoB levels predict the presence and extent of anatomical atherosclerotic cardiovascular disease, and elevated levels are associated with disease in multiple arterial beds; measurement of OxPL–apoB improves prognostication of peripheral artery disease, as well as incident and recurrent myocardial infarction and stroke, and improves risk reclassification, particularly in patients in intermediate risk categories, for whom improving decision-making is most impactful.
-
Plasma OxPL–apoB levels are reduced by treatment with antisense oligonucleotides aimed at reducing Lp(a) production and by lipoprotein apheresis, niacin therapy and bariatric surgery.
-
Plasma OxPL–apoB levels predict cardiovascular events with a potency similar to or greater than that of plasma Lp(a) levels, probably because OxPL–apoB levels reflect the levels of the most atherogenic and pro-inflammatory Lp(a) and apoB-100-containing particles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Sachdeva, A. et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am. Heart J. 157, 111–117.e2 (2009).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Schwartz, G. G. et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107 (2018).
Miller, Y. I. et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 (2011).
Tsiantoulas, D. et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 56, 440–448 (2015).
Witztum, J. L. & Lichtman, A. H. The influence of innate and adaptive immune responses on atherosclerosis. Annu. Rev. Pathol. 9, 73–102 (2014).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018).
Sun, X. et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 31, 189–206.e8 (2020).
van der Valk, F. M. et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 134, 611–624 (2016).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740 (2000).
Palinski, W. et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. invest. 98, 800–814 (1996).
Taleb, A., Witztum, J. L. & Tsimikas, S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 5, 673–694 (2011).
Glavinovic, T. et al. Physiological bases for the superiority of apolipoprotein B over low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol as a marker of cardiovascular risk. J. Am. Heart Assoc. 11, e025858 (2022).
Steinberg, D., Parthasarathy, S., Carew, T. E., Khoo, J. C. & Witztum, J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320, 915–924 (1989).
Gillotte, K. L., Horkko, S., Witztum, J. L. & Steinberg, D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. J. Lipid Res. 41, 824–833 (2000).
Steinberg, D. & Witztum, J. L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 2311–2316 (2010).
Chang, M. K. et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 200, 1359–1370 (2004).
Friedman, P., Horkko, S., Steinberg, D., Witztum, J. L. & Dennis, E. A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 277, 7010–7020 (2002).
van Dijk, R. A. et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J. Lipid Res. 53, 2773–2790 (2012).
Boonmark, N. W. & Lawn, R. M. The lysine-binding function of Lp(a). Clin. Genet. 52, 355–360 (1997).
Cushing, G. L. et al. Quantitation and localization of apolipoproteins [a] and B in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis 9, 593–603 (1989).
Skalen, K. et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754 (2002).
Ravandi, A. et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J. Am. Coll. Cardiol. 63, 1961–1971 (2014).
Upchurch, C. M. et al. Targeting oxidized phospholipids by AAV-based gene therapy in mice with established hepatic steatosis prevents progression to fibrosis. Sci. Adv. 8, eabn0050 (2022).
Yang, M. Q. et al. Interferon regulatory factor 1-Rab27a regulated extracellular vesicles promote liver ischemia/reperfusion injury. Hepatology 67, 1056–1070 (2018).
Yeang, C. et al. Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 115, 179–189 (2019).
Oehler, B. et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci. Rep. 7, 5447 (2017).
Mohammadi, M. et al. Antinociception by the anti-oxidized phospholipid antibody E06. Br. J. Pharmacol. 175, 2940–2955 (2018).
Ambrogini, E. et al. Oxidation-specific epitopes restrain bone formation. Nat. Commun. 9, 2193 (2018).
Palmieri, M. et al. A neutralizing antibody targeting oxidized phospholipids promotes bone anabolism in chow-fed young adult mice. J. Bone Miner. Res. 36, 170–185 (2021).
Palmieri, M. et al. Neutralization of oxidized phospholipids attenuates age-associated bone loss in mice. Aging Cell 20, e13442 (2021).
Taleb, A. et al. High immunoglobulin-M levels to oxidation-specific epitopes are associated with lower risk of acute myocardial infarction. J. Lipid Res. 64, 100391 (2023).
Bergmark, C. et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49, 2230–2239 (2008).
Leibundgut, G. et al. Determinants of binding of oxidin apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 54, 2815–2830 (2013).
Tsimikas, S., Tsironis, L. D. & Tselepis, A. D. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 2094–2099 (2007).
Rao, F. et al. Heritability of biomarkers of oxidized lipoproteins: twin pair study. Arterioscler. Thromb. Vasc. Biol. 35, 1704–1711 (2015).
McLean, J. W. et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 330, 132–137 (1987).
Laplaud, P. M., Beaubatie, L., Rall, S. C. Jr., Luc, G. & Saboureau, M. Lipoprotein[a] is the major apoB-containing lipoprotein in the plasma of a hibernator, the hedgehog (Erinaceus europaeus). J. Lipid Res. 29, 1157–1170 (1988).
Scanu, A. M. et al. Rhesus monkey lipoprotein(a) binds to lysine Sepharose and U937 monocytoid cells less efficiently than human lipoprotein(a). Evidence for the dominant role of kringle 4(37). J. Clin. Invest. 91, 283–291 (1993).
Belczewski, A. R. et al. Baboon lipoprotein(a) binds very weakly to lysine-agarose and fibrin despite the presence of a strong lysine-binding site in apolipoprotein(a) kringl IV type 10. Biochemistry 44, 555–564 (2005).
Chenivesse, X., Huby, T., Wickins, J., Chapman, J. & Thillet, J. Molecular cloning of the cDNA encoding the carboxy-terminal domain of chimpanzee apolipoprotein(a): an Asp57 → Asn mutation in kringle IV-10 is associated with poor fibrin binding. Biochemistry 37, 7213–7223 (1998).
Lim, E. T. et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 10, e1004494 (2014).
Emdin, C. A. et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J. Am. Coll. Cardiol. 68, 2761–2772 (2016).
Mora, S. et al. Lipoprotein(a) and risk of type 2 diabetes. Clin. Chem. 56, 1252–1260 (2010).
Kamstrup, P. R. & Nordestgaard, B. G. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 1, 220–227 (2013).
Ye, Z. et al. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes 63, 332–342 (2014).
Paige, E. et al. Lipoprotein(a) and incident type-2 diabetes: results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc. Diabetol. 16, 38 (2017).
Tsimikas, S. In search of a physiological function of lipoprotein(a): causality of elevated Lp(a) levels and reduced incidence of type 2 diabetes. J. Lipid Res. 59, 741–744 (2018).
Manten, G. T. et al. Changes of plasma lipoprotein(a) during and after normal pregnancy in Caucasians. J. Matern. Fetal Neonatal Med. 14, 91–95 (2003).
Doucet, C., Huby, T., Chapman, J. & Thillet, J. Lipoprotein[a] in the chimpanezee: relationship of apo[a] phenotype to elevated plasma Lp[a] levels. J. Lipid Res. 35, 263–270 (1994).
Loudon, I. Deaths in childbed from the eighteenth century to 1935. Med. Hist. 30, 1–41 (1986).
Berbée, J. F., Havekes, L. M. & Rensen, P. C. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 11, 97–103 (2005).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Brown, M. S. & Goldstein, J. L. Plasma lipoproteins: teaching old dogmas new tricks. Nature 330, 113–114 (1987).
Ruder, S. et al. Lp(a), oxidized phospholipids and oxidation-specific epitopes are increased in subjects with keloid formation. Lipids Health Dis. 21, 113 (2022).
Schneider, M. et al. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J. Lipid Res. 46, 769–778 (2005).
Torzewski, M. et al. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. JACC Basic. Transl. Sci. 2, 229–240 (2017).
Chen, Y. et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J. Immunol. 183, 1346–1359 (2009).
Chang, M. K., Binder, C. J., Torzewski, M. & Witztum, J. L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. PNAS 99, 13043–13048 (2002).
Dei, R. et al. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer’s disease. Acta Neuropathol. 104, 113–122 (2002).
Haider, L. et al. Oxidative damage in multiple sclerosis lesions. Brain 134, 1914–1924 (2011).
Dou, H. et al. Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation 144, 1940–1954 (2021).
Ikura, Y. et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology 43, 506–514 (2006).
Svenungsson, E. et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation 104, 1887–1893 (2001).
Muller, N. et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) exotein (a) synthesis in humans. J. Lipid Res. 56, 1034–1042 (2015).
McMahon, M. et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann. Rheum. Dis. 70, 1619–1624 (2011).
Leibundgut, G. et al. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J. Am. Coll. Cardiol. 59, 1426–1437 (2012).
DeFilippis, A. P. et al. Circulating levels of plasminogen and oxidized phospholipids bound to plasminogen distinguish between atherothrombotic and non-atherothrombotic myocardial infarction. J. Thromb. Thrombolysis 42, 61–76 (2016).
DeFilippis, A. P. et al. Atherothrombotic factors and atherosclerotic cardiovascular events: the multi-ethnic study of atherosclerosis. Eur. Heart J. 43, 971–981 (2022).
Clarke, R. et al. Oxidized phospholipids on apolipoprotein B-100 versus plasminogen and risk of coronary heart disease in the PROCARDIS study. Atherosclerosis 354, 15–22 (2022).
Schnitzler, J. G. et al. Atherogenic lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ. Res. 126, 1346–1359 (2020).
Tsimikas, S. et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 41, 360–370 (2003).
Tsimikas, S. et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 47, 2219–2228 (2006).
Faghihnia, N., Tsimikas, S., Miller, E. R., Witztum, J. L. & Krauss, R. M. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J. Lipid Res. 51, 3324–3330 (2010).
Bertoia, M. L. et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J. Am. Coll. Cardiol. 61, 2169–2179 (2013).
Tsimikas, S. et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386, 1472–1483 (2015).
Viney, N. J. et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 388, 2239–2253 (2016).
Tsimikas, S. et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 382, 244–255 (2020).
Young, S. G., Witztum, J. L., Casal, D. C., Curtiss, L. K. & Bernstein, S. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis 6, 178–188 (1986).
Byun, Y. S. et al. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J. Am. Coll. Cardiol. 65, 1286–1295 (2015).
Byun, Y. S. et al. Oxidized phospholipids on apolipoprotein B-100 and recurrent ischemic events following stroke or transient ischemic attack. J. Am. Coll. Cardiol. 69, 147–158 (2017).
Young, S. G. et al. Definition of a nonlinear conformational epitope for the apolipoprotein B-100-specific monoclonal antibody, MB47. J. Lipid Res. 35, 399–407 (1994).
Van Berkel, T. J., De Rijke, Y. B. & Kruijt, J. K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J. Biol. Chem. 266, 2282–2289 (1991).
Holvoet, P. et al. Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure. Thromb. Haemost. 76, 663–669 (1996).
Tsouli, S. G. et al. Autoantibody titers against OxLDL are correlated with Achilles tendon thickness in patients with familial hypercholesterolemia. J. Lipid Res. 47, 2208–2214 (2006).
Wu, T. et al. Is plasma oxidized low-density lipoprotein, measured with the widely used antibody 4E6, an independent predictor of coronary heart disease among U.S. men and women? J. Am. Coll. Cardiol. 48, 973–979 (2006).
Tsimikas, S. et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation 109, 3164–3170 (2004).
Tsimikas, S. et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 110, 1406–1412 (2004).
Tsimikas, S. et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353, 46–57 (2005).
Tsimikas, S. et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation 119, 1711–1719 (2009).
Tsimikas, S. et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J. Am. Coll. Cardiol. 56, 946–955 (2010).
Tsimikas, S. et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 60, 2218–2229 (2012).
Tsimikas, S. et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J. Am. Coll. Cardiol. 63, 1724–1734 (2014).
Capoulade, R. et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66, 1236–1246 (2015).
Nsaibia, M. J. et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J. Intern. Med. 280, 509–517 (2016).
Kamstrup, P. R., Hung, M. Y., Witztum, J. L., Tsimikas, S. & Nordestgaard, B. G. Oxidized phospholipids and risk of calcific aortic valve disease: the Copenhagen General Population Study. Arterioscler. Thromb. Vasc. Biol. 37, 1570–1578 (2017).
Capoulade, R., Yeang, C., Chan, K. L., Pibarot, P. & Tsimikas, S. Association of mild to moderate aortic valve stenosis progression with higher lipoprotein(a) and oxidized phospholipid levels: secondary analysis of a randomized clinical trial. JAMA Cardiol. 3, 1212–1217 (2018).
Despres, A. A. et al. Lipoprotein(a), oxidized phospholipids, and aortic valve microcalcification assessed by 18F-sodium fluoride positron emission tomography and computed tomography. CJC Open. 1, 131–140 (2019).
Zheng, K. H. et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J. Am. Coll. Cardiol. 73, 2150–2162 (2019).
Capoulade, R. et al. ApoCIII-Lp(a) complexes in conjunction with Lp(a)-OxPL predict rapid progression of aortic stenosis. Heart 106, 738–745 (2020).
Girard, A. et al. Impact of C-reactive protein levels on lipoprotein(a)-associated aortic stenosis incidence and progression. Eur. Heart J. Open. 3, oead032 (2023).
Hoekstra, M. et al. Genome-wide association study highlights APOH as a novel locus for lipoprotein(a) levels – brief report. Arterioscler. Thromb. Vasc. Biol. 41, 458–464 (2021).
Wade, D. P. et al. 5′ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. PNAS 90, 1369–1373 (1993).
Boffelli, D., Zajchowski, D. A., Yang, Z. & Lawn, R. M. Estrogen modulation of apolipoprotein(a) expression. Identification of a regulatory element. J. Biol. Chem. 274, 15569–15574 (1999).
Shlipak, M. G. et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA 283, 1845–1852 (2000).
Mooser, V. & Hobbs, H. H. Lipoprotein(a) and growth hormone: is the puzzle solved? Eur. J. Endocrinol. 137, 450–452 (1997).
Enkhmaa, B. & Berglund, L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis 349, 53–62 (2022).
Fefer, P. et al. The role of oxidized phospholipids, lipoprotein (a) and biomarkers of oxidized lipoproteins in chronically occluded coronary arteries in sudden cardiac death and following successful percutaneous revascularization. Cardiovasc. Revasc. Med. 13, 11–19 (2012).
Gilliland, T. C. et al. Lipoprotein(a), oxidized phospholipids, and coronary artery disease severity and outcomes. J. Am. Coll. Cardiol. 81, 1780–1792 (2023).
Penny, W. F. et al. Improvement of coronary artery endothelial dysfunction with lipid-lowering therapy: heterogeneity of segmental response and correlation with plasma-oxidized low density lipoprotein. J. Am. Coll. Cardiol. 37, 766–774 (2001).
Wu, M. D. et al. Lipoprotein apheresis acutely reverses coronary microvascular dysfunction in patients with severe hypercholesterolemia. JACC Cardiovasc. Imaging 12, 1430–1440 (2019).
Ahmadi, N. et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am. J. Cardiol. 105, 459–466 (2010).
Rader, D. J. & Bajaj, A. Lipoprotein(a) and oxidized phospholipids: partners in crime or individual perpetrators in cardiovascular disease? J. Am. Coll. Cardiol. 81, 1793–1796 (2023).
Lee, S. R. et al. LPA gene, ethnicity, and cardiovascular events. Circulation 135, 251–263 (2017).
Arai, K. et al. The I4399M variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein B-100 particles. Atherosclerosis 209, 498–503 (2010).
Saleheen, D. et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol. 5, 524–533 (2017).
Fraley, A. E. et al. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J. Am. Coll. Cardiol. 53, 2186–2196 (2009).
Kiechl, S. et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27, 1788–1795 (2007).
Arai, K. et al. Acute impact of apheresis on oxidized phospholipids in patients with familial hypercholesterolemia. J. Lipid Res. 53, 1670–1678 (2012).
Ho, J. H. et al. Effect of bariatric surgery on plasma levels of oxidised phospholipids, biomarkers of oxidised LDL and lipoprotein(a). J. Clin. Lipidol. 15, 320–331 (2021).
Yeang, C. et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J. Clin. Lipidol. 10, 594–603 (2016).
Roeseler, E. et al. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of follow-up and apolipoprotein(a) characterization. Arterioscler. Thromb. Vasc. Biol. 36, 2019–2027 (2016).
Khan, T. Z. et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur. Heart J. 38, 1561–1569 (2017).
Konishi, K. et al. Advanced fibrosis of non-alcoholic steatohepatitis affects the significance of lipoprotein(a) as a cardiovascular risk factor. Atherosclerosis 299, 32–37 (2020).
Tsimikas, S., Gordts, P., Nora, C., Yeang, C. & Witztum, J. L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 41, 2275–2284 (2020).
Choi, S. H. et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma volume – Observations Reversal study. J. Am. Coll. Cardiol. 52, 24–32 (2008).
Ky, B. et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J. Am. Coll. Cardiol. 51, 1653–1662 (2008).
Rodenburg, J. et al. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J. Am. Coll. Cardiol. 47, 1803–1810 (2006).
Yoshida, H. et al. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis 226, 161–164 (2013).
Watts, G. F. et al. PCSK9 inhibition with alirocumab increases the catabolism of lipoprotein(a) particles in statin-treated patients with elevated lipoprotein(a). Metabolism 107, 154221 (2020).
Berg, K. et al. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin. Genet. 52, 254–261 (1997).
Zhu, L. et al. Effect of an increase in Lp(a) following statin therapy on cardiovascular prognosis in secondary prevention population of coronary artery disease. BMC Cardiovasc. Disord. 22, 474 (2022).
Bhatia, H. S. et al. PCSK9 inhibition and oxidized phospholipids. J. Am. Coll. Cardiol. 78, 1288–1289 (2021).
O’Donoghue, M. L. et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N. Engl. J. Med. 387, 1855–1864 (2022).
Tsimikas, S. et al. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 27, 175–181 (2007).
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004).
Erlinge, D. et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet 397, 985–995 (2021).
Wu, R., de Faire, U., Lemne, C., Witztum, J. L. & Frostegard, J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension 33, 53–59 (1999).
Segev, A., Strauss, B. H., Witztum, J. L., Lau, H. K. & Tsimikas, S. Relationship of a comprehensive panel of plasma oxidized low-density lipoprotein markers to angiographic restenosis in patients undergoing percutaneous coronary intervention for stable angina. Am. Heart J. 150, 1007–1014 (2005).
Silaste, M. L. et al. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a). Arterioscler. Thromb. Vasc. Biol. 24, 498–503 (2004).
Pechlaner, R. et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 35, 229–236 (2015).
Bossola, M. et al. Oxidized low-density lipoprotein biomarkers in patients with end-stage renal failure: acute effects of hemodialysis. Blood Purif. 25, 457–465 (2007).
Bossola, M., Tazza, L., Luciani, G., Tortorelli, A. & Tsimikas, S. OxPL/apoB, lipoprotein(a) and OxLDL biomarkers and cardiovascular disease in chronic hemodialysis patients. J. Nephrol. 24, 581–588 (2011).
Budoff, M. J. et al. Aged garlic extract supplemented with B vitamins, folic acid and l-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev. Med. 49, 101–107 (2009).
Holleboom, A. G. et al. Lipid oxidation in carriers of lecithin:cholesterol acyltransferase gene mutations. Arterioscler. Thromb. Vasc. Biol. 32, 3066–3075 (2012).
Wiesner, P. et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J. Lipid Res. 54, 1877–1883 (2013).
Hartikainen, J. et al. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur. Heart J. 38, 2547–2555 (2017).
Naka, K. K. et al. Interleukin-1 genotypes modulate the long-term effect of lipoprotein(a) on cardiovascular events: the Ioannina Study. J. Clin. Lipidol. 12, 338–347 (2018).
Kothari, H. et al. Association of D-dimer with plaque characteristics and plasma biomarkers of oxidation-specific epitopes in stable subjects with coronary artery disease. J. Cardiovasc. Transl. Res. 11, 221–229 (2018).
Mefford, M. T. et al. PCSK9 loss-of-function variants and Lp(a) phenotypes among black US adults. J. Lipid Res. 60, 1946–1952 (2019).
Acknowledgements
The authors thank X. Yang (UCSD, USA) and E. Miller (UCSD, USA) for expert technical assistance in the SCOR UCSD biomarker laboratory. The many members of the SCOR/PPG laboratory for the past 30 years and the hundreds of worldwide collaborators are greatly acknowledged for their contributions to the work discussed in this Review. The authors are supported by NIH R01 HL159156 and HL170224 (S.T.) and PO HL147835 (J.L.W.).
Author information
Authors and Affiliations
Contributions
S.T. wrote the manuscript. Both authors researched data for the article, provided substantial contributions to discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.T. and J.L.W. are co-inventors and receive royalties from patents owned by UCSD on oxidation-specific antibodies and on biomarkers related to oxidized lipoproteins, and are co-founders and have an equity interest in Kleanthi Diagnostics and Oxitope. The terms of this arrangement have been reviewed and approved by the UCSD in accordance with its conflict-of-interest policies. S.T. has a dual appointment at UCSD and Ionis Pharmaceuticals. J.L.W. is a consultant to Ionis Pharmaceuticals.
Peer review
Peer review information
Nature Reviews Cardiology thanks Jeffrey Kroon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsimikas, S., Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nat Rev Cardiol 21, 170–191 (2024). https://doi.org/10.1038/s41569-023-00937-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00937-4