Abstract
All coral species in the genus Acropora are broadcast-spawning hermaphrodites. Fertilization in the ocean requires sufficient numbers of gametes from conspecifics and the contact time for fertilization is thought to be limited by the rapid diffusion of sperm. Many studies have reported a positive correlation between sperm concentration and fertilization success, but it is not clear how gametes diffuse in seawater to produce mixtures of gametes from many colonies, leading to fertilization that improves genetic diversity. To elucidate this, we analyzed the changes in sperm concentration of A. tenuis in situ after spawning and genotyped sperm and fertilized eggs from seawater using seven microsatellite (MS) markers. Results showed that most of the eggs were fertilized at below 106 sperm/mL in situ. MS genotyping showed that the alleles of released sperm were diverse and those alleles also appeared in the fertilized eggs. The MS fragment peak height in released sperm, which presumably reflects the allele frequency of the sperm, was positively correlated with the allele frequencies of the fertilized eggs. Collectively, synchronous spawning populations composed of highly fecund and genetically diverse colonies potentially increases genetic diversity and the number of descendants.
Similar content being viewed by others
Introduction
Sexual reproduction produces genetic diversity in offspring, which enables the selection of genotypes associated with higher fitness and adaptation1,2,3. Reef-building corals in the genus Acropora release gametes into seawater synchronously and this broadcast spawning system gives rise to genetic diversity in their offspring; eggs of one colony can potentially mate with sperm from many other colonies.
Climate change and heat wave potentially impact on the reproduction of the coral Acropora. Coral reefs are now under threat4, and reef degradation from bleaching events has increased4,5,6. Although the remaining corals have a higher thermal threshold for bleaching7 and can participate in sexual reproduction, it is plausible that their mating success has declined. The importance of sexual reproduction is noticed, but knowledge of how the process from spawning to fertilization generates genetic diversity in nature (in situ) is still limited.
Many marine invertebrates are benthic or sessile, releasing their gametes into the water column and spawning synchronously to facilitate fertilization8,9. Spawning synchronism in marine invertebrates has been widely reported10,11,12,13, and synchronism is associated with fertilization success14.
In broadcast-spawning Acropora corals, fertilization success is associated with sperm concentration, which is affected by spawning synchrony, gamete number, and other factors. For example, sympatric Acropora species release their gamete bundles synchronously10, and in vitro experiments15,16 have shown that the fertilization rate depends on sperm concentrations, which in nature are expected to be associated with spawning synchrony17, water currents18, colony densities19,20, and the distance from the sperm source21. The timing of spawning and variation in water currents lead to high variation in sperm concentrations that affects the selection of fertilization-related traits. Although data on sperm concentrations in situ after spawning are limited, several studies have examined sperm concentrations after spawning in broadcast spawning corals22,23.
When examining sperm concentrations and fertilization success in Acropora corals in situ, it can be difficult to isolate one species. However, in Okinawa, Japan, Acropora tenuis spawns at sunset, about 2.5 h earlier than most other Acropora species24. Moreover, at Sesoko Island, Okinawa, sunset spawning is dominated by A. tenuis25, making it a good model for investigating the time course from spawning to fertilization.
In this study, we obtained in situ data for A. tenuis on the time course of sperm concentrations, the genetic diversity of released sperm, and the non-biased fertilization success of released gametes. We discuss the relationship between the genetic diversity of the fertilized eggs that leads to the next generation, and the amount and genetic diversity of the released gametes.
Materials and methods
Underwater observation of Acropora tenuis
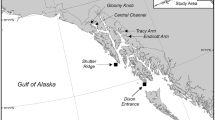
We observed spawning of the scleractinian coral A. tenuis (n = 11) on a section of the fringing reef of Sesoko Island, Okinawa, Japan (26° 37′ 43.9″ N 127° 51′ 43.3″ E), by snorkeling or SCUBA diving for six nights from May 26 to May 31, 2018. Floats were released from 4 of the 11 tagged colonies (ten15, ten21, ten27, and ten31). The area in which we monitored the floats was approximately 1 × 1 km2.
Sperm concentration in situ
To trace gamete bundles released from the tagged colonies, floats with fluorescent light bars (Fig. 1a) were released directly above the spawning colonies approximately 10 min after the colonies started spawning. We followed the floats by kayak, and collected 1 L seawater near each float at about 9 min intervals 4 to 6 times (during approximately 1 h) after the release of the floats (Fig. 1b). The collected seawater was brought back to Sesoko Station within 30 min, and the sperm concentrations were measured with a Thoma hemocytometer according to a previous study12. The sperm in the collected seawater was measured five times in 200 × 200 µm. When no sperm was found, the concentration was described as being below 2.5 × 104 sperm/mL. It took about 1 h from collecting the seawater to the start of sperm concentration measurement, and by this time most of the eggs in the seawater had already been fertilized.
Map of study site. (a) Floats used in this study had fluorescent light bars (ϕ 13.0 × 122 mm, Hapyson) attached. (b) Trajectory of the float measured in the daytime via GPS. The dot indicates the location of the spawning colony where the float was released. Asterisks indicate the location of water sampling at 9 min intervals.
Genetic diversity of spermatozoa in seawater and fertilized eggs
The genotypes of sperm and fertilized eggs were determined using microsatellite markers developed for Acropora26. Eggs in the collected seawater were transferred to fresh 0.22 µm filtered seawater, and fertilized eggs that had completed embryogenesis were fixed with 99.5% ethanol 3 days after collection. The remaining seawater was filtered and sperm were trapped on a membrane (mixed cellulose ester gridded at 0.45 μm, Merck Millipore, MA, USA). The membranes were soaked with 1 mL CHAOS solution (4 M guanidine thiocyanate, 0.1% [v/v] N-lauryl sarcosine sodium salt, 0.1 M β-mercaptoethanol, 10 mM Tris–HCl pH 8.0) and the DNA was extracted using a Wizard SV genomic DNA purification system (Promega, WI, USA). DNA was extracted from the fertilized eggs following a previous study with some modifications12. Fertilized eggs were kept in filtered seawater for 2 days and fixed in 99.5% EtOH. The fixed larvae were treated with 20 μL f lysis buffer (100 mM NaCl, 10 mM Tris–HCl [pH 8.0], 0.3% [w/v] Triton X-100, 0.3% [w/v] Tween 20) containing 1 g/mL proteinase K for 2.5 h at 55 °C and heated at 95 °C for 5 min. The supernatant was used for PCR reactions for genotyping. As a negative control for sperm detection in seawater, seawater was collected in the daytime following the same protocol. We sampled eight times at 9 min intervals after a float was released.
The sperm in seawater and fertilized eggs were genotyped with seven microsatellite markers with FAM or BIC (12406m3, 11543m5, 11401m4, 441m6, 11292m4, 10366m5, and 12130m5; Supplementary Information 1)26. The allele diversity of these seven markers was marked and the fragment amplification was stable. Subsequently, we used the seven markers to verify the presence of the alleles. Fragments were analyzed with a DNA sequencer (Applied Biosystems 3730xl or 3130xl) with GeneScan 500 LIZ dye size standard (Thermo Fisher, MA, USA). Peaks were measured with Microsatellite Analysis v1.0 software (Applied Biosystems by Thermo Fisher, MA, USA). For analyses of seawater containing sperm, peaks below 100 were excluded as alleles (Supplementary Fig. 1). The ratio of each peak in the samples (Rx) was calculated as Rx1 = (Hx1/H1 + H2 + ⋯ + Hx). Here, Hx1 indicates the height of one peak among the others, and H1 + ⋯ Hx is the sum of the heights of all peaks. In the negative seawater control, marker 12406m3 was used and three alleles (176, 179, and 185) were detected in one of the seven samples. These alleles were not detected in sperm in this study (153–174). Thus, the alleles detected in sperm from seawater were treated as those from the released sperm.
Statistical analyses
The Welch t-test was used to examine the differences in allele ratios. To test correlations between the peak height of MS alleles from sperm and the ratio of allele appearance in fertilized eggs, a GLMM was performed with the glmmML package in R ver. 3.127. Each allele was treated as a random effect and the binominal distribution was used as a family.
Results
Spawning of tagged colonies
In all, 10 of the 11 tagged A. tenuis colonies spawned on 29 May and 2 colonies spawned on 30 May. Although we did not record the spawning start times of all colonies, spawning occurred around 19:32 to 19:38 on 29 May and 19:33 to 19:37 on 30 May. Most A. tenuis colonies released > 1000 gamete bundles each.
In situ time course of sperm concentration and sperm genetic diversity
We followed the floats and collected seawater containing gametes. Most sperm concentrations in the collected seawater were < 105 sperm/mL (Fig. 2). We found no sperm in several samples although microsatellite analyses showed amplification of fragments in seawater in which no sperm were present (see below). The sperm concentrations varied over the course of sampling. Variation in sperm concentrations might have occurred because of differences between water currents and the movement of the floats, which might not represent precise water movements of the gametes from tagged colonies.
Time course of sperm concentration after the spawning in situ. Sperm concentrations were measured from seawater collected at 9 min intervals after the floats were released. Floats were released from the spawned colony approximately 10 min after they started releasing gametes. Water was collected for six times for Float 1 and four, five times for Float 2, and four times for Float 3.
Microsatellite genotyping showed that several alleles were amplified in each seawater sample. The numbers of alleles were indicated by the fragment lengths (bp). The allele numbers varied after spawning (Table 1, Supplementary Information 2). Although sperm were not found in many observations with the hemocytometer, microsatellite fragments were amplified from these samples and the eggs in these samples were fertilized. Most alleles found in sperm were detected in fertilized eggs (Table 1, alleles marked with *). The alleles detected only in seawater had lower MS fragment peak heights in the fragment analyses (Fig. 3).
Fragment peaks of alleles. The fragment peaks for each MS marker were compared. Alleles found in sperm/fertilized eggs (Larva/SW) or only in sperm (only SW) are plotted. *P < 0.0001 (Welch t-test, M4 t = 3.95, df = 91.8, M5 t = 4.88, df = 30.5, M7 t = 4.89, df = 47.9, M8 t = 8.39, df = 69.5, M9 t = 7.52, df = 45.0, M12 t = 3.22, df = 85.0, M13 t = 7.71, df = 34.1).
Genetic diversity of sperm and fertilized eggs in seawater
When genotyping the sperm samples, many alleles were detected for each marker, and the numbers varied by MS markers, sperm, and fertilized eggs (Table 1, Supplementary Information 2 and 3). We released floats from the spawning colonies to follow released gametes, but the alleles found in the collected seawater often did not match those of the spawned colony (Table alleles from tagged colonies are marked with †).
We examined 1 to 60 fertilized eggs for floats 1 to 3, but we could not analyze eggs found for float 4 due to loss of the samples. Many alleles were detected and most of these matched those in the same seawater sample. The alleles present changed over the sampling time (Table 1, Supplementary Information 3). Several alleles in sperm samples were not detected in the fertilized eggs (Table 1, alleles are not marked), and there were alleles that found only in the fertilized eggs (Table 1, alleles marked with ¶).
When comparing the fragment peaks of the alleles, MS fragment peak height was positively correlated with the frequency of the alleles in the fertilized eggs (Figs. 3 and 4, Supplementary Information 2 and 3). Several alleles were detected only in sperm samples and these had smaller peaks (Fig. 3, Supplementary Information 2 and 3). However, we could not distinguish which alleles in the fertilized eggs were derived from sperm or eggs. Alleles in the fertilized eggs were positively correlated with the peak heights in the fragment analyses (GLMM P < 0.001, coefficient = 6.9; Fig. 4).
Relationship between alleles in the fertilized eggs and sperm in situ. Ratio of the numbers of the each MS allele (e.g., 191) and those of all MS alleles (e.g., 191 and 197) in the fertilized eggs in each seawater sample (vertical line) (e.g., Allele 191 in Float1, third collection is 2/12 = 0.167 Supplementary Information 1). The horizontal line indicates the ratio of peak heights in fragment analyses of each seawater sample (e.g., ratio of allele 191 in Float 1, third collection was = 0.16). Alleles of each MS in this study are indicated by different symbols.
Discussion
The genetic diversity represented by allele frequencies in fertilized eggs was correlated with the genetic diversity of released sperm. After spawning, the sperm concentrations in situ varied and were often lower (< 105 sperm/mL) than the “ideal” level (106 sperm/mL)15,16, but fertilization was accomplished in situ. Microsatellite analyses showed that many alleles of the fertilized eggs matched those of sperm in situ. In addition, the frequencies of the alleles in fertilized eggs were positively correlated with MS fragment peak heights, which corresponds to the amount of sperm with those alleles. Therefore, mating success in reef-building Acropora depends on the amount of released sperm in situ, concurring with many previous in vitro fertilization trials; more sperm have greater chances of successfully fertilizing eggs.
The sperm concentration in situ was lower than we expected, but most eggs in the samples were fertilized. It took at least 1.5 h to examine egg fertilization. Many studies have indicated that fertilization in vitro decreases below 105 sperm/ML15,16,28. The sperm and eggs were kept in containers after collection, and so the condition for fertilization is not precisely represented in situ. The fertilization ratio is often dependent on the combination of colonies. For example, the fertilization rates of A. tenuis around Sesoko Station vary according to the combination of gametes from conspecific colonies; several combinations of colonies have very low fertilization rates25. While this needs to be examined, the alleles found in the fertilized eggs were correlated with the peak height of each allele, which is representative of sperm numbers.
Although the fertilization ratio in situ was potentially overestimated, fertilization might be accomplished soon after sperm and eggs are mixed29. Moreover, sperm–egg interactions due to chemoattractants may contribute to their successful fertilization at lower sperm concentrations30. If sperm can efficiently interact with unfertilized eggs with the assistance of chemoattractants and fertilization finishes quickly, fertilization with many different sperm from many colonies may occur, even at low sperm concentrations. However, we have no practical observations or information about gamete interactions, such as details of the release of bundles from colonies, bundles separated into sperm and eggs, and diffusion of these gametes.
The genetic inheritance of fertilized eggs, number of alleles, and their frequencies were positively correlated with sperm alleles found in the water. Most alleles found in the fertilized eggs were also found in the seawater containing sperm (Table 1, alleles marked with *). Presumably, most alleles found in both seawater and eggs represent the alleles in the fertilizing sperm, but several alleles were found only in the fertilized eggs (Table 1, alleles marked with ¶) and these alleles may be from both sperm and eggs. Although we could not distinguish the origin of alleles (sperm or eggs), the MS fragment peak heights of alleles found in seawater and fertilized eggs were slightly higher than those of alleles found only in seawater (Fig. 3), suggesting that the alleles found in more sperm in seawater completed fertilization in situ. There is almost no information on which alleles are passed to the next generation in corals.
Genetic inheritance in Acropora needs more study regarding the fertilization of gametes according to sperm concentration. In a previous study, eggs preferred conspecific sperm, while the proportion of fertilization by heterospecific sperm increased at lower sperm concentrations12. This implies that fertilization matching conspecific sperm and eggs is complicated and lower sperm concentrations might be associated with hybridization. In this study, we collected gametes in the ocean off Sesoko Island to follow the fertilization process in A. tenuis. Near this island, A. donei, which can potentially mate with A. tenuis, does not release a large number of eggs25. In addition, A. tenuis sperm does not fertilize eggs of A. donei in the presence of conspecifics24,25. Therefore, hybridization between A. tenuis and A. donei rarely occurs. We need to consider the breeding success of conspecifics or hybridization in later-spawning intercrossing species such as A. intermedia and A. florida. Fertilization in situ among intercrossing species may be associated with hybridization, which could be associated with adaptation to climate change31. Future studies need to address this.
References
Barton, N. Evolutionary biology. The geometry of adaptation. Nature 395, 751–752. https://doi.org/10.1038/27338 (1998).
Otto, S. P. & Lenormand, T. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 3, 252–261. https://doi.org/10.1038/nrg761 (2002).
Becks, L. & Agrawal, A. F. Higher rates of sex evolve in spatially heterogeneous environments. Nature 468, 89–92. https://doi.org/10.1038/nature09449 (2010).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. https://doi.org/10.1126/science.aan8048 (2018).
Thompson, D. M. & van Woesik, R. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc. Biol. Sci. 276, 2893–2901. https://doi.org/10.1098/rspb.2009.0591 (2009).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. https://doi.org/10.1126/science.1204794 (2011).
Sully, S., Burkepile, D. E., Donovan, M. K., Hodgson, G. & van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264. https://doi.org/10.1038/s41467-019-09238-2 (2019).
Yund, P. O. How severe is sperm limitation in natural populations of marine free-spawners?. Trends Ecol. Evol. 15, 10–13 (2000).
Levitan, D. R. & Petersen, C. Sperm limitation in the sea. Trend Ecol. Evol. 10, 228–231 (1995).
Baird, A., Guest, J. & Willis, B. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. https://doi.org/10.1146/Annurev.Ecolsys.110308.120220 (2009).
Wei, N. V. et al. Reproductive isolation among Acropora species (Scleractinia: Acroporidae) in a marginal coral assemblage. Zool. Stud. 51, 85–92 (2012).
Kitanobo, S., Isomura, N., Fukami, H., Iwao, K. & Morita, M. The reef-building coral Acropora conditionally hybridize under sperm limitation. Biol. Lett. 12, 20160511. https://doi.org/10.1098/rsbl.2016.0511 (2016).
Mercier, A. & Hamel, J.-F. Synchronized breeding events in sympatric marine invertebrates: Role of behavior and fine temporal windows in maintaining reproductive isolation. Behav. Ecol. Sociobiol. 64, 1749–1765 (2010).
Levitan, D. R. et al. Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58, 308–323 (2004).
Willis, B. L., Babcock, R. C., Harrison, P. L. & Wallace, C. C. Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16, S53–S65 (1997).
Nozawa, Y., Isomura, N. & Fukami, H. Influence of sperm dilution and gamete contact time on the fertilization rate of scleractinian corals. Coral Reefs 34, 1199–1206. https://doi.org/10.1007/s00338-015-1338-3 (2015).
Oliver, J. & Babcock, R. Aspects of the fertilization ecology of broadcast spawning corals: Sperm dilution effects and in situ measurements of fertilization. Biol. Bull. 183, 409–417. https://doi.org/10.2307/1542017 (1992).
Coma, R. & Lasker, H. R. Small-scale heterogeneity of fertilization success in a broadcast spawning octocoral. J. Exp. Mar. Biol. Ecol. 214, 107–120. https://doi.org/10.1016/S0022-0981(97)00017-8 (1997).
Teo, A. & Todd, P. A. Simulating the effects of colony density and intercolonial distance on fertilisation success in broadcast spawning scleractinian corals. Coral Reefs 37, 891–900. https://doi.org/10.1007/s00338-018-1715-9 (2018).
Marshall, D. J. In situ measures of spawning synchrony and fertilization success in an intertidal, free-spawning invertebrate. Mar. Ecol. Prog. Ser. 236, 113–119 (2002).
Babcock, R. C., Mundy, C. N. & Whitehead, D. Sperm diffusion-models and in-situ confirmation of long-distance fertilization in the free-spawning asteroid Acanthaster planci. Biol. Bull. 186, 17–28 (1994).
Omori, M., Fukami, H., Kobinata, H. & Hatta, M. Significant drop of fertilization of Acropora corals in 1999. An after-effect of heavy coral bleaching?. Limnol. Oceanogr. 46, 704–706. https://doi.org/10.4319/lo.2001.46.3.0704 (2001).
Levitan, D. R., Fogarty, N. D., Jara, J., Lotterhos, K. E. & Knowlton, N. Genetic, spatial, and temporal components of precise spawning synchrony in reef building corals of the Montastraea annularis species complex. Evolution 65, 1254–1270. https://doi.org/10.1111/j.1558-5646.2011.01235.x (2011).
Fukami, H., Omori, M., Shimoike, K., Hayashibara, T. & Hatta, M. Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Mar. Biol. 142, 679–684. https://doi.org/10.1007/S00227-002-1001-8 (2003).
Morita, M. et al. Reproductive strategies in the intercrossing corals Acropora donei and A. tenuis to prevent hybridization. Coral Reefs 38, 1211–1223. https://doi.org/10.1007/s00338-019-01839-z (2019).
Shinzato, C. et al. Development of novel, cross-species microsatellite markers for Acropora corals using next-generation sequencing technology. Front. Mar. Sci. 1, 11 (2014).
R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020).
Albright, R. & Mason, B. Projected near-future levels of temperature and pCO2 reduce coral fertilization success. PLoS One 8, e56468. https://doi.org/10.1371/journal.pone.0056468 (2013).
Iguchi, A., Morita, M., Nakajima, Y., Nishikawa, A. & Miller, D. In vitro fertilization efficiency in coral Acropora digitifera. Zygote 17, 225–227. https://doi.org/10.1017/S096719940900519X (2009).
Morita, M. et al. Eggs regulate sperm flagellar motility initiation, chemotaxis and inhibition in the coral Acropora digitifera, A. gemmifera and A. tenuis. J. Exp. Biol. 209, 4574–4579. https://doi.org/10.1242/jeb.02500 (2006).
Chan, W. Y., Hoffmann, A. A. & van Oppen, M. J. H. Hybridization as a conservation management tool. Conserv. Lett. https://doi.org/10.1111/conl.12652 (2019).
Acknowledgements
This study was supported by a JSPS KAKENHI Grant (#17K07414, 21H05304) to MM, 19J14375 and 21K15146 to SK. This work was partly supported by the Sasakawa Scientific Research Grant from The Japan Science Society (2021-4054) and Research Institute of Marine Invertebrates (KO2021-06) to SK. We thank Drs. N. Satoh and K. Nakashima at the Okinawa Institute of Science and Technology Graduate University for the usage of ABI sequencers. Dr. J.D. Reimer (University of the Ryukyus) is thanked for editing. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, see: http://www.textcheck.com/certificate/xHsfro.
Author information
Authors and Affiliations
Contributions
M.M. conceived and designed the experiments. S.K. and S.T. performed the experiments. M.M., S.K. wrote the paper. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitanobo, S., Toshino, S. & Morita, M. Genetic variation in released gametes produces genetic diversity in the offspring of the broadcast spawning coral Acropora tenuis. Sci Rep 12, 5026 (2022). https://doi.org/10.1038/s41598-022-08995-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08995-3
This article is cited by
-
Conservation of Protein Kinase A Substrates in the Cnidarian Coral Spermatozoa Among Animals and Their Molecular Evolution
Journal of Molecular Evolution (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.