Abstract
β-blocker therapy has been positively associated with improved survival in patients undergoing oncologic colorectal resection. This study investigates if the type of β-blocker used affects 90-day postoperative mortality following colon cancer surgery. The study was designed as a nationwide retrospective cohort study including all adult (≥ 18 years old) patients with ongoing β-blocker therapy who underwent elective and emergency colon cancer surgery in Sweden between January 1, 2007 and December 31, 2017. Patients were divided into four cohorts: metoprolol, atenolol, bisoprolol, and other beta-blockers. The primary outcome of interest was 90-day postoperative mortality. A Poisson regression model with robust standard errors was used, while adjusting for all clinically relevant variables, to determine the association between different β-blockers and 90-day postoperative mortality. A total of 9254 patients were included in the study. There was no clinically significant difference in crude 90-day postoperative mortality rate [n (%)] when comparing the four beta-blocker cohorts metoprolol, atenolol, bisoprolol and other beta-blockers. [97 (1.8%) vs. 28 (2.0%) vs. 29 (1.7%) vs. 11 (1.2%), p = 0.670]. This remained unchanged when adjusting for relevant covariates in the Poisson regression model. Compared to metoprolol, there was no statistically significant decrease in the risk of 90-day postoperative mortality with atenolol [adj. IRR (95% CI): 1.45 (0.89–2.37), p = 0.132], bisoprolol [adj. IRR (95% CI): 1.45 (0.89–2.37), p = 0.132], or other beta-blockers [adj. IRR (95% CI): 0.92 (0.46–1.85), p = 0.825]. In patients undergoing colon cancer surgery, the risk of 90-day postoperative mortality does not differ between the investigated types of β-adrenergic blocking agents.
Similar content being viewed by others
Introduction
Colorectal cancer is the second most common type of cancer in both men and women in Sweden1. The incidence of colorectal cancer has steadily increased in Sweden, partly due to a real increase but largely due to the fact that we are living longer and colorectal cancer affects older individuals in particular1,2. Although care associated with colorectal cancer has undergone major advancements over the past decades, including detection at earlier and more curable stages with screening programmes, improved diagnostic methods, and quality of treatment, colorectal cancer remains a major cause of morbidity and mortality3,4,5,6,7,8. It has been postulated that clinically important outcome benefits are gained by reducing the effects of surgery-induced hyperadrenergic activity in patients undergoing colon cancer surgery, through the use of β-blockade9.
In recent years, there has been considerable interest in the potential protective effects which preoperative beta-blockers may hold, since the immediate effect of beta-blockade is the blockage and mitigation of the sympathetic hyperactivity triggered by the trauma of surgery on several organs9,10,11,12. Results from two previously published studies concluded that preoperative beta-blocker use may be associated with a reduction in 30-day mortality following emergency and elective colonic cancer surgery9, and the same favorable effects were also seen after abdominal resection for rectal cancer11. In the previous large, nationwide, retrospective cohort study by Ahl et al. significantly fewer cardiovascular-related deaths were observed in patients treated with preoperative beta-blocker therapy (0.6% vs. 0.9%, p = 0.004), in spite of a higher prevalence of cardiovascular disease in this patient group9. On the other hand, in spite of these recent findings regarding the association between beta-blockers and reduced mortality, use of beta-blockers were not found to be associated with improved survival in the randomized controlled Perioperative Ischemic Evaluation (POISE) trial by Devereaux and colleagues. However, in the POISE trial the incidence of cardiovascular adverse events, such as i.e. cardiovascular death, non-fatal myocardial infarction and non-fatal cardiac arrest, were found to be reduced in their beta-blocked patient cohort13. Conflicting results in relation to mortality reduction similar to that of the POISE trial have also been reproduced in other studies13,14,15,16. This discrepancy could be attributed to the fact that these studies have tended to include several different surgical fields under the umbrella term ‘non-cardiac surgery’ i.e. orthopaedic, abdominal and vascular procedures which were included in the POISE trial. However, in the above large nationwide cohort study, only patients who underwent elective colon cancer surgery in Sweden over a 10 year period were included. The authors concluded that preoperative beta-blocker therapy is associated with considerable reductions in postoperative short-term and long-term mortality following elective colon cancer surgery9.
While all β-adrenoceptor blocking drugs share the common property of antagonizing the actions of the endogenous adrenergic agonists at the receptor level, there are however pharmacological differences between various agents17,18,19,20,21. For instance, in terms of pharmacokinetic variability (local anesthetic properties due to membrane stabilizing activity, K+-channel blocking activity, antioxidant properties, partial agonist activity, cardioselectivity) as well as pharmacodynamic differences, e.g. dose–response curves, drug-drug interactions, and in the presence of comorbidities17,18,19,20. This study aims to investigate and compare the effects of beta-blocker therapy on 90-day postoperative mortality risk following colon cancer surgery, based on the type of β-blocker administered. While there are pharmacological differences between the most commonly prescribed beta-blockers metoprolol, atenolol, bisoprolol and other beta-blockers, in terms of e.g. cardioselectivity and lipophilicity, it could be hypothesized that the clinically important effect is merely due to the attenuation of the hyperadrenergic response initiated due the trauma of surgery.
Methods
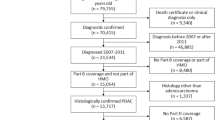
Ethical approval for the execution of the study was obtained from the Swedish Ethical Review Authority, Uppsala County, Sweden (reference: 2015/454 and 2015/454/1). Patient consent was waived and approved by the ethical review authority due to the retrospective nature of the study where only unidentified personal data was obtained and no changes to care were made. All patients ≥ 18 years old with ongoing β-blocker therapy that underwent colon cancer surgery between January 1, 2007 and December 31, 2017 were considered for inclusion. Patient data were retrieved from the Swedish Colorectal Cancer Registry (SCRCR)22 and the Swedish National Board of Health and Welfare National Patient Registry. Data on dispensed prescriptions of beta-blockers were collected from the Swedish Prescribed Drug Register (SPDR). Patients were considered to be receiving regular beta-blocker therapy if they filled a prescription for β-blockers within the year before surgery.
Patients with β-blocker therapy were subdivided into four groups according to the type of β-blocker used prior to surgery: metoprolol, atenolol, bisoprolol, and other β-blockers. The Pearson’s chi-squared test and Fisher’s exact test were used to determine the statistical significance of differences between categorical variables. For non-normally distributed continuous variables the Kruskal–Wallis test was applied in order to calculate the statistical significance of differences between the groups. The primary outcome of interest was 90-day postoperative mortality. A Poisson regression model with robust standard errors was employed to analyze the association between the type of β-blocker and risk of 90-day postoperative mortality risk. Adjustment for potential confounding in the model was performed by including the following covariates: age, sex, American Society of Anesthesiologists (ASA) classification, Charlson Comorbidity Index (CCI), cancer stage, surgical setting (acute versus elective presentation), type of surgical resection, and operative method (open versus laparoscopic). To compensate for missing data in the covariates in the regression model, multiple imputation by chained equations was applied. For binary variables logistic regression was implemented, for nominal variables a Bayesian polytomous regression model was used, and for ordinal variables a proportional odds model was applied. Analyses were performed using the statistical programming language R23.
Results
A total of 9254 patients met the specified inclusion criteria. Depicted in Tables 1 and 2 are patient demographics, comorbidity data, clinical characteristics, and surgical interventions in each beta-blocker group. Patients on bisoprolol were considered the least fit for surgery, with a larger proportion of the cohort having an ASA score of ≥ 3, followed by metoprolol, other β-blockers, and atenolol (ASA ≥ 3: 54.4% vs. 45.6% vs. 42.3% vs. 32.7%, P value < 0.001). In addition, patients administered bisoprolol suffered from the most comorbidities, followed by the metoprolol, other β-blocker, and atenolol cohorts (CCI ≥ 7: 34.2% vs. 28.1% vs. 24.6% vs. 19.4%, P value < 0.001). Most comorbidities were the most prevalent among bisoprolol users, with the exception for dementia, hemiplegia, liver disease, and cerebrovascular disease (Table 2). Furthermore, myocardial infarction, congestive heart failure, peripheral vascular disease, and chronic obstructive pulmonary disease were shown to be more prevalent in patients administered bisoprolol. Comparing the β-blocker cohorts’ characteristics, statistically significant differences were observed with regards to age, sex, ASA classification and CCI. No clinically significant differences were detected between the different beta-blocker cohorts regarding cancer stage, surgical setting, type of surgery, or operative method (Table 1).
There were no statistically significant differences regarding the crude 90-day postoperative mortality rate between the four cohorts of β-blocker therapy (Table 3). Table 4 shows the adjusted incidence rate ratio for 90-day postoperative mortality for the four cohorts of β-blockers. After adjustment for relevant covariates in the Poisson regression model, no difference in 90-day postoperative mortality risk could be detected. In comparison with metoprolol, there was no statistically significant change in the risk of 90-day postoperative mortality with atenolol [adj. IRR (95% CI): 1.45 (0.89–2.37), p = 0.132], bisoprolol [adj. IRR (95% CI): 0.83 (0.49–1.39), p = 0.490], or other β-blockers cohorts [adj. IRR (95% CI): 0.92 (0.46–1.85), p = 0.825]. However, there were statistically significant differences in risk of 90-day postoperative mortality in terms of ASA 1–2 (ref.) in comparison to ASA 3–5 [adj. IRR (95% CI): 2.62 (1.63–4.22), p = < 0.001] as well as in the surgical setting when comparing elective (ref.) with emergency surgery [adj. IRR (95% CI): 4.5 (3.05–6.65), p = < 0.001].
Ethics approval
Ethical approval was obtained from The Swedish Ethical Review Authority (reference number 2015/454 and 2015/454/1). The principles of the Declaration of Helsinki and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were complied with while conducting this study.
Discussion
The current study demonstrates that there is no difference in the risk of 90-day postoperative mortality after colon cancer surgery based on the type of β-blocker exposure. This observation may support the hypothesis that the positive and prophylactic effects associated with β-blocker use on postoperative mortality, following both elective and emergency colon cancer surgery, is attributable to the common property of β-adrenoceptor antagonism that these agents share. Thus, although they differ from one another in their additional pharmacological properties β-blockers may be considered a class of drugs with the ability to attenuate the effects of surgery-induced hyperadrenergic state.
All β-adrenoceptor blocking drugs share the common property of antagonizing the actions of the endogenous adrenergic agonists, norepinephrine and epinephrine, at the receptor level17,24. In addition, there are pharmacological differences between various agents in terms of pharmacokinetic variability (local anesthetic properties due to membrane stabilizing activity, K+- channel blocking activity, anti-oxidant properties, partial agonist activity, cardioselectivity) as well as pharmacodynamic differences, e.g. dose–response curves, drug-drug interactions, and presence of comorbidities17,18,19,20. Differences in the aromatic ring structure between various types of β-blockers causes variability in lipophilic characteristics, consequently impacting pharmacokinetic properties such as hepatic extraction ratio, protein binding, volume of distribution as well as the ratio of renal versus hepatic clearance21.
While β-blockers typically have been classified on the basis of their cardioselectivity, the beneficial effects of β-blockade may also be examined based on the degree to which they possess other pharmacological properties such as partial agonist properties, membrane stabilization and lipophilicity. In the multicenter, prospective, observational study by Ley et al. overall 30-day mortality was 15.8% with a significantly lower mortality observed in patients administered β-blockers following hospitalization after traumatic brain injury (13.8% vs. 17.7%, p = 0.013)25. Propranolol, a non-specific β-adrenergic receptor antagonist with ability to passively cross the blood–brain barrier due to its lipophilic nature, was observed to be associated with a lower mortality compared to other beta-blockers. Propranolol, metoprolol and nebivolol26,27 are all considered lipophilic agents primarily eliminated by hepatic metabolism, have shorter half-lives, and greater variations in plasma concentrations26,28. In comparison, more hydrophilic agents e.g. nadolol, sotalol and atenolol are subjected to excretion by the kidneys and thus dosage need to be adjusted with respect to renal function28. Furthermore, lipophilic beta-blockers might also improve cardiac vagal activity, which has been linked to mortality, after they cross the blood–brain barrier to reach the central nervous system26,29.
Third-generation β-blockers, such as nebivolol and carvedilol. are agents which unlike “classic β-blockers”, exhibit vasodilating activities by antagonizing α1-adrenoreceptors and activating β3-adrenergic receptors30,31, Additionally, third-generation β-blockers display anti-hypertrophic, anti-apoptotic, anti-proliferative, antioxidant and angiogenic properties still under examination31,32,33. Vasodilatory β-blockers provide benefits such as reduction of peripheral vascular resistance whilst maintaining or improving cardiac output, stroke volume, and left ventricular function34. On the contrary, β-blockers without vasodilating properties are inclined to increase peripheral vascular resistance, decrease cardiac output and reduce left ventricular function. Consequently, vasodilating β-blockers with favorable hemodynamic effects may prove more useful in decreasing central pressure and preventing cardiovascular events. Simultaneously as the second-generation agents, such as metoprolol, bisoprolol and atenolol, exhibit higher affinity binding to β1-receptors than β2-receptors35,36, the degree of selectivity are not definite and ranges widely between these agents26,37. Bisoprolol and nebivolol possesses the highest β1-selectivity38, in comparison to other β-blockers used in clinical practice such as e.g. atenolol and metoprolol which are both approximately fivefold selective for beta-1 versus beta-2-receptors39. Moreover, the extent of selectivity may be influenced by the magnitude of the dose37. In lower doses, β1-selective agents antagonize cardiac β1-receptors and have less effect on vascular smooth and bronchial muscles40. In contrast, administered at higher dosage (e.g. > 50 mg/d of metoprolol) even β1-selective agents also block β2-receptors, which imply that cardioselective beta-blockers may result in adverse effects on patients with obstructive airways disease26,40,41.
In the large nationwide retrospective cohort study by Ahl et al. preoperative beta-blockade was found to be associated with considerable reductions in short- and long-term postoperative mortality following elective colon cancer surgery. The study showed a 43% risk reduction in one-year all-cause mortality (adjusted HR 0.57, 95% CI 0.52 to 0.63, p < 0.001) in beta-blocker users9. Similarly, Ahl et al. demonstrated, in a cohort of 11,966 patients of whom 3513 were exposed to regular beta-blockers, a strong association between beta-blocker use and improved survival and morbidity rates following abdominal resection performed for rectal cancer11. Furthermore, the results of this study also indicate that mortality due to cardiovascular and respiratory illness, sepsis and multiorgan failure were significantly lower among beta-blocker users. In addition, beta-blocker therapy may also provide a protective effect against the development of postoperative complications after emergency colon cancer surgery, which was earlier demonstrated in another study on the topic by Ahl and colleagues42. The study revealed that there was a trend toward reduced overall incidence of major postoperative complications observed in beta-blocker positive patients (adjusted IRR 0.77, 95% CI 0.59–1.00, p = 0.055). Kwon et al. published, in their cohort of 8431 patients undergoing elective colorectal and bariatric surgical procedures of whom 23.5% were taking beta-blockers prior to surgery, that continuation of beta-blocker therapy on the day of, and after, surgery was associated with reduced 90-day mortality and fewer cardiac events43. In the current study, the finding that there was no significant difference in the crude risk of 90-day postoperative mortality with atenolol, bisoprolol, other beta-blockers, compared to metoprolol, remained unchanged even after adjustment for relevant covariates in the Poisson regression model. This indicates that beta-blocker therapy, irrespective of the agent used, may decrease mortality after elective and emergency colon cancer surgery.
The present study is strengthened by the use of information obtained from an externally validated, prospectively collected database with a > 99% national inclusion coverage of colon cancer patients in Sweden as well as a more homogenous surgical population treated with standardized surgery for a single type of cancer predominantly affecting the elderly population. Meanwhile, the retrospective design does result in limitations such as unknown confounders which are not controlled for, unknown indication for issued drug prescriptions, inability to determine whether favorable effects of β-blockade are attributable to pre-, peri- and/or postoperative administration of β -blockers, and lack of information on a strict daily β-blocker compliance during and after the hospitalization. It is unfortunate that the present study did not include a patient cohort without beta-blocker therapy. However, previous studies have been published on the outcomes after colorectal surgery between beta-blocker and non-beta-blocker users. The aim of the current study was to compare the 90-day postoperative mortality after colon cancer surgery based on the type of β-blocker explaining the absence of a reference group without beta-blocker therapy. Future research may overcome these limitations, through implementation of an interventional design with application of a randomized allocation procedure to prospectively investigate the effect of different types of β-blockers on the postoperative outcome.
Conclusion
This retrospective cohort study demonstrates that in patients undergoing elective and emergency colon cancer surgery, there was no statistically significant difference in the risk of 90-day postoperative mortality between common β-blockers.
References
Colorectal Cancer Report. A report from the Swedish Colorectal Cancer Registry aimed toward patients and the general public (Swedish) [Tarmcancerrapport 2019. En rapport från Svenska Kolorektalcancerregistret som riktar sig till patienter och allmänheten] Regional Cancer Centre North 2019. https://cancercentrum.se/globalassets/cancerdiagnoser/tjock-och-andtarm-anal/kvalitetsregister/tjock-och-andtarm-2020/patientrapport-2019-kolorektal.pdf. Last accessed 22 January 2021.
Colon Cancer. National quality report 2019 from the Swedish Colorectal Cancer Registry (Swedish) [Koloncancer 2019. Nationell kvalitetsrapport för år 2019 från Svenska Kolorektalcancerregistret] Regional Cancer Centre North 2019. Available at: https://cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/kvalitetsregister/tjock--och-andtarm-2020/kolonrapport-2019.pdf. Last accessed 21 January 2021) ’.
Dödlighet i tjock-och ändtarmscancer—Folkhälsomyndigheten. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/resultat/halsa/dodlighet-i-tjock-och-andtarmscancer/.
Rawla, P., Sunkara, T. & Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 14, 89–103 (2019).
Screening för tjock-och ändtarmscancer—Rekommendation och bedömningsunderlag. 20.
Lauby-Secretan, B., Vilahur, N., Bianchini, F., Guha, N. & Straif, K. The IARC perspective on colorectal cancer screening. N. Engl. J. Med. 378, 1734–1740 (2018).
Hultcrantz, R. et al. Nationella arbetsgruppen för utredning om införande av allmän tarmcancerscreening. 9.
The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017—The Lancet Gastroenterology & Hepatology. https://www.thelancet.com/journals/langas/article/PIIS2468-1253(19)30345-0/fulltext.
Ahl, R. et al. Effects of beta-blocker therapy on mortality after elective colon cancer surgery: A Swedish nationwide cohort study. BMJ Open 10, e036164 (2020).
Ahl, R. et al. Effect of beta-blocker therapy on early mortality after emergency colonic cancer surgery. BJS (Br. J. Surg.) 106, 477–483 (2019).
Ahl, R. et al. β-blockade in rectal cancer surgery: A simple measure of improving outcomes. Ann. Surg. 271, 140–146 (2020).
Ahl, R. et al. β-blocker after severe traumatic brain injury is associated with better long-term functional outcome: A matched case control study. Eur. J. Trauma Emerg. Surg. 43, 783–789 (2017).
POISE Study Group et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371, 1839–1847 (2008).
Blessberger, H. et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004476.pub2 (2014).
Friese, R. S., Barber, R., McBride, D., Bender, J. & Gentilello, L. M. Could beta blockade improve outcome after injury by modulating inflammatory profiles?. J. Trauma 64, 1061–1068 (2008).
Bangalore, S., Wetterslev, J., Pranesh, S., Sawhney, S. & Messerli, C. G. Perioperative beta blockers in patients having non-cardiac surgery: A meta-analysis. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet] (Centre for Reviews and Dissemination (UK), 2008).
Wood, A. J. Pharmacologic differences between beta blockers. Am. Heart J. 108, 1070–1077 (1984).
Ågesen, F. N., Weeke, P. E., Tfelt‐Hansen, P. & Tfelt‐Hansen, J. Pharmacokinetic variability of beta‐adrenergic blocking agents used in cardiology. Pharmacol Res Perspect 7, e00496 (2019).
Lemmer, B. Pharmacological basis for the therapy of cardiovascular disease with beta-adrenoceptor blocking drugs (author’s transl). Herz 7, 168–178 (1982).
Baker, J. G., Hill, S. J. & Summers, R. J. Evolution of β-blockers: From anti-anginal drugs to ligand-directed signalling. Trends Pharmacol. Sci. 32, 227–234 (2011).
Meier, J. Pharmacokinetic comparison of pindolol with other beta-adrenoceptor-blocking agents. Am. Heart J. 104, 364–373 (1982).
Swedish Colorectal Cancer Registry (SCRCR)—Nationella Kvalitetsregister [Internet]. [cited 2020 Nov 9]. https://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/swedishcolorectalcancerregistryscrcr.2156.html.
R Development Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2008. http://www.R-project.org/.
Prichard, B. N. & Tomlinson, B. The additional properties of beta adrenoceptor blocking drugs. J. Cardiovasc. Pharmacol. 8(Suppl 4), S1-15 (1986).
Ley, E. J. et al. Beta blockers in critically ill patients with traumatic brain injury: Results from a multicenter, prospective, observational American Association for the Surgery of Trauma study. J. Trauma Acute Care Surg. 84, 234–244 (2018).
Poirier, L. & Tobe, S. W. Contemporary use of β-blockers: Clinical relevance of subclassification. Can. J. Cardiol. 30, S9–S15 (2014).
Westerlund, A. Central nervous system side-effects with hydrophilic and lipophilic beta-blockers. Eur. J. Clin. Pharmacol. 28(Suppl), 73–76 (1985).
Borchard, U. Pharmacokinetics of beta-adrenoceptor blocking agents: Clinical significance of hepatic and/or renal clearance. Clin. Physiol. Biochem. 8(Suppl 2), 28–34 (1990).
Townend, J. N. & Littler, W. A. Cardiac vagal activity: A target for intervention in heart disease. The Lancet 345, 937–938 (1995).
Kalinowski, L. et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 107, 2747–2752 (2003).
do Vale, G. T., Ceron, C. S., Gonzaga, N. A., Simplicio, J. A. & Padovan, J. C. Three generations of β-blockers: History, class differences and clinical applicability. Curr. Hypertens. Rev. 15, 22–31 (2019).
Hess, M. L. & Varma, A. The third-generation beta-blocker: Have we found the elusive, effective antioxidant?. J. Cardiovasc. Pharmacol. 62, 443–444 (2013).
Vyssoulis, G. P. et al. The impact of third-generation Beta-blocker antihypertensive treatment on endothelial function and the prothrombotic state: Effects of smoking. Am. J. Hypertens. 17, 582–589 (2004).
Pedersen, M. E. & Cockcroft, J. R. The vasodilatory beta-blockers. Curr. Hypertens. Rep. 9, 269–277 (2007).
Tucker, W. D., Sankar, P. & Theetha Kariyanna, P. Selective beta-1-blockers. In StatPearls (StatPearls Publishing, 2021).
Ladage, D., Schwinger, R. H. G. & Brixius, K. Cardio-selective beta-blocker: Pharmacological evidence and their influence on exercise capacity. Cardiovasc. Ther. 31, 76–83 (2013).
Weber, M. A. The role of the new beta-blockers in treating cardiovascular disease. Am. J. Hypertens. 18, 169S-176S (2005).
Manrique, C., Giles, T. D., Ferdinand, K. C. & Sowers, J. R. Realities of newer beta-blockers for the management of hypertension. J. Clin. Hypertens. (Greenwich) 11, 369–375 (2009).
Schnabel, P. et al. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J. Cardiovasc. Pharmacol. 36, 466–471 (2000).
Frishman, W. H. & Saunders, E. β-Adrenergic blockers. J. Clin. Hypertens. (Greenwich) 13, 649–653 (2011).
McDevitt, D. G. Pharmacologic aspects of cardioselectivity in a beta-blocking drug. Am. J. Cardiol. 59, 10F-12F (1987).
Ahl, R. et al. The relationship between severe complications, beta-blocker therapy and long-term survival following emergency surgery for colon cancer. World J. Surg. 43, 2527–2535 (2019).
Kwon, S. et al. β-blocker continuation after noncardiac surgery: A report from the surgical care and outcomes assessment program. Arch. Surg. 147, 467–473 (2012).
Acknowledgements
I would like to express my sincere gratitude to my research supervisor and mentor, Dr. Shahin Mohseni, PhD, Associate Professor of Surgery, Senior Attending Surgeon, division of Trauma and Emergency Surgery, department of Surgery Örebro University Hospital, for giving me the opportunity to join his research group and providing invaluable guidance throughout this research in order to complete my Bachelor’s thesis. His enthusiasm, sincerity, curiosity and motivation has greatly inspired me to pursue not only my research interests, but to have the courage and determination to dare pursue unconventional ambitions as well as be bold enough to make the conscious effort required simply by working hard for my goals. I would like to acknowledge Dr. Maximilian Forssten for his statistical expertise and for him without any hesitance taking the time to share his knowledge as well as technical know-how. I want to give special thanks to Dr. Gabriel Sjölin for his friendly advice and support after I had first read the published studies which piqued my curiosity during the summer 2020 investigating the effects of beta-blockade on postoperative outcome as well as for being so kind to introduce me to his colleague Dr. Mohseni.
Funding
Open access funding provided by Örebro University.
Author information
Authors and Affiliations
Contributions
Study design: L.E., S.M. Data collection: G.S., S.M., M.P.F. Analysis and interpretation of data: L.E., M.P.F., S.M., P.M., R.A. Article draft: L.E., S.M., P.M., R.A. All authors have criticality reviewed and accepted the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ekestubbe, L., Bass, G.A., Forssten, M.P. et al. Pharmacological differences between beta-blockers and postoperative mortality following colon cancer surgery. Sci Rep 12, 5279 (2022). https://doi.org/10.1038/s41598-022-08736-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08736-6
This article is cited by
-
Beta-blocker adjunct therapy as a prospective anti-metastatic with cardio-oncologic regulation
Clinical & Experimental Metastasis (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.