Abstract
Most new cases and the highest mortality rates of breast cancer occur among middle-aged and old women. The recurrence rate of early-stage invasive ductal carcinoma (IDC) among women aged ≥ 50 years and receiving different treatments remains unclear. Therefore, this study was conducted to determine these rates. We used Surveillance, Epidemiology, and End Results (SEER) data for this nationwide population-based cohort study. All women aged ≥ 50 years and diagnosed with early-stage IDC between 2000 and 2015 were identified and divided into three treatment groups, namely, breast conservation therapy (BCT), mastectomy alone (MAS), and mastectomy with radiation therapy (MAS + RT). The recurrence rates of IDC among these groups were then compared. The BCT group had a lower short-term recurrence risk than the MAS and MAS + RT groups (hazard ratio [HR]: 1.00 vs. 2.90 [95% CI 1.36–2.66] vs. 2.07 [95% CI 0.97–4.44]); however, the BCT group also had a higher long-term recurrence risk than MAS and MAS + RT groups (HR 1.00 vs. 0.30 [95% CI 0.26–0.35] vs. 0.43 [95% CI 0.30–0.63]). The high long-term recurrence rate of the BCT group was especially prominent at the 10- and 15-year follow-ups. The results provide valuable evidence of the most reliable treatment strategy for this population. Further studies including more variables and validation in other countries are warranted to confirm our findings.
Similar content being viewed by others
Introduction
Breast cancer presents a great burden to public health and an important threat to women. According to breast cancer statistics in the United States, approximately 12% of all American women will develop invasive breast cancer in their lifetime1. Indeed, 276,480 new cases of female invasive breast cancer and 42,170 deaths from this disease are expected to occur in the country in 20201. Second only to that of lung cancer, the mortality rate of breast cancer is higher than the mortality rates of other types of cancer1. In Taiwan, breast cancer is the most common female cancer, and this cancer was third leading cause of female cancer-related deaths in 20192. The incidence rate of breast cancer is approximately 188–194 per 100,000 women2.
Breast cancer is more common in middle-aged and old women than in younger women, and most deaths are recorded in women aged ≥ 65 years3. In 2017, women aged > 50 years made up 81% of all new female cases of invasive breast cancer in the United States4. In Taiwan, 66.6% of the women diagnosed with breast cancer in 2017 were aged > 50 years5. Invasive ductal carcinoma (IDC) is the most common type of invasive breast cancer (80%); invasive lobular carcinoma (7%–15%) ranks a distant second in terms of invasiveness6. According to statistics in Taiwan, IDC comprises approximately 85.7% of all newly diagnosed breast cancer cases5. Therefore, the development of suitable treatment strategies, especially for early-stage IDC, is an important issue for the female population. Common treatments for early-stage IDC include (1) breast conservation therapy (BCT), which involves breast-conserving surgery (BCS) plus postsurgical radiation, (2) mastectomy alone (MAS), and (3) mastectomy with radiation therapy (MAS + RT)7.
A previous nationwide population-based study by the Surveillance, Epidemiology, and End Results (SEER) database revealed that women with early-stage IDC receiving BCT have better 5- and 10-year survival rates than those receiving MAS or MAS + RT7. However, this study included women aged ≥ 18 years, and the characteristics of this population may differ from those of middle-aged and old women. Few studies on recurrence rates following different treatments in middle-aged and old women with early-stage IDC have been published. Local recurrence is important to overall survival because local failure predicts distant metastasis in the future8. We conducted this nationwide population-based cohort study to assess the short- and long-term recurrence rates of early-stage IDC in middle-aged and old women following different treatments. Our hypothesis is that women receiving BCT will have a lower recurrence rate than those receiving MAS or MAS + RT.
Materials and methods
Data sources
We used SEER data reported by the National Cancer Institute for this study9. The SEER is a national population-based report of the most recent cancer incidence, prevalence, demographic characteristics, diagnosis time, tumor characteristics, surgery, RT, mortality, survival, and lifetime risk statistics in the United States10. It is published annually by the Surveillance Research Program of the National Cancer Institute in an effort to reduce the cancer burden among the United States population10. In the initial phase of the survey, seven registries (SEER 7) with epidemiologically significant population subgroups of racial and ethnic minorities were published. Since then, the database has been incrementally expanded to include 18 cancer registries (SEER 18)11. The SEER data can be applied for the analyses online.
Study design, setting, and participants
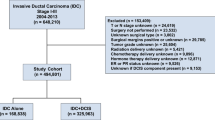
We used the SEER 18 database to conduct a nationwide population-based cohort study. Initially, all patients diagnosed with breast cancer as the primary cancer between 2000 and 2015 were identified (Fig. 1). The exclusion criteria were as follows: (1) male; (2) aged < 50 years; (3) ductal carcinoma in situ; (4) American Joint Committee on Cancer (AJCC) cancer staging was not T1-2, N0-1, or M0; (5) diagnosis was made only by autopsy or death certification; (6) survival < 1 month; (7) incomplete data (race, cancer stage, estrogen receptor [ER], progesterone receptor [PR], and marital status); (8) did not receive RT after BCS and did not receive MAS. Finally, middle-aged, and old women (age ≥ 50 years) diagnosed with early-stage IDC as the primary cancer between 2000 and 2015 were identified for the analyses. According to the AJCC, the definitions of early-stage IDC are as follows: (1) cancer stage: T1-2, N0-1, or M0; (2) positive lymph nodes ≤ 3 (patients with > 4 positive lymph nodes were excluded because RT is almost suggested in these patients); (3) tumor size < 5 cm12. Patients were divided into three treatment groups as follows: (1) BCT (BCS + RT), (2) MAS, and (3) MAS + RT.
Flowchart of this study. SEER, Surveillance, Epidemiology, and End Results; IDC, invasive ductal carcinoma; AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor; RT, radiotherapy; BCS, breast conservative surgery; BCT, breast conservative treatment (BCS + RT); MAS, mastectomy alone.
Definitions of variables and outcomes
Age was divided into the following subgroups: (1) 50–59 years, (2) 60–69 years, (3) 70–79 years, (4) 80–89 years, and (5) ≥ 90 years (Table 1). Race was classified as white, black, and others. Marital status was classified as married, never married, widowed, and others. Tumor size was classified as ≤ 2 cm, 2–3 cm, 3–4 cm, and 4–5 cm. Tumor grade was classified as I, II, III, and IV based on histological findings. Positive lymph node(s) was classified as 0, 1, 2, and 3. ER and PR status were classified as positive and negative.
The primary outcomes were short-term recurrence rate (< 1 year) and long-term recurrence rate (≥ 1 year). Recurrence times were tracked beginning on the day breast cancer was first diagnosed. Recurrence was defined as local tumor recurrence in the breast (after BCT), chest wall (after MAS), ipsilateral/parasternal/infra- or supraclavicular lymph nodes, and skin of the chest wall (not breast)13. Because the time of surgical resection of tumors is not available in the database we used, we choose to use the time of diagnosis as the beginning of recurrence-free survival according to previous study using the same database14.
Statistical analysis
For descriptive statistics, we used frequencies and percentages to represent categorical variables and means with standard deviations (SDs) to represent continuous variables. For inferential statistics, we used the chi-squared test to investigate associations between the three treatment groups and categorical variables in the demographic and clinical characteristics. One-way ANOVA (analysis of variance) was used to investigate associations between the three treatment groups and continuous variables in the demographic characteristics. Kaplan–Meier analysis and log-rank tests were used to compare differences in the recurrence curves of the three treatment groups. The Cox proportional hazard model was used to investigate predictors for recurrence. We used SAS 9.4 to obtain descriptive and inferential statistics and STATA SE13.0 to draw the recurrence curves. The significance level was set to 0.05 (two-tailed).
Ethics approval and consent to participate
This study protocol was approved by the Institutional Review Board of Kaohsiung Medical University (Approval No. KMUHIRB-EXEMPT(II)-20190018). Informed consent was waived because we used deidentified secondary data from the SEER. The waiver does not affect the rights and welfare of the participants.
Results
Overall, 184,964 patients were included in this study (Table 1). The BCT group included 132,510 patients (71.6%), the MAS group included 46,580 patients (25.2%), and the MAS + RT group included 5874 patients (3.2%). The mean age was 64.9 years, more patients were in the 60–69-year subgroup than in other subgroups, and patients in the MAS group tended to be older than those in other groups. Most patients were white (82.7%). Most of the patients were married (59.4%) or widowed (17.4%). The most common tumor size was ≤ 2 cm (74.7%), and most patients in the BCT group had tumors of this size (80.8%). In terms of tumor grade, grade II tumors were the most common (44.0%), followed by grade III tumors (30.7%). In terms of lymph node involvement, zero positive lymph nodes (78.5%) were the most common, especially in the BCT group (82.8%). The MAS + RT group had a higher percentage of three positive lymph nodes than the BCT and MAS groups. ER- and PR-positive tumors were present in 82.2% and 71.0%, respectively, of the total population and more common in the BCT group than in other groups.
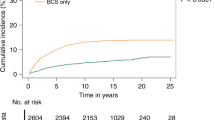
The BCT group had a lower short-term recurrence rate than the MAS and MAS + RT groups (0.07% vs. 0.14% vs. 0.14%; Table 2). Multivariate Cox regression analysis also showed that the BCT group has a lower short-term recurrence risk than the MAS and MAS + RT groups (hazard ratio [HR]: 1.00 vs. 2.90 [95% CI 1.36–2.66] vs. 2.07 [95% CI 0.97–4.44]; Table 3 and Fig. 2). By contrast, the BCT group had a higher long-term recurrence rate than the MAS and MAS + RT groups (1.2% vs. 0.4% vs. 0.5%) (Table 2). Multivariate Cox regression analysis showed that the BCT group has a higher long-term recurrence risk than the MAS and MAS + RT groups (HR 1.00 vs. 0.30 [95% CI 0.26–0.35] vs. 0.43 [95% CI 0.30–0.63]; Table 4 and Fig. 2). In addition, in the short-term recurrence analysis, only age 80–89 years was an independent predictor of recurrence (Table 3). Age ≥ 90 years, black race, tumor grade II, and PR-negative tumors reasonably predicted long-term recurrence (Table 4).
Subgroup analysis of short-term recurrence showed that the BCT group has a lower recurrence rate at 6 months (Supplementary Table 1 and Supplementary Fig. 1) than the other groups. Long-term recurrence analysis showed that the BCT group has a higher recurrence rate than the MAS and MAS + RT groups at the 10- and 15-year follow-ups (Supplementary Table 2 and Supplementary Fig. 2). The follow-up rates and numbers of subjects at risk at each follow-up time-points was showed in the Supplementary Table 3. The 1-yr, 3-yr, 5-yr cumulative recurrence rates and 95% CIs of three treatment groups was showed in the Supplementary Table 4. Competing risk analysis with adjustment for demographic and clinical characteristics revealed that the BCT group (reference) has a lower short-term recurrence risk than the MAS (HR 1.90, p < 0.001) and MAS + RT (HR 2.08, p = 0.048) groups. Moreover, the BCT group (reference) had a higher long-term recurrence risk than the MAS (HR 0.28, p < 0.001) and MAS + RT (HR 0.42, p < 0.001) groups. The proportionality assumption of Cox proportional hazard model was checked by plotting log minus log (log(−log(S(t))) vs. t) in the model. The parallelism in the plot indicates the proportionality assumption was satisfied (Supplementary Fig. 3).
Discussion
The present study showed that most breast cancer patients receive BCT, followed by MAS and MAS + RT. Patients who received BCT were more likely to be of white race and have a smaller tumor size, lower tumor grade, fewer positive lymph nodes, and larger number of ER- and PR-positive tumors than patients in other groups. Compared with the MAS and MAS + RT groups, the BCT group had a lower short-term recurrence risk but a higher long-term recurrence risk, especially at the 10 and 15-year follow-ups. Age ≥ 90 years, black race, tumor grade II, and PR-negative tumors were independent predictors for long-term recurrence.
We confirmed that the BCT group has a higher long-term recurrence risk than the MAS and MAS + RT groups; this result sheds some light on what a long-disputed issue in the literature has been. The possible explanations for the higher long-term recurrence risk in the BCT group are incomplete surgical removal of tumor cells of precancerous lesions, subclinical lesions, or malignant cells not eradicated by RT15,16. A previous study using the same database with this study showed that the BCT group has a higher long-term survival rate than MAS group and MAS + RT group7. However, the present study focused on middle-aged and old women, different from the study recruiting all women ≥ 18 years7. Another difference is that the present study was conducted to investigate recurrence, not survival. Therefore, using BCT for prevention of recurrence rate in middle-aged and old women with early-stage IDC should be more cautious. Further investigation about the different effect is warranted.
Risk of recurrence has a great influence on patients with breast cancer because this risk causes patients to live with a constant fear of death17. The reasons behind local recurrence remain largely unknown17, but the possible mechanisms include the existence of cancer stem cells and transformation of cancer cells into a relatively aggressive phenotype17. Cancer stem cells and transformed cancer cells are highly metastatic and resistant to conventional therapies17. A high percentage of aggressive cells is a feature of recurrent breast cancers17. Many clinical predictors for recurrence, including ER-negative, PR-negative, human epidermal growth factor receptor 2 (HER-2)-positive, triple-negative breast cancers, age, race, menopausal status, smoking, mammographic features, tumor morphology, tumor size, tumor stage, lymph node metastases, and gene expression profiling, have been proposed17,18.
Age ≥ 90 years, which has not been fully studied in the literature, was identified to be an independent predictor for long-term recurrence in the present study. A large population-based study in the Netherlands in 2020 reported that patients aged 75–79 years were at higher risk of distant recurrence than patients aged 70–74 years (subdistribution HR 1.25; 95% CI 1.11–1.41); however, age ≥ 80 years did not show this higher risk19. The authors attributed their findings to several reasons: (1) patients in the aged 75–79 years were undertreated, (2) the risk of death without recurrence increases with age, and (3) patients with a high competing mortality risk were overtreated19. Another population-based study in Germany in 2019 revealed that patients aged < 70 years have higher 5- and 10-year locoregional recurrence and distant metastasis rates than those aged ≥ 70 years (17% vs. 13%)20. More evidence is needed to clarify this finding. Black race was a risk factor for cancer recurrence in the present study, consistent with findings in previous studies18. Racial disparities may be due to socioeconomic factors and a more aggressive tumor biology among African–Americans18. Tumor grade was also associated with poor outcomes18. The present study revealed that tumor grade II is associated with long-term recurrence. While patients with tumor grades III and IV were at higher risk for long-term recurrence than those with tumor grade I, the difference between grades was not significant. PR-negative is a predictor for recurrence, and the results between the present and previous studies are consistent18. In general, breast cancers that are single hormone receptor-positive appear to have a poorer prognosis than those that are both ER- and PR-positive18. The present study also revealed a higher long-term recurrence risk in patients with ER-negative breast cancer than in those with ER-positive breast cancer; however, the difference was not significant (HR 1.13; 95% CI 0.97–1.33).
The major strengths of the present study include its nationwide population-based design, large sample size, and clear delineation of the knowledge gap in research on the recurrence rate of early-stage IDC in women aged ≥ 50 years. The limitations are as follows. First, the data were obtained from various institutions and may have bias in terms of treatment and quality. Second, because the present study conducts a secondary analysis of data, the results can only suggest associations between variables rather than causal relationships. Third, some variables, including genetic data, lymphovascular invasion, size of metastatic lymph nodes, resection margins, adjuvant therapies (e.g., chemotherapy and endocrine therapy), and HER2, were not considered in the present study because data on these variables were made available only after 2010. Fourth, because the data used for our analyses are from the United States, their generalization to other countries requires further validation. In the future, we plan to use the breast cancer database in Taiwan to validate the finding in this study.
Conclusion
This nationwide population-based cohort study revealed that, among middle-aged and old women with early-stage IDC, the BCT group has a lower short-term recurrence risk but a higher long-term recurrence risk than the MAS and MAS + RT groups, especially at the 10- and 15-year follow-ups. Using BCT should be cautious for its higher long-term recurrence in middle-aged and old women with early-stage IDC. The results fill the knowledge gap in research on the long- and short-term recurrence rates of IDC and provide valuable evidence of the most reliable treatment strategy for this population. Further studies, including more variables and validation in other countries, are warranted to confirm our findings.
Data availability
The data of SEER are publicly available. Please see the website https://seer.cancer.gov/archive/csr/1975_2014/.
Abbreviations
- IDC:
-
Invasive ductal carcinoma
- BCT:
-
Breast conservation therapy
- BCS:
-
Breast-conserving surgery
- MAS:
-
Mastectomy
- RT:
-
Radiation therapy
- SEER:
-
Surveillance, epidemiology, and end results
- AJCC:
-
American Joint Committee on Cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- HER-2:
-
Human epidermal growth factor receptor 2
References
BREASTCANCER.ORG. U.S. Breast Cancer Statistics. https://www.breastcancer.org/symptoms/understand_bc/statistics (2020).
Welfare, M. o. H. a. 2019 Taiwan death statistics. https://dep.mohw.gov.tw/DOS/cp-4927-54466-113.html (2020).
Shachar, S. S., Hurria, A. & Muss, H. B. Breast cancer in women older than 80 years. J. Oncol. Pract. 12, 123–132. https://doi.org/10.1200/JOP.2015.010207 (2016).
Society, A. C. Breast Cancer Facts & Figures. https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html (2020).
Health Promotion Adminstration, M. o. H. a. W. 2017 Cancer registry report. https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=12235 (2020).
Sharma, G. N., Dave, R., Sanadya, J., Sharma, P. & Sharma, K. K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 1, 109–126 (2010).
Agarwal, S., Pappas, L., Neumayer, L., Kokeny, K. & Agarwal, J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 149, 267–274. https://doi.org/10.1001/jamasurg.2013.3049 (2014).
Belkacemi, Y., Hanna, N. E., Besnard, C., Majdoul, S. & Gligorov, J. Local and regional breast cancer recurrences: Salvage therapy options in the new era of molecular subtypes. Front. Oncol. 8, 112. https://doi.org/10.3389/fonc.2018.00112 (2018).
Howlader, N. N. A., Krapcho, M., Miller, D., Bishop, K., Kosary, C. L, Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D. R., Chen, H. S., Feuer, E. J. & Cronin, K. A. (eds). SEER Cancer Statistics Review, 1975–2014. National Cancer Institute. https://seer.cancer.gov/archive/csr/1975_2014/ (2017).
Howlader, N. N. A., Krapcho, M., Miller, D., Brest, A., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D. R., Chen, H. S., Feuer, E. J. & Cronin, K. A. (eds). SEER Cancer Statistics Review, 1975–2017. National Cancer Institute. https://seer.cancer.gov/csr/1975_2017/ (2020).
Duggan, M. A., Anderson, W. F., Altekruse, S., Penberthy, L. & Sherman, M. E. The Surveillance, Epidemiology, and End Results (SEER) program and pathology: Toward strengthening the critical relationship. Am. J. Surg. Pathol. 40, e94–e102. https://doi.org/10.1097/PAS.0000000000000749 (2016).
Cancer, A. J. C. o. Breast Cancer Staging. https://cancerstaging.org/references-tools/quickreferences/Pages/default.aspx (2020).
Wapnir, I. L. et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J. Clin. Oncol. 24, 2028–2037. https://doi.org/10.1200/JCO.2005.04.3273 (2006).
Adambekov, S. et al. Survival and recurrence after intraperitoneal chemotherapy use: Retrospective review of ovarian cancer hospital registry data. Cancer Med. 9, 7388–7397. https://doi.org/10.1002/cam4.3340 (2020).
Jordan, R. M. & Oxenberg, J. in StatPearls (2021).
He, X. M. & Zou, D. H. The association of young age with local recurrence in women with early-stage breast cancer after breast-conserving therapy: A meta-analysis. Sci Rep. 7, 11058 (2017).
Ahmad, A. Pathways to breast cancer recurrence. ISRN Oncol. 2013, 290568. https://doi.org/10.1155/2013/290568 (2013).
Theodoros Foukakis, J. B. Prognostic and predictive factors in early, non-metastatic breast cancer. https://www-uptodate-com.lib.chimei.org.tw/contents/prognostic-and-predictive-factors-in-early-non-metastatic-breast-cancer?search=early%20breast%20cancer&topicRef=737&source=see_link#H1390695845 (2020).
de Boer, A. Z. et al. Impact of older age and comorbidity on locoregional and distant breast cancer recurrence: A large population-based study. Oncologist 25, e24–e30. https://doi.org/10.1634/theoncologist.2019-0412 (2020).
Holleczek, B., Stegmaier, C., Radosa, J. C., Solomayer, E. F. & Brenner, H. Risk of loco-regional recurrence and distant metastases of patients with invasive breast cancer up to ten years after diagnosis—Results from a registry-based study from Germany. BMC Cancer 19, 520. https://doi.org/10.1186/s12885-019-5710-5 (2019).
Acknowledgements
We thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative and funding support. We thank Enago for their English revision of our manuscript. The manuscript has been presented as Pre-print to Research Square as per the following source https://www.researchsquare.com/article/rs-80598/v1.
Funding
This study was supported by the Chi Mei Medical Center (Grant No. 108CM-KMU-05), the Ministry of Health and Welfare, Taiwan (MOHW110-TDU-B-212-144014, MOHW110-TDU-B-212-144020), and the Ministry of Science and Technology, Taiwan (MOST 110-2320-B-037-019). The funding agency was not involved in any aspect of the study design, including data collection, data interpretation, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
K.Y., Y.J.W., C.C.H., and S.F.W. designed the study and wrote the manuscript. S.F.W. performed the data analysis and wrote the manuscript. C.C.H., H.J.L., J.J.W., and Y.F.T. provided clinical experience and wrote the manuscript. C.C.H. and S.F.W. supervised the entire study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kao, Y., Wu, YJ., Hsu, CC. et al. Short- and long-term recurrence of early-stage invasive ductal carcinoma in middle-aged and old women with different treatments. Sci Rep 12, 4422 (2022). https://doi.org/10.1038/s41598-022-08328-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08328-4
This article is cited by
-
Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment
Medical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.