Abstract
Halyomorpha halys has been recognized as a global cross-border pest species. Along with well-established pheromone trapping approaches, there have been many attempts to utilize botanical odorant baits for field monitoring. Due to sensitivity, ecological friendliness, and cost-effectiveness for large-scale implementation, the selection of botanical volatiles as luring ingredients and/or synergists for H. halys is needed. In the current work, botanical volatiles were tested by olfactometer and electrophysiological tests. Results showed that linalool oxide was a potential candidate for application as a behavioral modifying chemical. It drove remarkable attractiveness toward H. halys adults in Y-tube assays, as well as eliciting robust electroantennographic responsiveness towards antennae. A computational pipeline was carried out to screen olfactory proteins related to the reception of linalool oxide. Simulated docking activities of four H. halys odorant receptors and two odorant binding proteins to linalool oxide and nerolidol were performed. Results showed that all tested olfactory genes were likely to be involved in plant volatile-sensing pathways, and they tuned broadly to tested components. The current work provides insights into the later development of field demonstration strategies using linalool oxide and its molecular targets.
Similar content being viewed by others
Introduction
We have been fighting against agricultural and forestry pest insects for centuries. Among them, invasive, cross-border species are drawing emerging attention along with the changing of global climate, agronomic changes, and human activities1. Due to lack of local natural enemies, invasive pest can easily spread and cause severe damage on crop/vegetation over the world2. Despite intensive management efforts, choices for monitoring and control are limited. Years of experience have resulted in several promising strategies such as biological control, transgenic variation, and more importantly, ecological approaches e.g., luring technology based on understanding of key volatile cues and chemical communications of target species3,4,5. Efforts to develop effective attractants have been huge, but the development of trap technology can result in more environmentally friendly management strategies and are worth the cost6,7.
The brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), is native to Eastern Asia and has spread globally with localized strains identified in North America and Europe. Recent occurrences of this species have been reported also in Oceania and South America8. Over 120 host plants from fruit crops, vegetables, ornamentals, shrubs, and forest trees were confirmed to be damaged by this polyphagous pest huge annual crop losses have been reported9. In order to conduct monitoring and control methods, aspects of chemical ecology have been well tackled for H. halys and its sibling species10,11,12. Utilizations of aggregation pheromones, alarm pheromones, attract-and-kill, push–pull strategies along with integrated management approaches are under development13,14,15,16,17. Meanwhile, a monitoring protocol for H. halys has matured since the two-component attractant was developed from Pentatomidae pheromone components7,9,18,19. Nevertheless, new studies are emerging to identify and improve synergism of additional chemicals with undergoing attractants, as well as to increase the cost-effectiveness of the baiting methods20.
Of the semiochemical research done on H. halys during 2019–2021 (Table S1), most studies (64.7%) explored using pheromone and synergist components of H. halys or other hemipteran species. The major focuses of such works were evaluating traps, densities, periods, pheromone recipes, trapping-based IPMs, etc.20,21,22,23,24,25,26,27,28,29,30,31. The second most tackled approach was physical interventions, including photoselective exclusion netting, and vibrational signals, which compiled 41.2% of recent works32,33. Host plants were evaluated in some works for direct trapping of H. halys (17.6%)22,26,34,35,36. Relatively few studies concerned botanical volatile attractant development (11.8%). However, some novel attractant and/or synergistic cues were identified in these works, including hexanal, (±)-α-pinene, (−)-sabinene, and others34,37. Host plant volatiles may expand the trapping ability of current lures, and they are usually good candidates for pest baiting by screening the key ingredients and the best combinations. To date, most host plant odorants are designed as synergists for already existing lure products, in order to increase luring efficiency and/or lower the costs for large scale implementations38,39,40. Attempts for improvements and optimizations of undergoing attractants are still needed for pest management41,42.
It is challenging to start from scratch to select natural products as attractants or agonists for pests43. One cost-effective strategy was to focus on available host cues which have been proved bioactive toward common insect species44. The chemical linalool oxide, which serves as a generalist plant-based lure, has been applied toward many insects45. As a tetrahydrofuran, linalool oxide was presented in various orchard fruits as a universal flavor within volatile blends46. This component was implemented as an odorant bait ingredient for different insect families including malaria vectors, lepidopteran adults, and beetles46,47,48. It could be a potential additive for odorant baiting in stink bugs, but has hardly been tested toward the family Pentatomidae. We have evaluated linalool oxide in a field trial, and this component exhibited equivalent attractiveness to H. halys compared to an odor bait mixture49. However, the reception of this volatile by H. halys olfaction was still elusive.
Chemical communications among H. halys, plants, and natural enemies have been studied, and a lot of background information has been reported in terms of the ecological and molecular basis of olfaction50,51,52. However, works need to be done on localization of the peripheral and central neural pathways in which key volatiles and proteins interact with each other. We have previously used a DREAM-like method (Deorphanization of receptors based on expression alterations in mRNA levels) to pre-screen odorant binding proteins (OBPs) for sensing E-2-decenal in H. halys53,54. Another strategy to evaluate candidate volatiles is to conduct homological alignments, so that tested spectra for both olfactory genes and ligand volatiles can be narrowed55. Benefiting from simulation technology, molecular docking has been widely used to assess bindings of olfactory proteins to botanical ligands in insects56,57. In summary, based on the continuously improving modeling database for insect receptor proteins, in silico methods have provided us with trial and error opportunities before lab tests58,59.
In the current work, a series of indoor olfactometer assays and electrophysiological tests were done to further select additional ingredients for improvements in bait recipes for H. halys. Homological selection and simulated molecular docking were carried out to locate potential H. halys olfactory receptors (HahlORs) and HhalOBPs for screened chemical components, in order to provide insights to deorphanization attempts in the future.
Materials and methods
Insects
Nymphs and adults of H. halys were obtained from laboratory colonies at the Institute of Plant Protection and Agro-products Safety, Anhui Academy of Agricultural Sciences, Hefei. They were continuously reared on a diet of organic green beans (Phaseolus vulgaris L.) and corn (Zea mays L.) in rearing cages (60 × 60 × 60 cm) at 25 ± 1 °C, 65 ± 5% RH and 16 L: 8 D photoperiod. Insects were fasted for 1–2 h prior to the tests.
Chemicals
Synthetic standard chemicals used within the study for bioassays and electrophysiological tests were commodities including n-hexane (95%, Sigma-Aldrich, St. Louis, MO, USA) as solvent, and linalool oxide (99%, Sigma-Aldrich, St. Louis, MO, USA), nerolidol (99%, Sigma-Aldrich, St. Louis, MO, USA), methyl (E,E,Z)-2,4,6-decatrienoate (95%, Sigma-Aldrich, St. Louis, MO, USA), and n-dodecane (99%, Sigma-Aldrich, St. Louis, MO, USA) as treatments.
Olfactometer assay
Olfactometer assays were done using a Y-tube system which was previously described17. Parameters for the Y-tube were: stem length at 30 cm, arm length at 20 cm, stem diameter at 3 cm, arm diameter at 2.5 cm, and arm angle at 90°. Airflow was constantly fixed at 0.5 L/min with purification and humidify done by successive connections to activated carbon and double distilled water. Laddered solutions were done by mixing standard chemicals with n-hexane solvent. The control arm was set by providing the same volume of n-hexane solvent to compare with each treatment. Single H. halys adult was introduced from the end of the stem tube and allowed to choose within 5 min during each trial. All tests were done during scotophase under infrared light at 25 ± 2 °C and 40–60 RH. A total of 30 replicates were done for each chemical at each dosage toward each gender.
Electroantennogram (EAG) recording
EAG was used to identify electrophysiological activities of adult H. halys to linalool oxide. Each H. halys antenna was prepared following standard procedures by cutting the tip and base of the antenna and immediately mounting the excised antenna between two ends of a recording probe (Ockenfels SYNTECH GmbH, Buchenbach, Germany). The other end of the recording probe was directly connected via an interface box to a signal acquisition interface board (IDAC 2; Ockenfels SYNTECH GmbH, Buchenbach, Germany). Stimulations were manually driven by a gas stimulator (CS-55, Ockenfels SYNTECH GmbH, Buchenbach, Germany). Linalool oxide was tested at dosages of 1 μg, 10 μg, and 100 μg, respectively, and n-hexane was used as control. Each stimulation record contained successive measurements of air–control–treatment–control–air. Continuous air flow was set at 150 mL/min, and stimulate flow velocity was 20 mL/min for 0.1 s.
n-Hexane (95%, Sigma-Aldrich, St. Louis, MO, USA) was used as control. One μg/μl (E)-2-decenal (95%, Sigma-Aldrich, St. Louis, MO, USA) and the measure dosage was 10 μg, and the measure order of antenna was air–n-hexane–(E)-2-decenal. The replication was 10, direct voltage was 2 mv, continuous flow velocity was 150 ml/min, stimulate flow velocity was 20 ml/min, stimulation time was 0.1 s, and stimulus intervals was 10 s. Raw data of voltages were transferred by: Relative response value (mV) = sample response value (mV)—control response value (mV) before statistical analysis was done.
Olfactory gene characterization
Gene families of OBPs and ORs of H. halys were collected from previous reported works53,60,61,62. Translated amino acid sequences (Dataset S1) of selected ORs from Halyomorpha halys, Apolygus lucorum, Sogatella furcifera, Cimex lectularius, Drosophila melanogaster, Bombyx mori, Mythimna separata, and Helicoverpa armigera were firstly aligned with MUSCLE and phylogenetic tree was developed using the Neighbor-Joining method63 in MEGA-X 10.1.8 software64 before formatted with FigTree v 1.4.465. Structural predictions were done using SWISS-MODEL (https://swissmodel.expasy.org, Basel, Switzerland). Amino acid alignment was done using PRALINE multiple sequence alignment (https://www.ibi.vu.nl/programs/pralinewww, Amsterdam, The Netherlands).
Simulated molecular docking
Docking studies were done to predict binding of selected H. halys olfactory proteins toward linalool oxide and nerolidol, respectively. All known 30 OBPs and 4 ORs of H. halys were SWISS-MODEL-ed and assessed by GMQE and QMEAN values. Specifically, the confidential interval for GMQE was set at 0–1, and QMEAN was set at [− 4, 0]. Higher GMQE values indicated more promising modeling, and lower QMEAN values indicated better binding possibilities of ligands and the selected proteins. For OBPs, a lower cutting threshold of 30% identity was used to initially screen from 30 proteins before docking was done.
Three-dimensional structures of tested volatiles were downloaded from Pubchem (https://pubchem.ncbi.nlm.nih.gov). Data were transferred to PDB formats via OpenBabel V3.066 before energies of ligand structures were minimized by Molecular Operating Environment (MOE; CCG ULC., Montreal, Canada). The docking algorithm was conducted by using AutoDockTools (ADT)67. Docking affinities of selected proteins to ligands were automatically evaluated by ADT. Docking results were visualized and exported as vector images by PyMol (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.) before edited in Adobe Illustrator CS6 software (Adobe, San Jose, CA, USA).
Statistics and data processing
Comparison of means was done using SPSS with GLM and Tukey HSD at α = 0.05. Counts data was compared with Chi-square test at α = 0.05. All statistics were carried out using IBM SPSS Statistics 22.0.0 (SPSS, Chicago, IL, USA). Bar and plot charts were developed using Prism 5 for Windows ver. 5.01 (GraphPad software, San Diego, CA, USA). Correlation matrix was developed with Statgraphics Centurion XVII (Statpoint Technologies, Inc., VA, USA).
Data accessibility
All described data in this work have been included in the manuscript and online supplementary materials (Table S1–S3, Dataset S1).
Results
Behavioral valence of H. halys to selected allelochemicals
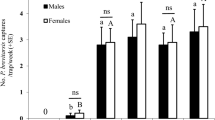
Within all four tested chemicals, linalool oxide elicited significant choice behaviors at the lowest dosages (Fig. 1). Both female and male adults of H. halys chose linalool oxide in the Y-tube assays when the chemical was applied at 1 µg and 10 µg. While higher dosages of 100 µg drove contrary choice results in both genders, reflecting the potential repellency role of linalool oxide at high dosages. Other tested chemicals did not drive observable choice behaviors until they were applied at 40 µg dosages. Furthermore, linalool oxide at 10 μg and nerolidol at 40 μg stimulated significant gender-biased behavioral preferences, showing that female H. halys adults were more sensitive than males under these tested dosages (Fig. 1). In sum, H. halys adults are most sensitive toward linalool oxide within all tested chemicals.
Behavioral valence of H. halys to various tested volatiles. Results of Y-tube assays with H. halys by applied selected botanical and pheromone-related chemicals. Asterisk indicates significant choice preferences of H. halys to either tested chemicals or solvent control (Chi-square test, P < 0.05, n = 30 for each gender at each dosage toward each treatment). Hashtag indicates significant choice preferences between genders to the same dosage treatment (Chi-square test, P = 0.0285 for linalool oxide at 10 μg and P = 0.0195 for nerolidol at 40 μg).
Electrophysiological responses of H. halys antennae toward linalool oxide
The resolutions of EAG tests were lower for linalool oxide compared with the behavioral assays (Fig. 2A). Female antennae showed responsiveness to linalool oxide at 10 µg dosages and the responding level increased dramatically along with the increase in dosage. On the other hand, male antennae only started to respond to linalool oxide when applied at 100 µg (Fig. 2A). Overall, gender bias was observed in the EAG assays, as also shown in the Y-tube assays that females were more sensitive than male H. halys adults (Figs. 1, 2A).
Olfactory evidences of H. halys in sensing linalool oxide. (A) Results of electroantennogram tests with linalool oxide. Lower-case letters indicate significant differences among tested dosages in either male or female adults. (GLM and Tukey HSD multiple comparison. P < 0.05. Error bars indicate ± s.e.m.) (B) Phylogenetic analysis of putative linalool sensing odorant receptors of H. halys by referring to ORs from A. lucorum, S. furcifera, C. lectularius, D. melanogaster, B. mori, M. separata, and H. armigera. The evolutionary history was inferred by using the Maximum Likelihood method and the JTT matrix-based model. The tree with the highest log likelihood (− 2,980,075.06) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, and then selecting the topology with superior log likelihood value. This analysis involved 374 amino acid sequences. There were a total of 1344 positions in the final dataset. Evolutionary analyses were conducted in MEGA X. (C) Structural predictions of four putative linalool sensing ORs in H. halys, with modeling re-constructed referring to known Cryo-EM structures of insect ORco (Apocrypta bakeri ORco: 6c70.1.A) Schematic shows representative 7-TMD structure of insect OR. All four ORs showed 7-TMD structures and potentially tetramer binding activities. Predictions were done with SWISS-MODEL. (D) Alignment of the four H. halys ORs with referring to most related linalool sensing ORs in Drosophila and cotton bollworm. Conservations of amino acid residues were indicated with colors.
Characterization of putative odorant receptors for linalool oxide
A total of four putative receptors for linalool oxide were screened by BLASTP against known linalool oxide sensing ORs in Drosophila and H. armigera. Reference ORs were HarmOr12, DmelOr19a, DmelOr69aB, DmelOr69aA, DmelOr98a, and DmelOr9a, which, were reported to have tuned to ( ±)-linalool and/or linalool oxide stimuli68,69. The selected HhalOR24a, HhalOr45b, HhalOr82a, and HhalOr4-like were separated into four major clusters. Among them, HhalOr82a was more similar with lepidopteran ORs other than hemipteran ones (Fig. 2B). Structural predictions of these four proteins all showed a representative 7-TMD structure of insect ORs (Fig. 2C), and they were able to form the tetramer structure which was proved to be the functional basis of ORs. When the four ORs were aligned with reference ORs, it showed that they were not conserved in the N– part. However, much more conservation was observed during the C– end (Fig. 2D). Since all referred insect ORs to linalool oxide were broadly tuning ORs toward plant odorants, we carried out docking simulations using the four HhalORs in order to investigate H. halys attractiveness to this botanical volatile at the molecular level.
Simulated docking to linalool oxide and nerolidol
Simulated docking studies were conducted using HhalOR4-like, HhalOR24a, HhalOR45b, and HhalOR82a, comparing with the parameters of HarmOR12 (Fig. 3A–F, Table S2). The tested ligands were the tetrahydrofuran linalool oxide and the sesquiterpene alcohol nerolidol (Fig. 3A). The H. armigera OR12 was checked for binding affinity to linalool oxide by stimulated docking as reference. It showed that binding energies for HarmOr12 with nerolidol was − 4.25, and it was − 3.26 with linalool-oxide (Fig. 3B, Table S 2). Similar results were observed for HhalOR24a, HhalOR45b, and HhalOR82a, which presented lower binding energies for nerolidol than for linalool-oxide (Fig. 3D–F, Table S 2). While HhalOR4-like exhibited better predicted binding affinity with linalool-oxide (− 3.22) than nerolidol (− 2.97) (Fig. 3C). Among, HhalOR82a was predicted to be the best matched receptor for nerolidol with binding energy at − 4.49. For linalool-oxide, the best predicted receptors were HhalOR4-like and HhalOR82a, both with a binding energy at − 3.22 (Table S 2).
Molecular docking studies of H. halys olfactory proteins to linalool oxide and nerolidol. (A) Chemical structures of linalool oxide (left) and nerolidol (right). (B) Schematics showing docking poses of HarmOR12 binding with linalool oxide (left) and nerolidol (right), respectively. (C–F) Schematics showing docking poses of HhalOR4-like (C), HhalOR24a (D), HhalOR45b (E), and HhalOR82a (F) binding with linalool oxide (up) and nerolidol (down), respectively. (G) Sequence alignment between HhalOBP8, HhalOBP30, and CpalOBP4 (Chrysopa pallens OBP4: 6jpm.1.A). Conservations of amino acid residues were indicated with colors. Identities of HhalOBP8 with CpalOBP4 was 36.97%, and for HhalOBP30 and CpalOBP4 it was 33.04%. (H–I) Schematics showing docking poses of HhalOBP8 (H) and HhalOBP30 (I) binding with linalool oxide (left) and nerolidol (right), respectively.
In order to draw a promising conclusion, investigations on OBPs were also done with similar protocols and algorithms. However, among 30 HhalOBPs, we only identified two qualified OBPs namely HhalOBP8 and HhalOBP30 which had > 30% identities with reference model (Chrysopa pallens OBP4: 6jpm.1.A; CpalOBP4). Both HhalOBP8 and HhalOBP30 were typical 6-C OBPs as CpalOBP4, and the identities for comparing with CpalOBP4 were 36.97% for HhalOBP8 and 33.04% for HhalOBP30, respectively (Fig. 3G). We have observed that binding energies for HhalOBP8 with nerolidol was − 4.81, and it was − 3.71 for HhalOBP30 with nerolidol. For another ligand linalool oxide, simulated binding energies were − 3.17 with HhalOBP8 and HhalOBP30, respectively (Fig. 3H,I, Table S3). Due to the results, it was suggested that both HhalOBP8 and HhalOBP30 could be general OBPs which were involved in plant odorant sensation. HhalOBP8 had better binding potential to nerolidol than HhalOBP30 did.
Discussion
Shelter plants of insect pests can exhibit various volatile compounds68. In the current study, we revisited a series of botanical ingredients, and revealed attractiveness of linalool oxide under mid dosages during bioassays and electrophysiological tests toward H. halys adults, especially females. Higher dosages of linalool oxide and nerolidol repelled the adults from the odorant sources. As a common additional ingredient in bait formulations for moths, linalool oxide was assessed by simulated molecular docking with phylogenetically selected H. halys ORs and OBPs. The computational results showed that similar binding affinities were found for HarmOR12 reference and four H. halys ORs including HhalOR4-like, HhalOR24a, HhalOR45b, and HhalOR82a. Furthermore, HhalOBP8 and HhalOBP30 out of 30 HhalOBPs showed moderate binding potentials to linalool oxide and nerolidol. These six olfactory genes should be prioritized for further functional tests in order to identify relevant reception basis for sensing linalool oxide and nerolidol in H. halys.
Utilization of hemipteran olfaction
Pheromone lures have been one of the most popular monitoring methods for H. halys and other Pentatomidae around the globe70. Many aggregation pheromones of this family share the bisabolene backbone including the H. halys which employ a two-component recipe of 3S6S7R10S-murgantiol and 3R6S7R10S-murgantiol7. This recipe was improved later by adding synergists such as methyl (2E,4E,6Z)-2,4,6-decatrienoate (MDT)20,71. The downside of such practices was that they represented species-specific biomarker volatiles that enhance aggregation and retention of H. halys, and this may result in heavier crop damage at the implementation site72. As this species of stink bug had high sensitivity toward certain botanical volatile resources, cost-effective alternatives/additives of the H. halys odor bait may be developed from host plant emissions34,37. Nevertheless, most of the trapping works for H. halys have not considered plant volatiles for olfactory perception. Compared to other Hemiptera to which molecular regulations based on olfaction have been applied, works for H. halys are relatively rare62,73,74. Future studies may involve identifications of key host cues, their molecular targets, and reliable implementation methods, e.g., the RNA interference approach75. Furthermore, the mechanism by which key volatile signals are coded through central olfactory systems of this stink bug is still unknown and a fascinating area to explore.
Linalool oxide as additional ingredient for odorant bait
The tetrahydrofuran linalool oxide was reported to have enriched botanical bait formulations, and originated from plant volatiles/essence76,77. Lures containing linalool oxide have been widely applied for trapping insects including moths, mosquitoes, and beetles47,48,78. As a common emission from natural botanical products, linalool oxide was found to be related to plant injury77,78. The secondary metabolite role of linalool oxide has implied its potential functions in plant defense79. In fact, this component has also been used as a control reagent for houseflies and coffee bugs80,81. For H. halys and other stink bugs, attraction by linalool oxide has not yet been reported. Literature has shown that this species is behaviorally modified by plant essential oils, which have presented this chemical in mixed volatile blends12. Some works have reported that selected key ingredients of volatiles may work better than a full spectrum of plant volatile blend82. It may support that linalool oxide has the potential to be a vital addition for optimization of current commercial luring recipes for H. halys as this chemical has outperformed other tested components in the Y-tube assays. The results from the current study have raised the possibility that linalool oxide can be used as an ecological insect behavioral modifying chemical in stink bugs, as is revealed in lepidopteran and dipteran species. Future field trials and implementation of fully-established botanical blend recipes for testing the final effectiveness of artificial baiting approaches for H. halys could benefit from screening on more terpenoids, tetrahydrofurans, esters, and aromatics.
Narrowed-down spectra for receptor protein deorphanization
One important move in the field of chemical ecology is to identify functional genes (mostly receptors) for understanding the olfactory reception of selected bioactive odorants, or “deorphanization”55. The so-called reversed chemical ecology sought to solve this matter by providing peripheral coding information for later sorting signaling from brain innervated patterns in higher neuropils of insects83. However, most of the volatile ligands did not activate all receptors in a species. A sensing spectrum provided by an OR limits the firing pattern of the corresponding odorant sensory neuron and thus influenced behavioral outputs of insects84. All in all, if a previous selection could be done, it would not always be necessary to functionally demonstrate all existing receptor genes against a known volatile ligand. Because thousands of candidate plant volatiles would generate endless research, and we would like our hypothesis tested swiftly and accurately by saving time, labor, and cost. Fortunately, there are several approaches for narrowing down candidate spectra of ORs and OBPs ready for deorphanization. For example, DREAM technology was developed in Mammalia and Drosophila and was reported to have worked in stink bugs53,85,86, and we successfully utilized this method to allocate pheromone OBPs in H. halys54. Benefiting from abundant gene annotations, another straight-forward method was to conduct homology alignment combined with molecular docking simulation. Works have shown that docking results could fit well with functional tests. Thus, a total of two HhalOBPs and 4 HhalORs were highlighted using this approach from the current research, and can become vital candidates for testing binding and reception of linalool oxide as well as nerolidol in further studies.
Combinatorial coding by multiple-receptor decision toward allelochemicals
It was intriguing that linalool oxide attracted H. halys with lower to middle range dosages while repelling them when applied at a high dosage. This phenomenon was identified in Drosophila, which employed a sensory switch to drive contrary behaviors in sour and salt reception87,88. It is possible that linalool oxide was coded via a combinatorial pathway through the olfactory systems in H. halys, as it has been shown in the vinegar fly. As a common botanical volatile component, linalool oxide was not likely to be involved in the labeled line circuits, which were mostly used by insects to decide life-and-death issues89. While higher concentrations in the air of linalool oxide could mean damage already done to the plants77, and this repellent modality may help this species to balance their populations within the distributed areas78,90. This potential ecological significance of dose may be useful for development of linalool oxide- and nerolidol-based push–pull strategies in a more precise way. On the other side, multiple pathways decision system by the insects’ olfaction involved several ORs and glomeruli in the neuropil91,92. In this work, selected OBPs and ORs shared similar docking affinities with linalool oxide and nerolidol ligands, which also implied that the olfactory reception pathways for these components involved more than one receptor to be functionally operated69. Furthermore, tuning spectra for linalool oxide of the H. halys ORs may also be broad, as it has been shown in docking simulation results, indicating that additional botanical behavioral modification chemicals may exist.
References
Paini, D. R. et al. Global threat to agriculture from invasive species. Proc. Nat. Acad. Sci. USA 113, 7575–7579. https://doi.org/10.1073/pnas.1602205113 (2016).
Sakai, A. K. et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037 (2001).
Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Annu. Rev. Entomol. 43, 243–270. https://doi.org/10.1146/annurev.ento.43.1.243 (1998).
Dent, D. & Binks, R. H. Insect Pest Management 377 (CABI, 2020). https://doi.org/10.1079/9780851993409.0000.
Leftwich, P. T. et al. Genetic pest management and the background genetics of release strains. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 376, 20190805. https://doi.org/10.1098/rstb.2019.0805 (2021).
El-Sayed, A., Suckling, D., Wearing, C. & Byers, J. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 99, 1550–1564. https://doi.org/10.1603/0022-0493-99.5.1550 (2006).
Khrimian, A., Zhang, A. J., Weberet, D. C., Ho, H. Y. & Jeffrey,. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 77, 1708–1717. https://doi.org/10.1021/np5003753 (2014).
EPPO. EPPO Global database. https://gd.eppo.int/taxon/HALYHA/distribution (2020).
Lee, D. H., Short, B. D., Joseph, S. V., Bergh, J. C. & Leskey, T. C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 42, 627–641. https://doi.org/10.1603/EN13006 (2013).
Khrimian, A. The geometric isomers of methyl-2,4,6-decatrienoate, including pheromones of at least two species of stink bugs. Tetrahedron 61, 3651–3657. https://doi.org/10.1016/j.tet.2005.02.032 (2005).
Aldrich, J., Khrimian, A., Chen, X. & Camp, M. J. Semiochemically based monitoring of the invasion of the brown marmorated stink bug and unexpected attraction of the native green stink bug (Heteroptera: Pentatomidae) in Maryland. Fla. Entomol. 92, 483–491. https://doi.org/10.1653/024.092.0310 (2009).
Weber, D. C. et al. Chemical ecology of Halyomorpha halys: Discoveries and applications. J. Pest Sci. 90, 989–1008. https://doi.org/10.1007/s10340-017-0876-6 (2017).
Schoonhoven, L. M., van Loon, J. J. A. & Dicke, M. Insect–Plant Biology Vol. 421 (Oxford University Press, 2005).
Noge, K., Prudic, K. L. & Becerra, J. X. Defensive roles of (E)-2-alkenals and related compounds in Heteroptera. J. Chem. Ecol. 38, 1050–1056. https://doi.org/10.1007/s10886-012-0166-y (2012).
Zhang, Q. H. et al. Essential oils as spatial repellents for the brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). J. Appl. Entomol. 138, 490–499. https://doi.org/10.1111/jen.12101 (2014).
Tognon, R. et al. Volatiles mediating parasitism of Euschistus conspersus and Halyomorpha halys eggs by Telenomus podisi and Trissolcus erugatus. J. Chem. Ecol. 42, 1016–1027. https://doi.org/10.1007/s10886-016-0754-3 (2016).
Zhong, Y. Z. et al. Behavioral responses of the egg parasitoid Trissolcus japonicus to volatiles from adults of its stink bug host, Halyomorpha halys. J. Pest Sci. 90, 1097–1105. https://doi.org/10.3389/fphys.2018.01610 (2017).
Borges, M. & Aldrich, J. Instar-specific defensive secretions of stink bugs (Heteroptera: Pentatomidae). Experientia 48, 893–896. https://doi.org/10.1007/BF02118429 (1992).
Kuhar, T. P. & Kamminga, K. Review of the chemical control research on Halyomorpha halys in the USA. J. Pest Sci. 90, 1021–1031. https://doi.org/10.1007/s10340-017-0859-7 (2017).
Weber, D. C., Leskey, T. C., Walsh, G. C. & Ashot, K. Synergy of aggregation pheromone with methyl (E, E, Z)-2, 4, 6-decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 107, 1061–1106. https://doi.org/10.1603/EC13502 (2014).
Morrison, W. R. et al. Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag. Sci. 75, 104–114. https://doi.org/10.1002/ps.5156 (2019).
Kirkpatrick, D. M. et al. Estimating monitoring trap plume reach and trapping area for nymphal and adult Halyomorpha halys (Hemiptera: Pentatomidae) in crop and non-crop habitats. Environ. Entomol. 48, 1104–1112. https://doi.org/10.1093/ee/nvz093 (2019).
Suckling, D. M. et al. Live traps for adult brown marmorated stink bugs. Insects 10, 376. https://doi.org/10.3390/insects10110376 (2019).
Suckling, D. M. et al. Trapping brown marmorated stink bugs: “The Nazgȗl” lure and kill nets. Insects 12, 433. https://doi.org/10.3390/insects10120433 (2019).
Acebes-Doria, A. L. et al. Season-long monitoring of the brown marmorated stink bug (Hemiptera: Pentatomidae) throughout the United States using commercially available traps and lures. J. Econ. Entomol. 113, 159–171. https://doi.org/10.1093/jee/toz240 (2020).
Formella, A., Dorman, S. J., Taylor, S. V. & Kuhar, T. P. Effects of aggregation lure and tree species on Halyomorpha halys (Hemiptera: Pentatomidae) seasonal oviposition. J. Econ. Entomol. 113, 203–210. https://doi.org/10.1093/jee/toz281 (2020).
Weber, D. C. et al. Attractiveness of pheromone components with and without the synergist, methyl (2E,4E,6Z)-2,4,6-decatrienoate, to brown marmorated stink bug (Hemiptera: Pentatomidae). J. Econ. Entomol. 113, 712–719. https://doi.org/10.1093/jee/toz312 (2020).
Leskey, T. C., Short, B. D. & Ludwick, D. Comparison and refinement of integrated pest management tactics for Halyomorpha halys (Hemiptera: Pentatomidae) management in apple orchards. J. Econ. Entomol. 113, 1725–1734. https://doi.org/10.1093/jee/toaa067 (2020).
Leskey, T. C. et al. Refining pheromone lures for the invasive Halyomorpha halys (Hemiptera: Pentatomidae) through collaborative trials in the United States and Europe. J. Econ. Entomol. 114, 1666–1673. https://doi.org/10.1093/jee/toab088 (2021).
Chartois, M. et al. A crowdsourcing approach to track the expansion of the brown marmorated stinkbug Halyomorpha halys (Stål, 1855) in France. Biodivers. Data J. 9, e66335. https://doi.org/10.3897/BDJ.9.e66335 (2021).
Bergh, J. C. et al. Border habitat effects on captures of Halyomorpha halys (Hemiptera: Pentatomidae) in pheromone traps and fruit injury at harvest in apple and peach orchards in the Mid-Atlantic, USA. Insects 12, 419. https://doi.org/10.3390/insects12050419 (2021).
Candian, V. et al. Photoselective exclusion netting in apple orchards: Effectiveness against pests and impact on beneficial arthropods, fungal diseases and fruit quality. Pest Manag. Sci. 76, 179–187. https://doi.org/10.1002/ps.5491 (2020).
Caorsi, V. et al. Design of ideal vibrational signals for stinkbug male attraction, through vibrotaxis experiments. Pest Manag. Sci. https://doi.org/10.1002/ps.6590 (2021).
Wong, H. L. et al. Attraction of brown marmorated stink bugs, Halyomorpha halys, to blooming sunflower semiochemicals. J. Chem. Ecol. 47, 614–627. https://doi.org/10.1007/s10886-021-01281-y (2021).
Blaauw, B. R. et al. Plant stimuli and their impact on brown marmorated stink bug dispersal and host selection. Front. Ecol. Evol. 7, 414. https://doi.org/10.3389/fevo.2019.00414 (2019).
Akotsen-Mensah, C. et al. Behavioral response of Halyomorpha halys (Hemiptera: Pentatomidae) and its egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to host plant odors. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2021.696814 (2021).
Noge, K. Hexanal, a major volatile found in fresh peanut seed, elicits foraging behavior in the laboratory-reared brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae). J. Pest Sci. 44, 15–19. https://doi.org/10.1584/jpestics.D18-053 (2019).
Light, D. M. et al. A pear-derived kairomone with pheromonal potency that attracts male and female codling moth, Cydia pomonella (L.). Naturwissenschaften 88, 333–338. https://doi.org/10.1007/s001140100243 (2001).
Tang, R. et al. Identification and testing of oviposition attractant chemical compounds for Musca domestica. Sci. Rep. 6, 33017. https://doi.org/10.1038/srep33017 (2016).
Tang, R., Zhang, J. P. & Zhang, Z. N. Electrophysiological and behavioral responses of male fall webworm moths (Hyphantria cunea) to herbivory-induced mulberry (Morus alba) leaf volatiles. PLoS ONE 7, e49256. https://doi.org/10.1371/journal.pone.0049256 (2012).
Varela, N., Avilla, J., Anton, S. & Gemeno, C. Synergism of pheromone and host plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 141, 114–122. https://doi.org/10.1111/j.1570-7458.2011.01171.x (2011).
Bai, P. H. et al. Botanical volatiles selection in mediating electrophysiological responses and reproductive behaviors for the fall webworm Moth Hyphantria cunea. Front. Physiol. 11, 486. https://doi.org/10.3389/fphys.2020.00486 (2020).
Jiang, N. J. et al. Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. https://doi.org/10.1111/1744-7917.12956 (2021).
Melo, N. et al. The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr. Biol. 31, 1988–1994. https://doi.org/10.1016/j.cub.2021.02.010 (2021).
Nyasembe, V. O. et al. Linalool oxide: Generalist plant based lure for mosquito dsease vectors. Parasit. Vectors 8, 581. https://doi.org/10.1186/s13071-015-1184-8 (2015).
Song, Q. S., Yang, D. R., Zhang, G. M. & Yang, C. R. Volatiles from Ficus hispida and their attractiveness to fig wasps. J. Chem. Ecol. 27, 1929–1942. https://doi.org/10.1023/A:1012226400586 (2001).
Knight, A. L., Mujica, V., Herrera, S. L. & Tasin, M. Addition of terpenoids to pear ester plus acetic acid increases catches of codling moth (Lepidoptera: Tortricidae). J. Appl. Entomol. 143, 813–821. https://doi.org/10.1111/jen.12646 (2019).
Nehme, M. et al. Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ. Entomol. 39, 169–176. https://doi.org/10.1603/EN09177 (2010).
Tang, R., Zhong, Y. Z., Han, R. C., Cao, L. & Chen, H. L. The invention relates to an attractant composition using a Halyomorpha halys attractant synergist and its application and attractant lure. China, CN112514900B [P] (2021).
Morrison, W. R., Blaauw, B. R., Leskey, T. C., Nielsen, A. L. & Talamas, E. Predation and parasitism by native and exotic natural enemies of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) eggs augmented with semiochemicals and differing host stimuli. Biol. Control 121, 140–150. https://doi.org/10.1016/j.biocontrol.2018.02.016 (2018).
Zhang, J. et al. Olfactory reception of host alarm pheromone component by the odorant-binding proteins in the samurai wasp, Trissolcus japonicus (Hymenoptera: Scelionidae). Front. Physiol. 11, 1058. https://doi.org/10.3389/fphys.2020.01058 (2020).
Sun, D. et al. Identification of candidate olfactory genes in the antennal transcriptome of the stink bug Halyomorpha halys. Front. Physiol. 11, 876. https://doi.org/10.3389/fphys.2020.00876 (2020).
Paula, D. et al. Identification and expression profile of odorant-binding proteins in Halyomorpha halys (Hemiptera: Pentatomidae). Insect Mol. Biol. 25, 580–594. https://doi.org/10.1111/imb.12243 (2016).
Zhong, Y. Z. et al. Behavioral evidence and olfactory reception of a single alarm pheromone component in Halyomorpha halys. Front. Physiol. 9, 1610. https://doi.org/10.3389/fphys.2018.01610 (2018).
Montagné, N., de Fouchier, A., Newcomb, R. D. & Jacquin-Joly, E. Advances in the identification and characterization of olfactory receptors in insects. In: Progress in Molecular Biology and Translational Science 55–80. (Elsevier, 2015). https://doi.org/10.1016/bs.pmbts.2014.11.003.
Li, D. Z. et al. Structure-based analysis of the ligand-binding mechanism for DhelOBP21, a C-minus odorant binding protein, from Dastarcus helophoroides (Fairmaire; Coleoptera: Bothrideridae). Int. J. Biol. Sci. 11, 1281. https://doi.org/10.7150/ijbs.12528 (2015).
Venthur, H. & Zhou, J. J. Odorant receptors and odorant-binding proteins as insect pest control targets: A comparative analysis. Front. Physiol. 9, 1163. https://doi.org/10.3389/fphys.2018.01163 (2018).
Kepchia, D. et al. Use of machine learning to identify novel, behaviorally active antagonists of the insect odorant receptor co-receptor (Orco) subunit. Sci. Rep. 9, 4055. https://doi.org/10.1038/s41598-019-40640-4 (2019).
Li, T. T. et al. Crystal structure and ligand identification of odorant binding protein 4 in the natural predator Chrysopa pallens. Int. J. Biol. Macromol. 141, 1004–1012. https://doi.org/10.1016/j.ijbiomac.2019.09.043 (2019).
Sparks, M. E. et al. Brown marmorated stink bug, Halyomorpha halys (Stål), genome: Putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest. BMC Genomics 21, 1–26. https://doi.org/10.1186/s12864-020-6510-7 (2020).
Sparks, M. E., Shelby, K. S., Kuhar, D. & Gundersen-Rindal, D. E. Transcriptome of the invasive brown marmorated stink bug, Halyomorpha halys (Stål)(Heteroptera: Pentatomidae). PLoS ONE 9, e111646. https://doi.org/10.1371/journal.pone.0111646 (2014).
Zhou, Y. L. et al. Silencing in Apolygus lucorum of the olfactory coreceptor Orco gene by RNA interference induces EAG response declining to two putative semiochemicals. J. Insect Physiol. 60, 31–39. https://doi.org/10.1016/j.jinsphys.2013.10.006 (2014).
Saitou, N. & Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454 (1987).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Rambaut, A. FigTree v1.4.2 (accessed 5 September 2021). http://tree.bio.ed.ac.uk/software/figtree/.
O’Boyle, N. M. et al. Open Babel: An open chemical toolbox. J. Cheminformatics. 3, 33. https://doi.org/10.1186/1758-2946-3-33 (2011).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. https://doi.org/10.1002/jcc.21256 (2009).
Di, C., Ning, C., Huang, L. Q. & Wang, C. Z. Design of larval chemical attractants based on odorant response spectra of odorant receptors in the cotton bollworm. Insect Biochem. Mol. 84, 48–62. https://doi.org/10.1016/j.ibmb.2017.03.007 (2017).
Münch, D. & Galizia, C. G. DoOR 2.0-comprehensive mapping of Drosophila melanogaster odorant responses. Sci. Rep. 6, 21841. https://doi.org/10.1038/srep21841 (2016).
Borges, M. et al. Field responses of stink bugs to the natural and synthetic pheromone of the Neotropical brown stink bug, Euschistus heros, (Heteroptera: Pentatomidae). Physiol. Entomol. 23, 202–207. https://doi.org/10.1046/j.1365-3032.1998.233086.x (1998).
Sugie, H. et al. Identification of the aggregation pheromone of the brown winged green bug Plautia stali Scott (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 31, 427–431. https://doi.org/10.1303/aez.31.427 (1996).
Sargent, C., Martinson, H. M. & Raupp, M. J. Traps and trap placement may affect location of brown marmorated stink bug (Hemiptera: Pentatomidae) and increase injury to tomato fruits in home gardens. Environ. Entomol. 43, 432–438. https://doi.org/10.1603/EN13237 (2014).
Jain, R. G., Robinson, K. E., Fletcher, S. J. & Mitter, N. RNAi-based functional genomics in hemiptera. Insects 11, 557. https://doi.org/10.3390/insects11090557 (2020).
Finetti, L. et al. Characterization of Halyomorpha halys TAR1 reveals its involvement in (E)-2-decenal pheromone perception. J. Exp. Biol. 224, jeb238816. https://doi.org/10.1242/jeb.238816 (2021).
Li, J., Wang, X. P., Wang, M. Q., Ma, W. H. & Hua, H. X. Advances in the use of the RNA interference technique in Hemiptera. Insect Sci. 20, 31–39. https://doi.org/10.1111/j.1744-7917.2012.01550.x (2013).
El-Sayed, et al. Floral scent of Canada thistle and its potential as a generic insect attractant. J. Econ. Entomol. 101, 720–727. https://doi.org/10.1093/jee/101.3.720 (2008).
Piesik, D., Weaver, D. K., Peck, G. E. & Morrill, W. L. Mechanically-injured wheat plants release greater amounts of the secondary metabolites linalool and linalool oxide. J. Plant. Res. 46, 29–39. https://doi.org/10.1016/j.tet.2005.02.032 (2006).
Zhou, Y. C. et al. Frugivory by brown marmorated stink bug (Hemiptera: Pentatomidae) alters blueberry fruit chemistry and preference by conspecifics. Environ. Entomol. 45, 1227–1234. https://doi.org/10.1093/ee/nvw110 (2016).
Boachon, B. et al. CYP76C1 (Cytochrome P450)-mediated linalool metabolism and the formation of volatile and soluble linalool oxides in Arabidopsis flowers: A strategy for defense against floral antagonists. Plant Cell 27, 2972–2990. https://doi.org/10.1105/tpc.15.00399 (2015).
Cossetin, L. F. et al. In vitro safety and efficacy of lavender essential oil (Lamiales: Lamiaceae) as an insecticide against houseflies (Diptera: Muscidae) and blowflies (Diptera: Calliphoridae). J. Econ. Entomol. 111, 1974–1982. https://doi.org/10.1093/jee/toy145 (2018).
Njihia, T. N. et al. Identification of kairomones of second instar nymphs of the variegated coffee bug Antestiopsis thunbergii (Heteroptera: Pentatomidae). Chemoecology 27, 239–248. https://doi.org/10.1007/s00049-017-0248-y (2017).
Meza, F. C. et al. Behavioural and electrophysiological responses of female Anopheles gambiae mosquitoes to volatiles from a mango bait. J. Chem. Ecol. 46, 387–396. https://doi.org/10.1007/s10886-020-01172-8 (2020).
Leal, W. S. Reverse chemical ecology at the service of conservation biology. PNAS 114, 12094–12096. https://doi.org/10.1073/pnas.1717375114 (2017).
Leal, W. S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. https://doi.org/10.1146/annurev-ento-120811-153635 (2013).
Koerte, S. et al. Evaluation of the DREAM technique for a high-throughput deorphanization of chemosensory receptors in drosophila. Front. Mol. Neuro. 11, 366. https://doi.org/10.3389/fnmol.2018.00366 (2018).
Von Der Weid, B. et al. Large-scale transcriptional profiling of chemosensory neurons identifies receptor-ligand pairs in vivo. Nat. Neurosci. 18, 1455–1463. https://doi.org/10.1038/nn.4100 (2015).
Ai, M. et al. Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. https://doi.org/10.1038/nature09537 (2010).
Zhang, Y. V., Ni, J. & Montell, C. The molecular basis for attractive salt-taste coding in Drosophila. Science 340, 1334–1338. https://doi.org/10.1126/science.1234133 (2013).
Hansson, B. S. & Stensmyr, M. C. Evolution of insect olfaction. Neuron 72, 698–711. https://doi.org/10.1016/j.neuron.2011.11.003 (2011).
Hahn, M. G., Rodriguez-Saona, C. & Hamilton, G. C. Characterizing the spatial distribution of brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae), populations in peach orchards. PLoS ONE 12, e0170889. https://doi.org/10.1371/journal.pone.0170889 (2017).
Semmelhack, J. L. & Wang, J. W. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 459, 218–223. https://doi.org/10.1038/nature07983 (2009).
Tang, R. et al. The olfactory reception of acetic acid and ionotropic receptors in the Oriental armyworm, Mythimna separata Walker. Insect. Biochem. Mol. Biol. 118, 103312. https://doi.org/10.1016/j.ibmb.2019.103312 (2020).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFD0300105; 2021YFD1400100, 2021YFD1400102, 2021YFD1400101), Guangzhou Science and Technology Project (2022-01-01-05-2053-0012), the Key-Area Research and Development Program of Guangdong Province (2020B020224002), and China Postdoctoral Science Foundation (Grant No. 2020M683001). We thank engineer Qiong-Fang Yang for technical supports on electrophysiological tests. We thank B.F.A. Yorda for technical supports on visual presentation development.
Author information
Authors and Affiliations
Contributions
R.T., F.H.W., and R.C.H. conceived the project. Y.Z.Z. and R.T. designed the study. Y.Z.Z., M.H.X., and L.C. conducted the behavioral assays. Y.Z.Z. and H.L.C. conducted the electrophysiological tests. C.H., X.Z., and R.T. conducted the protein computation analysis. Y.Z.Z., L.C., F.Z., and R.T. analyzed the data. R.T., F.H.W., and R.C.H. drafted the manuscript with inputs from all. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, YZ., Xie, MH., Huang, C. et al. Characterizations of botanical attractant of Halyomorpha halys and selection of relevant deorphanization candidates via computational approach. Sci Rep 12, 4170 (2022). https://doi.org/10.1038/s41598-022-07840-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07840-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.