Abstract
Few studies assessed the association between major adverse cardiovascular events and adherence to warfarin and direct oral anticoagulants (DOACs) in patients with atrial fibrillation (AF). Therefore, we aimed to evaluate the effects of adherence to oral anticoagulants (OACs) in patients with AF using claims data (July 2014–April 2019). Using the initial 3-month medication possession rate (MPR), patients were categorized into adherent (MPR ≥ 0.8) or non-adherent (MPR < 0.8) groups. Propensity score matching of non-adherent group to adherent group was conducted for warfarin (1:1) and DOAC (1:3), respectively. Incidence of ischemic stroke, myocardial infarction (MI), intracranial hemorrhage, and all-cause death was assessed in the matched cohort (67,147 patients). The hazard ratio (HR) for adherence to OAC was estimated using the Cox proportional hazard model with adjusting covariate including age and sex. The risk for ischemic stroke, MI, and all-cause death was lower in the DOAC adherent group than in the DOAC non-adherent group (HR: 0.78; 95% confidence intervals: 0.73–0.84; 0.75, 0.60–0.94; 0.54, 0.51–0.57, respectively). Adherence to OAC was not associated with the risk of intracranial hemorrhage (1.01, 0.85–1.20). Commitment programs to improve adherence in patients with AF could maximize drug effectiveness and safety.
Similar content being viewed by others
Introduction
In an ever-aging era, the prevalence of atrial fibrillation (AF), characterized by irregular and rapid heart rate, is expected to grow by 2.5 times over the next 50 years1,2. AF is known to increase the risk of stroke and can lead to thromboembolism and heart failure3,4,5. In AF, complications such as stroke and myocardial infarction (MI) are reportedly associated with high mortality. Comorbidities such as hypertension, history of a previous stroke, and coronary heart disease are not only associated with the development of AF, but also elevate the risk of stroke6. According to clinical guidelines, managing the risk of fatal AF complications such as stroke and myocardial infarction is important7. Patients should be treated with oral anticoagulants (OACs) to prevent the occurrence of these complications. A risk-factor-based assessment should precede initiating medication treatment to manage the risk of stroke. Using the CHA2DS2-VASc clinical stroke risk score, AF patients who are eligible for OACs are identified, and those with “low stroke risk” are not recommended for antithrombotic treatment. For selecting OACs, direct oral anticoagulants (DOACs) are preferred over vitamin K antagonists, mostly warfarin, as DOACs result in a lower risk of bleeding and are less affected by time in the therapeutic range (TTR) while securing similar effectiveness when compared with warfarin7,8. Nevertheless, patients with AF taking anticoagulants need to be carefully observed owing to the increased risk of bleeding9,10,11.
Along with regular monitoring of warfarin use, guidelines emphasize the importance of actively promoting adherence to and persistence of DOAC treatment7. In previous studies, adherent use of DOACs revealed superior clinical outcomes without bleeding risk. In a retrospective observational study, the adherent DOAC users reportedly showed lower risks of ischemic stroke and systemic embolism than the non-adherent ones12. Conversely, the risk of stroke increased 3–4 times when patients discontinued oral anticoagulant (OAC) administration13,14,15. Increasing age, presence of comorbidities, and frequent dosing schedules have been reported as risk factors for poor adherence to OACs16,17. Although adherence to OACs is important in preventing stroke, studies on clinical outcomes other than stroke are limited. Besides stroke, AF can also lead to MI and even death; however, studies assessing clinical outcomes other than stroke remain limited. Considering these complications, it is crucial to evaluate the benefits of OAC adherence. Moreover, intracranial hemorrhage must be assessed to verify the safety of adherence7. Therefore, we aimed to evaluate the risk of ischemic stroke, MI, intracranial hemorrhage, and death following adherence to DOACs or warfarin.
Methods
Data source
Herein, we employed the claims data from July 1, 2014 to April 30, 2019, provided by the Health Insurance Review and Assessment Service (HIRA). The HIRA data are medical claims data covering approximately 98% of the total Korean population. The database contains information about demographic characteristics, including sex, age, insurance type, and healthcare resource utilization data, such as the type of medical procedure, diagnosis of disease, costs, and medication use. Information on medication use includes the generic name, prescription date, daily dosage, quantity, duration, and general codes18. Disease diagnoses were coded according to the International Classification of Disease-10th revision (ICD-10).
Study design and population
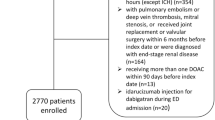
The target population in this retrospective cohort study (Fig. 1) included newly treated patients with AF who were taking OACs. To identify eligible patients from claims data, information of patients with (1) an OAC prescription and (2) a diagnosis of AF (ICD-10: I48) was extracted between July 1, 2015 and April 30, 201819. OACs included warfarin and DOACs (rivaroxaban, apixaban, dabigatran, and edoxaban). The first day of OAC prescription was set as the cohort entry date. The index date was defined as 90 days after the cohort entry date. We measured adherence during this 90-day period, according to a previous study20. We included only those patients who did not switch drugs or did not die within 90 days of the cohort entry date.
OAC initiation is recommended based on the CHA2DS2-VASc score; this score predicts the risk of stroke and is calculated based on patient characteristics21. As described previously14, the CHA2DS2-VASc score was calculated based on the diagnosis code within a year before the cohort entry date (Supplementary Table S1). Only males with a CHA2DS2-VASc score ≥ 2 and females with a CHA2DS2-VASc score ≥ 3 were included. Only adult patients (age > 18 years) were included; patients who received OACs within 1 year before the cohort entry date were excluded, and only newly treated patients were selected. Patients diagnosed with valvular diseases (ICD-10: I05, Z952–Z954) were excluded; only patients with non-valvular AF were included22. Patients diagnosed with ischemic stroke, MI, and intracranial hemorrhage within 90 days of adherence determination were also excluded.
Intervention
The medication possession rate (MPR) is used to evaluate medication adherence23,24 and is calculated by dividing the total number of prescription days for the initial 90 days by 90 days. Based on the MPR, the population was categorized into the adherent (MPR ≥ 0.8) or non-adherent (MPR < 0.8) groups.
As patients were categorized by early adherence, we established another cohort to determine whether early adherence to OACs continued for the later adherence. Newly treated patients with AF taking OACs were included using the same criteria as mentioned above. Among them, patients censored due to switching or death within 365 days were excluded (Supplementary Fig. S1). Pearson’s correlation test was used to assess the correlation between early and later adherence. If the Pearson correlation coefficient was close to + 1 and the p-value was less than 0.05, the MPR of the initial 3 months was considered to be positively correlated with the MPR in the last 3 months. The linearity was determined using a scatter plot. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by performing univariable logistic regression analysis to assess causation between the two variables. Initial adherence was considered to affect later adherence when the p-value was less than 0.05.
Outcome measurement
The patients were followed up to observe outcomes from the index date to the end of the study, death, or discontinuation of initial OACs. Outcomes were determined on the first record of the following four events independently, using ICD-10 and procedure codes: (1) ischemic stroke, (2) MI, (3) intracranial hemorrhage, and (4) death. The diagnostic codes were confirmed based on a previous study that validated the codes for clinical outcomes25. The operational definitions of outcomes are listed in Supplementary Table S2.
Patient characteristics
Demographic characteristics, including sex, age group, and type of insurance on the cohort entry date, were assessed. Within 1 year before the cohort entry date, pre-clinical history of diabetes mellitus, hypertension, dyslipidemia, MI, prior percutaneous coronary intervention, prior coronary artery bypass graft, chronic renal failure, chronic obstructive pulmonary disease, unstable angina, cognitive disease, heart failure, stroke, vascular disease, intracranial hemorrhage, and cancer, which could affect the adherence to OACs or outcomes, was evaluated17,26,27. Concomitant medications, such as aspirin, antiplatelets, beta-blockers, and calcium channel blockers, were considered potential confounders. The operation definitions of pre-clinical history and concomitant medications are presented in Supplementary Tables S3 and S4.
The CHA2DS2-VASc and HAS-BLED scores, representative risk scores for stroke and bleeding, respectively, were calculated based on diagnosis codes within a year before the cohort entry date (Supplementary Table S1), as described previously14. The Charlson comorbidity index (CCI) was used to estimate the burden of underlying diseases that could affect OAC adherence28. Additionally, the number of emergency room (ER) visits and outpatient visits was included as a covariate for each patient.
Statistical analysis
Propensity score (PS) matching was performed to minimize the potential impact of confounders on outcomes. Multivariable logistic regression estimated the PS for the adherent group using the following variables within a year before the cohort entry date or at the cohort entry date: age group, sex, type of insurance, pre-clinical history, co-medication, CHA2DS2-VASc, HAS-BLED, CCI, and the number of ER and outpatient visits29,30,31,32,33. Matching was performed using a greedy algorithm34. Non-adherent warfarin users were matched 1:1 with adherent warfarin users, owing to the insufficient number of patients in the adherent group. For rivaroxaban, apixaban, dabigatran, and edoxaban, we performed 1:3 matching for each DOAC (i.e., one rivaroxaban non-adherent user was matched to three rivaroxaban adherent users). A standardized mean difference (SMD) between the adherent and non-adherent groups was estimated to compare the distribution of variables used for matching. Covariates with SMD ˃ 0.1, which can provide evidence of an imbalance between matched groups, were included in the survival analysis.
The incidence of outcomes was calculated by dividing the number of individual events by the total follow-up period and presented as 100 person-years (PY). The Cox proportional hazards model was used to estimate the adjusted hazard ratio (aHR). Age, OAC type, sex, CCI, and CHA2DS2-VASc score were included as confounders in the model. These models are presented in the Supplementary Table S6. A subgroup analysis was performed by grouping the patients as follows: (1) a group of patients aged 75 years or above, (2) a group of patients with a CHA2DS2-VASc score ≥ 4, and (3) a group of patients with CCI ≥ 4. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Ethics declarations
The study was approved by the Institutional Review Board of Sungkyunkwan University (SKKU-201910031-UE001), South Korea. As patient claims data from the Health Insurance Review and Assessment Service (HIRA) had been anonymized and de-identified, the Institutional Review Board of Sungkyunkwan University waived the requirement for informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Informed consent
As patient claims data which support the findings of this study had been anonymized and de-identified publicly available information, the institutional review board waived the requirement for informed consent.
Results
In total, 67,147 patients were included after PS matching (Fig. 2). Table 1 presents the baseline characteristics of overall and matched cohorts. Additionally, patient characteristics for each drug have been shown in Supplementary Table S5. After PS matching, all SMDs, except the age group and CCI, were less than 0.1. The mean (SD) CHA2DS2-VASc score was 3.1 (0.9) in both adherent and non-adherent groups in the matched cohort.
The incidence rates per 100PY and aHR for events according to the adherent and non-adherent groups are shown in Fig. 3. The incidence of ischemic stroke was lower in the adherent group than in the non-adherent group (3.13 per 100PY vs. 4.23 per 100PY for DOACs or warfarin). MI and death rates were lower in the adherent group. The risk of ischemic stroke (aHR = 0.78, 95% CI 0.73–0.84), MI (0.75, 0.60–0.94), and death (0.54, 0.51–0.57) was significantly low among adherent DOAC users. Adherence to warfarin was not associated with a lower risk of ischemic stroke (0.85, 0.71–1.03) and MI (0.82, 0.46–1.45); however, a lower risk of death was noted (0.55, 0.47–0.64). The risk of intracranial hemorrhage, a known adverse outcome of OACs, was not associated with OAC adherence (1.01, 0.85–1.20). Shown in Supplementary Table S7, the aHR for ischemic stroke was not significant for the apixaban (0.87, 0.75–1.01) and dabigatran (0.85, 0.72–1.01) while it was significant for the rivaroxaban (0.76, 0.68–0.84) and edoxaban (0.66, 0.55–0.79). For MI, only edoxaban was significant (0.46, 0.24–0.86).
Figure 4 shows the aHR of events in subgroups according to age group, the CHA2DS2-VASc score, and CCI. In all subgroups, the risk of ischemic stroke and death was lower in the adherent group than in the non-adherent group. The aHR for death was 0.46 (95% CI 0.41–0.51) in the younger group and 0.57 (0.53–0.60) in the elderly group. Adherence to OACs improved efficacy against MI in the lower CCI group (aHR = 0.60, 95% CI 0.45–0.80), but not in the higher CCI group (0.99, 0.72–1.35). As the type of OAC was included as a covariate in the subgroup analysis, the hazard ratio for each OAC is presented in Supplementary Table S8.
Furthermore, we assessed the association between the initial 3-month adherence and subsequent 3-month adherence (Supplementary Fig. S2). Among 76,078 patients, the Pearson correlation coefficient between the two MPR measures in the early and later periods was 0.55 (p < 0.0001), revealing a significantly positive correlation; this has been depicted through a scatter plot presented in Supplementary Fig. S3. Patients with high initial adherence were more likely to adhere to the prescribed treatment during the later period than patients with low initial adherence (OR 15.28; 95% CI 14.68–15.90).
Discussion
Herein, adherence to DOACs for the initial 3 months was associated with a low risk of ischemic stroke and MI, with no increase in bleeding risk when compared with non-adherent usage. Adherence to warfarin was not associated with a reduced risk of stroke or MI, but was related to a reduced risk of death when compared with non-adherent usage.
Although the OACs themselves have suboptimal protective effects for AF patients from stroke risk, adherence was associated with better benefit of DOACs. The protective effect of adherent DOAC usage in our study was similar to that observed in previous studies. In the United States, the risk of ischemic stroke was found to be comparatively high following non-adherent DOAC usage (aHR = 1.50, 95% CI 1.30–1.73)15. Kim et al. also have reported results similar to those of our study. The adherent use of DOACs could be associated with the lower risk of ischemic stroke (0.73, 0.69–0.79) and MI (0.82, 0.72–0.93) without the risk of bleeding (1.01, 0.91–1.11), when compared with non-adherent use12. A previous retrospective study, using Medicare claims data, reported a lower HR for ischemic stroke in the adherent group (0.62) than in the non-adherent group (0.74), compared with the non-use group14 although it was difficult to directly compare our findings with these results. Beyond previous studies, we observed the benefit of adherence with stratifying the type of DOACs, which were on not only ischemic stroke (0.79, 0.74–0.85), MI (0.76, 0.61–0.94) but also all-cause death (0.54, 0.51–0.57).
AF complications still occurred in patients with good adherence in this study, even if the incidence rates of the adherent group were lower than those of the non-adherent group. These may also be affected by factors other than adherence, such as age or disease burden. In the subgroup analysis, the protective effect of OACs, which reduced the risk of ischemic stroke and MI, was found to be considerably high in younger patients and patients with a lower disease burden. As shown in our study, the benefit of adherent use of OACs was smaller in the older patient group than in the younger patient group when comparing point estimations. Additionally, the group with a high disease burden showed less reduction in the risk of adverse events than the low disease burden group.
Consistent with our results, Rutherford et al. have reported that the risk of stroke or bleeding is affected by age and comorbidities, as elderly patients or those with substantial comorbidities receive a reduced dose35. Kachroo et al. have reported that elderly patients with AF are more likely to discontinue treatment36. The presence of comorbidities affects not only the rate of OAC prescription, but also adherence, as previously reported that patients with high CCI are likely to demonstrate low adherence to OACs37,38. Previous studies also showed that stroke patients with AF who took DOACs were to be more likely to have non-modifiable factors such as being old and being female, and factors that favor thrombotic effects such as hypertension, diabetes mellitus, and dyslipidemia compared to stroke patients without AF39,40. Therefore, monitoring adherence in these high-risk patients is important, as recommended in clinical guidelines. Achieving optimal adherence in this patient group is essential to avoid any additional cardiovascular events, as the aforementioned patients who took DOACs were vulnerable to thrombotic factors. As reported in a recent study41, adding a statin to anticoagulant therapies would be worthwhile.
Interestingly, the statistical significance of aHR on adherence differed depending on the type of DOACs. Rivaroxaban and edoxaban seemed to lower the risk of ischemic stroke but apixaban and dabigatran didn’t. The difference between two types is dosing schedule. Apixaban and dabigatran are common on taking twice daily whereas rivaroxaban and edoxaban take once daily. Adherence itself can be affected by frequency of dosing42. This may be explored in further study.
The incidence rates of ischemic stroke attributed to DOAC adherence deviated from those previously reported. In our study, the incidence rates of ischemic stroke were 3.51 and 4.07 per 100PY in the adherent and non-adherent users, respectively, while a previous study has revealed that these rates were 1.20 and 1.92 per 100PY in the adherent and non-adherent users, respectively14. The deviation in incidence between studies could be attributed to patient inclusion criteria, as our study only included intermediate to high-risk patients: male patients with a CHA2DS2-VASc score ≥ 2 only and female patients with a CHA2DS2-VASc score ≥ 3 only. The study by Kim et al., which included patients with a mean CHA2DS2-VASc score of approximately 5, showed that the incidence rates of ischemic stroke were 4.21 and 5.84 per 100PY in the adherent and non-adherent groups, respectively12. Furthermore, racial differences may affect the incidence of stroke43.
Our study had several strengths. The risk of adverse health outcomes due to blood clots and the risk of bleeding, implying suboptimal control, were traced within the same dataset; therefore, the benefit of adherent OAC usage was examined from various angles. By applying the simplified study design, the findings can be interpreted easily in a cause-and-effect manner to deliver a straightforward message that adherence is related to adverse health outcomes in patients. Additionally, the results were representative of the Korean population with AF, as we used a population-level database covering the entire Korean population.
A few limitations should be considered when interpreting the present findings. First, we could not capture the data of patients who failed to administer prescribed medications despite the dispensed prescription, as adherence was estimated by prescription days using claims data. Especially for warfarin, the MPR could not serve as an alternative indicator to the period in the therapeutic range, which could be verified by the international normalized ratio (INR). However, as guidelines for patients with AF recommend that patients taking OACs should be regularly monitored by the clinician7,44, prescription days can be used to assess adherence to OACs, and patients with AF should be assumed to be under optimal control. In addition, as this study aimed to elucidate the clinical effect of adherence and not the therapeutic effect of OACs, factors other than adherence would have little effect on the results. Second, a misclassification bias may be present, as we used diagnostic codes of claims data to define outcomes and covariates operationally. To verify that the procedure was executed for disease remission, we limited the diagnostic codes. Furthermore, all claims of procedure codes are reviewed by HIRA to assess the appropriateness of the executing procedure before reimbursement; therefore, the procedure codes have their own accuracy and completeness. Third, the protective effect of adherence to OACs might have been overestimated, as the adherent group was older and had a slightly greater disease burden than the non-adherent group. However, the SMD revealed a balance between the adherent and non-adherent groups. Moreover, to derive the HRs of health outcomes related to adherence, we used the Cox proportional hazard model to adjust for patient characteristics, including age, sex, CCI, and the CHA2DS2-VASc score. Furthermore, we successfully verified the robustness of the study results, as the protective effect of adherent OAC use was uniform, regardless of patient characteristics, such as age group, the CHA2DS2-VASc score, and CCI, in various one-way sensitivity analyses. Comedication such as a statin may affect cardiovascular events through blood-thinning effects41. Although we did not observe statin use, we included dyslipidemia as one of the matching variables, and the adherent and non-adherent groups were well balanced, as shown by the SMD.
It is challenging to illustrate the impact of adherence on health outcomes because adherence is a time-varying covariate. To overcome this limitation, Hernandez et al. previously conducted a Cox proportional hazard analysis using time-dependent exposure, while Brown et al. performed the same analysis for diverse study periods, such as 3, 6, and 9 months14,17. In our study, we simplified the study design and simultaneously performed Pearson’s correlation test to support the assumption that adherence persisted within the first 3 months of the follow-up period. Patients with high initial adherence were shown to be more likely to adhere in the later period than those with low initial adherence. Similarly, a previous study using medical records from the outpatient clinic of a cardiology department in Korea reported that adherence to OACs was similar, regardless of the treatment period16. Based on the assumption of adherence persistence, we demonstrated the differences in protective effects of OACs between the adherent and non-adherent groups.
Conclusions
Adherent use of DOACs in patients with AF could be beneficial for reducing the risk of ischemic stroke, MI, and death, without increasing the risk of bleeding. Warfarin adherence presented a lower risk of death. Adherence to OACs could be more effective in younger patients and patients with lower CCI, particularly in preventing MI. Efforts to improve adherence in patients with AF taking OACs may help reduce the burden of cardiovascular diseases.
Data availability
We used claims data from the Health Insurance Review and Assessment Service (HIRA). The claims data may be available from HIRA with permission.
References
Go, A. S. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 285, 2370–2375 (2001).
Naccarelli, G. V., Varker, H., Lin, J. & Schulman, K. L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 104, 1534–1539 (2009).
Benjamin, E. J. et al. Impact of atrial fibrillation on the risk of death: the framing heart study. Circulation 98, 946–952 (1998).
Trulock, K. M., Narayan, S. M. & Piccini, J. P. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J. Am. Coll. Cardiol. 64, 710–721 (2014).
Wolf, P. A., Mitchell, J. B., Baker, C. S., Kannel, W. B. & D’Agostino, R. B. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch. Intern. Med. 158, 229–234 (1998).
Wańkowicz, P., Nowacki, P. & Gołąb-Janowska, M. Atrial fibrillation risk factors in patients with ischemic stroke. Arch. Med. Sci. 17, 19–24 (2019).
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498 (2021).
Schmitt, J., Duray, G., Gersh, B. J. & Hohnloser, S. H. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur. Heart J. 30, 1038–1045 (2009).
January, C. T. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 74, 104–132 (2019).
Halvorsen, S. et al. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur. Heart J. Cardiovasc. Pharmacother. 3, 28–36 (2017).
Jalota, A. et al. Novel anticoagulants for stroke prevention in patients with atrial fibrillation. Cardiovasc. Drugs Ther. 28, 247–262 (2014).
Kim, D. et al. The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients. Europace 22, 547–557 (2020).
Yao, X. et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J. Am. Heart Assoc. 5, e003074 (2016).
Hernandez, I., He, M., Brooks, M. M., Saba, S. & Gellad, W. F. Adherence to anticoagulation and risk of stroke among medicare beneficiaries newly diagnosed with atrial fibrillation. Am. J. Cardiovasc. Drugs 20, 199–207 (2020).
Alberts, M. J. et al. Association between once- and twice-daily direct oral anticoagulant adherence in nonvalvular atrial fibrillation patients and rates of ischemic stroke. Int. J. Cardiol. 215, 11–13 (2016).
Hwang, J. et al. NOAC adherence of patients with atrial fibrillation in the real world: dosing frequency matters?. Thromb. Haemost. 120, 306–313 (2020).
Brown, J. D., Shewale, A. R. & Talbert, J. C. Adherence to rivaroxaban, dabigatran, and apixaban for stroke prevention for newly diagnosed and treatment-naive atrial fibrillation patients: an update using 2013–2014 data. J. Manag. Care Spec. Pharm. 23, 958–967 (2017).
Kim, J. A., Yoon, S., Kim, L. Y. & Kim, D. S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 32, 718–728 (2017).
Johnson, E. S. et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol. Drug Saf. 22, 1–6 (2013).
Tsivgoulis, G. et al. Neuroimaging and clinical outcomes of oral anticoagulant-associated intracerebral hemorrhage. Ann. Neurol. 84, 694–704 (2018).
Lip, G., Freedman, B., De Caterina, R. & Potpara, T. S. Stroke prevention in atrial fibrillation: past, present and future. Thromb. Haemost. 117, 1230–1239 (2017).
Ray, W. A. Evaluating medication effects outside of clinical trials: new-user designs. Am. J. Epidemiol. 158, 915–920 (2003).
Komen, J. J. et al. Long-term persistence and adherence with non-vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur. Heart J. Cardiovasc. Pharmacother. 7, f72–f80 (2021).
Hess, L. M., Raebel, M. A., Conner, D. A. & Malone, D. C. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann. Pharmacother. 40, 1280–1288 (2006).
Park, J. et al. Validation of diagnostic codes of major clinical outcomes in a National Health Insurance database. Intern. J. Arrhythm. 20, 5 (2019).
Beyer-Westendorf, J. et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients-results from the Dresden non-interventional oral anticoagulation registry. Europace 17, 530–538 (2015).
Lee, C. J. et al. Assessing absolute stroke risk in patients with atrial fibrillation using a risk factor-based approach. Eur. Heart J. Cardiovasc. Pharmacother. 7, f3–f10 (2021).
Quan, H. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 43, 1130–1139 (2005).
Matarese, A., Sardu, C., Shu, J. & Santulli, G. Why is chronic obstructive pulmonary disease linked to atrial fibrillation? A systematic overview of the underlying mechanisms. Int. J. Cardiol. 276, 149–151 (2019).
Zhang, C. et al. Association of atrial fibrillation and clinical outcomes in adults with chronic kidney disease: a propensity score-matched analysis. PLoS ONE 15, e0230189 (2020).
Olesen, J. B. et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 367, 625–635 (2012).
Rivera-Caravaca, J. M. et al. A propensity score matched comparison of clinical outcomes in atrial fibrillation patients taking vitamin K antagonists: comparing the “real-world” vs clinical trials. Mayo Clin. Proc. 93, 1065–1073 (2018).
Lynch, K. T. et al. Bariatric surgery reduces incidence of atrial fibrillation: a propensity score-matched analysis. Surg. Obes. Relat. Dis. 15, 279–285 (2019).
Parsons, L. S. Reducing bias in a propensity score matched-pair sample using greedy matching techniques, in Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference 214–226 (2001).
Rutherford, O. W., Jonasson, C., Ghanima, W., Söderdahl, F. & Halvorsen, S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: a nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 6, 75–85 (2020).
Kachroo, S. et al. Oral anticoagulant discontinuation in patients with nonvalvular atrial fibrillation. Am. J. Manag. Care 22, e1–e8 (2016).
Perreault, S. et al. Oral anticoagulant prescription trends, profile use, and determinants of adherence in patients with atrial fibrillation. Pharmacotherapy 40, 40–54 (2020).
Proietti, M. et al. Long-term relationship between atrial fibrillation, multimorbidity and oral anticoagulant drug use. Mayo Clin. Proc. 94, 2427–2436 (2019).
Jani, B. D. et al. Multimorbidity and co-morbidity in atrial fibrillation and effects on survival: findings from UK Biobank cohort. Europace 20, f329–f336 (2018).
Wańkowicz, P. et al. Ischemic stroke risk factors in patients with atrial fibrillation treated with new oral anticoagulants. J. Clin. Med. 10, 1223 (2021).
Wańkowicz, P. et al. Pre-stroke statin therapy improves in-hospital prognosis following acute ischemic stroke associated with well-controlled nonvalvular atrial fibrillation. J. Clin. Med. 10, 3036 (2021).
Pandya, E. Y. & Bajorek, B. Factors affecting patients’ perception on, and adherence to, anticoagulant therapy: anticipating the role of direct oral anticoagulants. Patient 10, 163–185 (2017).
Shen, A. Y. et al. Racial/Ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke 39, 2736–2743 (2008).
Steffel, J. et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 39, 1330–1393 (2018).
Acknowledgements
This work was supported by a Grant (21153MFDS607) from Ministry of Food and Drug Safety of South Korea in 2021-2025.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Author information
Authors and Affiliations
Contributions
S.-Y.Y., J.-Y.S., and S.-H.K. participated in the conception and design of the study; S.-Y.Y., J.H.N. collected and analyzed the data; All authors interpreted the results; D.-W.K. and S.-Y. Y. wrote the first draft; E.-K.L. and E.-K.C. critiqued the manuscript for important intellectual content; All authors contributed to revising the draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, SY., Kang, DW., Nam, J.H. et al. Adherence is an optimal factor for maximizing the effective and safe use of oral anticoagulants in patients with atrial fibrillation. Sci Rep 12, 3413 (2022). https://doi.org/10.1038/s41598-022-07316-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07316-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.