Abstract
In this research, we assessed mortality after major bleeding events in atrial fibrillation (AF) patients taking four direct oral anticoagulants (DOACs). Drawing data from the Taiwan National Health Insurance Research Database between 2016 and 2019, we focused on AF patients on DOACs who had major bleeding episodes. Using propensity score stabilized weighting, we established four comparable pseudo-DOAC groups. Among 2770 patients (460 dabigatran, 1322 rivaroxaban, 548 apixaban, 440 edoxaban), 85.3% were prescribed low-dose regimens. The 7-day mortality rate was 9.0%, surging to 16.0% by the 30th day. Compared with dabigatran, there was a distinct divergence in 7-day mortality of factor Xa inhibitors (p = 0.012), with hazard ratios of 1.83 (95% CI 1.11–3.00, p = 0.017) for rivaroxaban, 2.13 (95% CI 1.23–3.66, p = 0.007) for apixaban, and 2.41 (95% CI 1.39–4.19, p = 0.002) for edoxaban. This pattern remained consistent when analyzing the subgroup that received lower dosages of DOACs. In conclusion, factor Xa inhibitors were associated with a significantly higher risk of 7-day mortality following major bleeding events than dabigatran among AF patients.
Similar content being viewed by others
Introduction
In atrial fibrillation (AF) patients, direct oral anticoagulants (DOACs) have been associated with comparable or even lower risks of stroke, major bleeding, and mortality compared to warfarin1,2. Further, a meta-analysis of randomized trials highlighted that all four DOAC types, including dabigatran, rivaroxaban, apixaban, and edoxaban, exhibit a better safety profile than warfarin3. Despite the lower bleeding risk, managing such events remains challenging for physicians in the emergency department (ED)4. Therefore, low-dose DOACs are frequently prescribed in real-world clinical practice5,6,7, reflecting physicians' concerns about major bleeding.
While extensive research has compared the safety profiles of DOACs to warfarin, the safety profile in terms of short-term mortality due to major bleeding among different DOACs remains limited8. Furthermore, most of these studies have focused on the incidence of major bleeding in AF patients, rather than short-term mortality following bleeding events9,10,11. Hence, we used the Taiwan National Health Insurance Research Database for a nationwide cohort study. We aimed to compare the short-term mortality after major bleeding in Asian patients with AF and receiving four DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban).
Methods
Data source
The primary data sources are the Taiwan National Health Insurance Research Database (NHIRD) and Taiwan Death Registry, available in the Health and Welfare Data Science Center. All the datasheets have been linked with individual personal identification numbers (PINs) encrypted for research. To avoid any disclosure of patient identity, the center reviews all analyzed results before releasing them12. The Institutional Review Board of China Medical University Hospital, Taiwan, approved this study and waived informed consent (CMUH109-REC2-146). All methods were performed in accordance with the relevant guidelines and regulations. Our study was conducted in compliance with Good Clinical Practice guidelines and adhered to the ethical standards outlined in the Declaration of Helsinki.
Study design
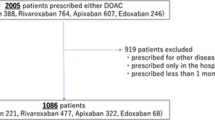
In Taiwan, dabigatran was first approved on June 1, 2012, followed by rivaroxaban (February 1, 2013), apixaban (June 1, 2014), and edoxaban (September 1, 2016), respectively. This nationwide retrospective cohort included patients with AF who received one of the DOACs and experienced following major bleeding events and visited ED during 2016–2019. Major bleeding events included intracranial hemorrhage (ICH), gastrointestinal (GI) bleeding, and bleeding at other critical sites. Noted that some patients had more than one bleeding sites. Please refer to the Supplementary Table S1 for International Classification of Diseases (Ninth and Tenth Revisions) Clinical Modification (ICD-9-CM and ICD-10-CM) codes used to define major bleeding. The index date is the ED visit for major bleeding management. We excluded patients: (1) those aged < 18 years old, (2) mild bleeding events, which did not need blood transfusion within 48 h after the ED visit except for ICH, or were discharged and alive within 48 h after the ED visit, (3) pulmonary embolism or deep vein thrombosis, mitral stenosis, or received a joint replacement or valvular surgery within 6 months before ED visit, (4) end-stage renal disease, (5) receiving more than one DOAC type within 90 days before the index date or switched from one DOAC to another before ED visit to reduce misclassification, (6) receiving idarucizumab for reversal of dabigatran during ED visit because the reversal agents for those on Factor Xa inhibitors were unavailable in Taiwan (Fig. 1). We assessed the use of DOACs which included dabigatran, rivaroxaban, apixaban, and edoxaban. The investigated dosages comprised either the standard-dose regimen or the low-dose regimen. Guided by the Taiwan NHIRD for AF treatment, the standard-dose regimen is specified as follows: 150 mg of dabigatran twice daily, 20 mg of rivaroxaban once daily, apixaban 5 mg twice daily, and 60 mg of edoxaban once daily. Patients who were administered dosages below these specified standard doses were categorized into the low-dose group.
In this study, we utilized the coding system defined in the Taiwan National Health Administration to identify surgical and medical hemostasis approaches employed in managing major bleeding events. These approaches are detailed in Supplementary Table S2.
Study outcomes
The primary outcome of this study was all-cause mortality within the first 7 and 30 days following ED visit due to major bleeding events. The 7-day mark was selected for its criticality in evaluating immediate mortality risk13,14. Evidence suggests that the risk of death is particularly high in the days immediately following ED admission, increasing cumulatively over time15. Notably, research indicates that approximately half of all deaths following major bleeding events occur within the first month, with a significant proportion—around one-third—occurring on the first day itself8. Consequently, the 7-day period provides vital insight into the acute phase of mortality risk. On the other hand, the 30-day timeframe was selected to offer a broader view of mortality outcomes, which is especially pertinent in conditions like intracranial hemorrhage where longer-term risks are relevant8,16,17.
Covariates
The covariates were relevant comorbidities or medications prior to the index date. The CHA2DS2-VASc score (congestive heart failure, hypertension, age 75 years or older, diabetes, previous stroke/transient ischemic attack, vascular disease, age 65–74 years, female) had been derived to predict the risk of thromboembolism in patients with AF18. The HAS-BLED score (hypertension, abnormal renal or liver function, stroke, bleeding history, labile INR, age 65 years or older, and antiplatelet drug or alcohol use) has been derived to predict the risk of bleeding in AF patients treated with DOACs19. Medications were confined to prescription at least once within 3 months before the index date, including NSAIDs (nonsteroidal anti-inflammatory drugs), PPI (proton pump inhibitor), ACEI (angiotensin-converting-enzyme inhibitor)/ARB (angiotensin II receptor antagonists, β-blocker, DHP-CCB (dihydropyridine calcium channel blockers), nonDHP-CCB (diltiazem/verapamil). Please refer to the Supplemental Table S1 for ICD-9-CM and ICD-10-CM codes used to define comorbidities.
Statistical analysis
We used propensity score stabilized weighting (PSSW) to create four pseudo- DOAC groups with similar baseline covariates20. The propensity score (PS) was estimated using the generalized boosted model (GBM) with all covariates in Table 1 included, except for CHA2DS2-VASc and HAS-BLED scores because these two scores were already a combination of other covariates. GBM involves an iterative process with multiple regression trees to capture complex and nonlinear associations to maximize the balance in the covariates across the four DOAC groups21. Furthermore, the PS provided by GBM is less affected by model misspecification and outlying weights22. We used the maximum of absolute standardized mean difference (max(ASMD)) to assess whether the four DOAC groups were well-balanced in covariates if an ASMD value of ≤ 0.123. We also examined the overlap of the PS distribution for the four DOAC groups because PSSW shows bias and excessive variance in the presence of PS tails24.
The mortality rate was computed as the number of patients dying after ED admission divided by the total number of eligible patients. The Cox proportional hazards regression model was made to show the hazard ratio (HR) with the 95% confidence interval (CI) to show the risk of mortality for DOACs, and the reference was dabigatran. Only the DOAC grouping was included in the Cox model because the 4 DOAC groups were well-balanced in covariates after PSSW. Subgroup analysis was made to examine whether the mortality of various types of major bleeding and dosing (low vs. standard) was similar to the overall group. For each subgroup analysis, we reconducted PSSW to ensure the well-balanced covariates among the 4 DOAC groups. A 2-sided p < 0.05 indicated statistical significance. All statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc).
Results
Patient characteristics and baseline demographics
From June 1, 2016, to December 31, 2019, our research enrolled patients receiving four DOACs, including dabigatran (460 patients), rivaroxaban (1322 patients), apixaban (548 patients), and edoxaban (440 patients). We initially noticed some heterogeneities between study groups (Supplementary Table S3). However, after performing propensity score weighting across all study groups, these heterogeneities were effectively mitigated, resulting in a well-balanced cohort (ASMD < 0.1) (Table 1).
Mortality Outcomes between DOACs
Throughout the 30-day observation period, there was no statistical difference in all-cause mortality across the four DOACs (Fig. 2a, log-rank p = 0.241). However, the dabigatran group had lower mortality than those receiving factor Xa inhibitors, including rivaroxaban, apixaban, and edoxaban, during the first 7-day interval (Fig. 2b, log-rank p = 0.012). There were no statistically significant differences across different DOAC groups for the 8-30 day interval (log-rank p = 0.732).
Mortality Rate Comparison Among Four DOACs. (a) Illustrates divergent trajectories, though these differences did not achieve statistical significance (log-rank p = 0.2411). In (b), the observation period is divided into two intervals: the initial 7 days and the subsequent 8–30 days. During the first 7-day interval, a statistically significant separation of mortality rates was observed (log-rank p = 0.0115). Conversely, the mortality rates for the 8–30 days period converged, with no statistical significance observed (DOACs, direct oral anticoagulants).
Table 2 compares mortality rates and hazard ratios among the four DOACs, using dabigatran as the reference group. Before PSSW was implemented, the lowest 7-day mortality rate was observed in the dabigatran group at 4.6%, followed by rivaroxaban at 9.0%, apixaban at 10.8%, and edoxaban at 11.1%. The hazard ratios, which indicate the risk of 7-day mortality, were approximately two times higher for the factor Xa inhibitors than dabigatran. Specifically, after applying PSSW, the hazard ratios were 1.83 (95% CI 1.11–3.00, p = 0.017) for rivaroxaban compared to dabigatran, 2.13 (95% CI 1.23–3.66, p = 0.007) for apixaban compared to dabigatran, and 2.41 (95% CI 1.39–4.19, p = 0.002) for edoxaban compared to dabigatran.
Cause of death following major bleeding events
The crude mortality rate was 9% at the 7-day interval and reached 16% at 30 days. In other words, 56% of death occurred during the first 7 days after ED admission, as shown in Table 3. In this initial 7-day period, cerebrovascular disease accounted for 54.4% of the mortality causes, surpassing other causes (26.2%), cardiac disease (12.9%), and cancer (6.5%). In the subsequent 8–30 day period, the proportion of deaths attributable to cerebrovascular disease decreased to 36.9%, followed by other causes (31.3%), cardiac disease (16.9%), and cancer (14.9%). Patients treated with Dabigatran had lower cerebrovascular-related mortality proportion (23.8% within 7 days and 37.5% in the 8–30 day interval) in comparison to those receiving factor Xa inhibitors, though these findings did not reach statistical significance.
Subgroup analysis of mortality outcomes among different bleeding sites and dose-regimen
Figure 3 displays a subgroup analysis highlighting 7-day mortality rates across different bleeding sites. Among patients who experienced ICH, those treated with dabigatran demonstrated the lowest 7-day mortality rate of 12.09%. In comparison, the rates were 20.95% for rivaroxaban (HR 1.84, 95% CI 0.94–3.60, p = 0.074), 22.09% for apixaban (HR 1.85, 95% CI 0.88–3.87, p = 0.103), and 29.81% for edoxaban (HR 2.70, 95% CI 1.27–5.74, p = 0.009). The only statistically significant difference among these comparisons was observed between dabigatran and edoxaban. Regarding GI bleeding, there was no statistically significant difference in the 7-day mortality rates among the four DOACs.
Subgroup Analysis of Cumulative 7-Day Mortality Rates by Bleeding Sites and Dosage. Among patients who experienced ICH, those treated with dabigatran had a 7-day mortality rate of 12.09%, the lowest among the group. For patients on a low-dose regimen, Factor Xa inhibitors exhibited higher hazard ratios for 7-day mortality compared to dabigatran. (DOACs, direct oral anticoagulants; ICH, intracranial hemorrhage; GI, gastrointestinal; R, rivaroxaban; A, apixaban; E, edoxaban; HR, hazard ratio; CI, confidence interval).
Overall, 14.7% of the patients received standard-dose DOACs, whereas 85.3% received low-dose DOACs. The proportion of patients who received low-dose DOACs was 93% in the dabigatran group, 96% in the rivaroxaban group, 68% in the apixaban group, and 67% in the edoxaban group (Fig. 1). Low-dose regimens of four DOACs exhibit considerable disparities in outcomes, as depicted in Fig. 3. Mainly, dabigatran presents a significantly lower mortality risk than the other three DOACs. In contrast, low-dose rivaroxaban, apixaban, and edoxaban were associated with increased mortality risks, with hazard ratios of 1.71 (95% CI 1.05–2.77, p = 0.030), 2.21 (95% CI 1.27–3.83, p = 0.005), and 2.18 (95% CI 1.22–3.89, p = 0.008), respectively. These findings were starkly contrasted to those observed with standard-dose dabigatran, which showed no statistically significant differences from the other DOACs. Furthermore, in an investigation of individual DOACs (Fig. 4), no significant difference in mortality rates was observed between standard and low-dose regimens.
Management of major bleeding
Among the 2770 patients who experienced DOAC-related major bleeding events, 11.3% underwent surgical intervention, while 35.3% received medical interventions such as endoscopic hemostasis or transarterial embolization (TAE). Additionally, 7.9% required blood transfusions of more than four units, as detailed in Table 4. Patients treated with dabigatran were more likely to receive endoscopic hemostasis, TAE, and blood transfusions. Notably, the differences in medical intervention and blood transfusion rates were statistically significant when comparing the dabigatran group with the apixaban group.
Of these 2770 patients, 60.3% experienced GI bleeding, 28.4% ICH, and the remaining 12.5% suffered bleeding in other sites. The incidence of ICH as a major bleeding type was 19.8% in the dabigatran group, 31.8% in the rivaroxaban group, 31.4% in the apixaban group, and 23.6% in the edoxaban group. The dabigatran group exhibited a higher proportion of GI bleeding (70.2%), followed by edoxaban (63.9%), rivaroxaban (57.3%), and apixaban (56.6%). Notably, a significantly higher proportion in the dabigatran group needed blood transfusions (> 4 units) for GI and other sites bleeding compared to the apixaban group. Similarly, for other site bleeding events, the need for blood transfusions (> 4 units) was significantly higher in the dabigatran group than the rivaroxaban group.
Discussion
In this nationwide retrospective cohort study of 2770 major bleeding events among Asian AF patients treated with DOACs, we found that the hazard ratio for 7-day mortality in those treated with factor Xa inhibitors was twice those treated with dabigatran. Additionally, the 7-day mortality rate was lower following dabigatran bleeding events than factor Xa inhibitors, although the differences in 30-day mortality did not reach statistical significance. Furthermore, the initial 7-day mortality rates varied between 4.6 and 11.1% among different DOACs, but decreased to 7.0% in the 8–30 day period after the bleeding event. This finding underscores the importance of prompt identification and expedited management, particularly during the first week after a major bleeding event.
The mortality rate after major bleeding events in our study involving DOACs aligns with previous research16,17. In our study, the 30-day mortality rate related to major bleeding was 16%, which is consistent with a cohort study that included 2002 patients presenting to the hospital with DOAC or warfarin-related bleeding, reporting a 30-day mortality rate of 12.6% for DOAC-related major bleeding17. In our research, the 7-day and 30-day mortality rates in patients receiving dabigatran were 4.6% and 11.5%, respectively. These rates are compatible with a phase III trial that indicated a 7-day and 30-day mortality rate of 5.3% and 9.1% for dabigatran16. Although the consistency of these findings across studies is noteworthy, there remains a gap in the research regarding direct comparisons among different DOACs. As a result, our study fills this gap by highlighting differences in the 7-day mortality rate among DOACs. Specifically, we observed that Factor Xa inhibitors had a mortality rate of 9.2–11.1%, twice of dabigatran (4.6%).
NOACs-related ICH is the most feared major bleeding event due to its associated morbidity and mortality. In our study, the 7-day ICH-related mortality rate for NOACs ranged from 12.0 to 29.8%, which is higher than the GI-bleeding-related mortality rate of 2.79–5.48%. Among different types of bleeding events, ICH accounted for 28.4% of cases but accounted for nearly half of the deaths. This finding aligns with a multinational study where ICH represented 24.8% of patients and was identified as the primary cause of death within one month following a major bleeding event with NOACs8. Of note, the dabigatran group showed a lower ICH incidence (19.8%) compared to factor Xa inhibitors (23.6–31.8%), suggesting a potential link between lower ICH rates and reduced 7-day mortality. In contrast to ICH, GI bleeding usually leads to earlier medical intervention. The dabigatran group not only experienced more GI bleeding events (70.2%) compared to factor Xa inhibitors (56.6%-63.9%) but also underwent more medical interventions (41.5%). This proactive approach in treating GI bleeding may explain the lower 7-day mortality seen in the dabigatran group after major bleeding events.
The physiological mechanisms underlying the lower 7-day mortality of dabigatran compared to Xa inhibitors remain unclear. However, one potential explanation could be attributed to inherent differences between dabigatran and Xa inhibitors. For instance, in a study involving 9769 patients with nonvalvular atrial fibrillation, dabigatran was found to be associated with a lower risk of acute kidney injury (AKI) when compared to warfarin25. In contrast, the Xa inhibitor apixaban did not demonstrate a statistically significant relationship with renal outcomes. It is worth noting that AKI is closely linked to mortality outcomes in patients experiencing bleeding events26,27. Therefore, we cannot exclude the possibility of different AKI outcomes among NOACs contributing to the varying mortality rates after major bleeding. Further research is needed to elucidate the underlying mechanisms.
Previous studies demonstrated that even low-dose DOACs have a favorable safety profile compared to warfarin in patients with atrial fibrillation3. The use of low-dose DOACs in treating AF becomes particularly notable in East Asia, where specific factors such as a lower average body mass index, ethnicity, and renal clearance of DOACs have influenced the preference for prescribing low-dose DOACs28,29. This trend is evident in nationwide cohorts from Taiwan and Korea, where nearly 90% and more than 60% of patients are prescribed low-dose dabigatran and rivaroxaban, respectively30,31. Of note, in our study including patients with NOACs-related major bleeding, there was also a higher prevalence of low-dose use of DOACs than standard-dose. Even though, low-dose regimens of four DOACs exhibit considerable disparities in outcomes. Dabigatran still presents a significantly lower mortality risk than the other three DOACs of Xa inhibitors.
This study stands out for its robust methodology and significant insights into the short-term mortality outcomes associated with different DOACs in Asian AF patients. The use of the Taiwan NHIRD, which covers 99% of the Taiwanese population, enabled a large-scale, nationwide retrospective cohort study. The study's propensity score stabilized weighting ensures well-balanced comparison groups to enhance the validity of our findings. Furthermore, our focus on both 7-day and 30-day mortality provides a nuanced understanding of the immediate and short-term risks following major bleeding events. The subgroup analyses across different bleeding sites and dose regimens offer valuable insights, especially in the context of East Asian patient populations where low-dose DOACs are prevalently prescribed. By accessing the death registry system, we demonstrated the cause of death, categorized by the specific type of oral anticoagulant administered. Our findings fill a critical gap in existing literature by comparing short-term mortality outcomes among all four major DOACs, providing crucial information for clinicians in managing AF patients at risk of major bleeding.Our study has some limitations. First, our study lacks information about post-bleeding management, such as administration of prothrombin complex concentrates, which may have an impact on clinical outcomes after bleeding. Second, there were only a small number of patients (n = 20) who received the reversal agent idarucizumab during the study period. Additionally, the reversal agent for factor Xa inhibitors was not available in Taiwan. Therefore, we excluded patients who received idarucizumab for the reversal of dabigatran effect in our study. Third, the NHIRD does not capture information such as creatinine level or body weight data, which may affect the clinical mortality outcomes.
In conclusion, our study found a higher hazard ratio for 7-day mortality in Asian AF patients with major bleeding related to Factor Xa inhibitors than dabigatran, although differences in 30-day mortality did not reach statistical significance. The mortality rate mainly occurred within the initial 7-day period, emphasizing the urgent need for prompt identification and intervention in cases of major bleeding.
Data availability
The data for this study were sourced from the National Health Insurance Research Database Taiwan (NHIRD), which is housed exclusively in the Health and Welfare Data Science Center. Due to restrictions on data sharing, we are unable to make the research data publicly available. However, the datasets used and/or analyzed in this study can be made available by the corresponding author upon reasonable request.
References
Yao, X. et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J. Am. Heart Assoc. 5, e003725 (2016).
Chan, Y. H. et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J. Am. Heart Assoc. 7, e008150 (2018).
Ruff, C. T. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 383, 955–962 (2014).
Baugh, C. W. et al. Anticoagulant reversal strategies in the emergency department setting: Recommendations of a multidisciplinary expert panel. Ann. Emerg. Med. 76, 470–485 (2020).
Cheng, W.-H. et al. Low-dose rivaroxaban and risks of adverse events in patients with atrial fibrillation. Stroke 50, 2574–2577 (2019).
Murata, N. et al. Clinical outcomes of off-label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF Registry. Circ. J. 83, 727–735 (2019).
Yu, H. T. et al. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J. Am. Heart Assoc. 9, e014177 (2020).
Bassand, J.-P. et al. Bleeding and related mortality with NOACs and VKAs in newly diagnosed atrial fibrillation: Results from the GARFIELD-AF registry. Blood Adv. 5, 1081–1091 (2021).
Chan, Y.-H. et al. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest 156, 529–543 (2019).
Wolfe, Z., Khan, S., Nasir, F., Subramanian, C. R. & Lash, B. A systematic review and Bayesian network meta-analysis of risk of intracranial hemorrhage with direct oral anticoagulants. J. Thromb. Haemost. 16, 1296–1306 (2018).
Lip, G. Y., Larsen, T. B., Skjøth, F. & Rasmussen, L. H. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 60, 738–746 (2012).
Hsieh, C.-Y. et al. Taiwan’s national health insurance research database: past and future. Clinical epidemiology, 349–358 (2019).
Brabrand, M., Lassen, A. T., Knudsen, T. & Hallas, J. Seven-day mortality can be predicted in medical patients by blood pressure, age, respiratory rate, loss of independence, and peripheral oxygen saturation (the PARIS score): A prospective cohort study with external validation. PLoS One 10, e0122480 (2015).
Hsieh, M.-J. et al. Developing and validating a model for predicting 7-day mortality of patients admitted from the emergency department: An initial alarm score by a prospective prediction model study. BMJ Open 11, e040837 (2021).
Conway, R., Cournane, S., Byrne, D., O’Riordan, D. & Silke, B. Time patterns in mortality after an emergency medical admission; relationship to weekday or weekend admission. Eur. J. Intern. Med. 36, 44–49 (2016).
Majeed, A. et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 128, 2325–2332 (2013).
Xu, Y. et al. Direct oral anticoagulant-or warfarin-related major bleeding: characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest 152, 81–91 (2017).
Pamukcu, B., Lip, G. Y. & Lane, D. A. Simplifying stroke risk stratification in atrial fibrillation patients: Implications of the CHA2DS2–VASc risk stratification scores. Age Ageing 39, 533–535 (2010).
Shah, S. et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2, 200–209 (2018).
Xu, S. et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 13, 273–277 (2010).
McCaffrey, D. F., Ridgeway, G. & Morral, A. R. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol. Methods 9, 403 (2004).
Lee, B. K., Lessler, J. & Stuart, E. A. Weight trimming and propensity score weighting. PloS One 6, e18174 (2011).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Li, F., Thomas, L. E. & Li, F. Addressing extreme propensity scores via the overlap weights. Am. J. Epidemiol. 188, 250–257 (2019).
Yao, X. et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J. Am. Coll. Cardiol. 70, 2621–2632 (2017).
Wang, I.-K. et al. Renal function is associated with one-month and one-year mortality in patients with intracerebral hemorrhage. Plos One 18, e0269096 (2023).
Fiaccadori, E. et al. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int. 59, 1510–1519 (2001).
Hori, M. et al. Rivaroxaban vs warfarin in Japanese patients with atrial fibrillation–the J-ROCKET AF study. Circul. J. 76, 2104–2111 (2012).
Delgado-Fernández, M. The J-ROCKET AF study: A matter of ethnicity or a matter of weight?. Circul. J. 77, 2636 (2013).
Chan, Y.-H. et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 68, 1389–1401 (2016).
Cho, M. S. et al. Outcomes after use of standard-and low-dose non–vitamin K oral anticoagulants in Asian patients with atrial fibrillation. Stroke 50, 110–118 (2019).
Funding
This study was supported by a grant [CMRPD1M0211] from Chang Gung Memorial Hospital.
Author information
Authors and Affiliations
Contributions
J.H.Y. and D.Y.C. were instrumental in conceiving and structuring the study. L.C.S. has a grant support to access the data in Health and Welfare Data Science Center, Taiwan. L.C.S. and P.R.L. undertook the task of data processing and statistical analysis. The data curation and interpretation tasks were jointly handled by J.H.Y., D.Y.C., W.K.H., and L.C.S. The first draft of the manuscript was written by J.H.Y. and L.C.S. All authors made significant contributions to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, JH., Li, PR., Chen, DY. et al. Mortality after major bleeding in Asian atrial fibrillation patients receiving different direct oral anticoagulants: a nationwide, propensity score study. Sci Rep 14, 4771 (2024). https://doi.org/10.1038/s41598-024-55500-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55500-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.