Abstract
To solve the problems of eutrophication and resource crisis, the recovery of phosphorus by struvite (NH4MgPO4·6H2O) precipitation has become a focus of recent research. The feasibility of using Kraft lignin powder as a seed to promote struvite precipitation has been demonstrated in the previous study. In this study, the effect of lignin in promoting struvite precipitation in synthetic wastewater with different characteristics was investigated. Lignin-induced struvite crystallization was tested under various initial concentrations of PO4–P and NH4–N, total suspended solids (TSS) and alkalinity. At pH 7.9, the enhancement of PO4–P recovery remains around 45% under different PO4–P and NH4–N concentrations. Moreover, lignin is more effective under relatively lower alkalinity and still workable to reduce co-precipitates potential under higher alkalinity. Also, the effect of TSS on PO4–P recovery is not significant. Overall, the effect of lignin in promoting phosphorus recovery is relatively stable and can be used in synthetic wastewater with different characteristics.
Similar content being viewed by others
Introduction
Phosphorus is a critical raw material for the fertilizer, detergent and pesticide industries, and the demand for phosphorus is met by continuous and intensive mining of phosphate rocks1,2,3. However, phosphate rocks are a limited and non-renewable resource4, and their reservation is being depleted5,6. A great amount of effort has been made in searching for more sustainable sources to substitute phosphate rocks7,8. Meanwhile, significant levels of phosphates are released into wastewater contributing to eutrophication9,10,11. Phosphate-containing wastewater can be a viable resource for phosphorus recovery and recycling12,13,14. Recovering and recycling phosphorus from wastewater is a sustainable approach for phosphorus resource management, and this approach can achieve two benefits simultaneously: prevention of phosphorus enrichment in water bodies and conservation of a finite phosphorus resource15,16. Various technologies have been developed to recover and recycle phosphorus from wastewater. These include chemical precipitation, ion exchange, biological removal and recovery, and struvite crystallization17. In chemical precipitation, a divalent or trivalent metal salt like aluminum or iron sulphate is added to wastewater to precipitate phosphorus in the form of metal salt sludge, then phosphorus is recovered from the precipitated sludge. The drawback of this technology is that it is very difficult to separate chemically-bonded phosphorus, making phosphorus recovery inefficient18. In ion exchange, phosphorus is selectively removed and recovered by using ion exchange media. The application of this technology has been hampered due to: (a) high chemical requirement for the phosphorus recovery, and (b) the performance of the ion exchange media is highly dependent on pH19. In biological phosphorus removal and recovery systems, phosphorus in wastewater is assimilated by plants or algae. After harvesting, the plants or algae are processed through specific technologies to release phosphorus. The main disadvantages of this technology are: need for skilled man power and fluctuating performance leading to difficulty in operation20. In struvite crystallization, both nutrients (nitrogen and phosphorus) are recovered simultaneously as struvite crystal. Among these technologies, phosphorus crystallization as struvite is the most attractive one21,22, as it not only achieves high phosphorus removal from wastewater but also recovers phosphorus in the form of struvite crystal using a simple process. Struvite has a low risk of contamination by pathogens and is a product that can be easily transported and directly applied to soil23. Furthermore, struvite is a valuable and excellent fertilizer, because it minimizes the nutrients loss because of its slow releasing rate and its low water solubility24.
As for phosphorus recovery and recycling from wastewater as struvite, two approaches have been taken to enhance the efficiency. The first one is optimizing the struvite formation conditions including pH, molar ratio of Mg/P, competing ions and their concentrations, mixing speed, reactor shape and size, and temperature25. The second one is adding seed to promote struvite nucleation and crystallization26. Our previous study27 demonstrates that phosphorus recovery from the waste stream through struvite precipitation using Kraft lignin as the seed is a sustainable approach and a cleaner production process. It has been shown that at relatively low pH of 7.9, using the Kraft lignin as the seed can enhance phosphorus recovery through struvite precipitation by 44.6%, also, lignin addition at pH 7.9 has the advantage of producing high quality struvite crystals by mitigating co-precipitation. This underlines the benefit of using the Kraft lignin as the seed, because producing high purity struvite from wastewater is essential as it can increase the market value of struvite without costly subsequent purification process15. Compared with the previously reported seed materials including sand28, stainless steel mesh29, pumice stone30, and biochars31, using Kraft lignin as the seed has the following advantages: lignin materials are generated as waste from pulp and paper industries, are biodegradable and environmentally friendly, and are an ideal soil amendment for enhancing plant growth32. In addition, Kraft lignin is more efficient in promoting struvite formation27.
Phosphorus recovery through struvite formation is a process of chemical crystallization and precipitation33, therefore wastewater characteristics, such as pH, ammonium concentration, phosphate concentration, the presence of suspended solids and their concentration, and alkalinity, are possible factors influencing struvite properties34. In general, suspended solids and alkalinity were reported to negatively affect struvite formation. For example, Barnes et al.35 demonstrated that total suspended solids (TSS) above 1000 mg/L interfered with struvite precipitation process; Desmidt et al.36 studied the effect of volatile suspended solids (VSS) on urease driven struvite precipitation and found that struvite crystals could only agglomerate at low VSS concentration; Ping et al.37 found that as the concentration of TSS increased, the PO4–P recovery efficiency decreased linearly, and the purity of struvite decreased with increasing TSS concentration; Liu et al.38 have shown that alkalinity inhibited phosphorus recovery, but the adverse effect of alkalinity on phosphorus recovery had a threshold which was dependent on the initial concentration of PO4–P, in addition, alkalinity negatively affected struvite purity; Wei et al.39 reported that alkalinity inhibited struvite formation. Recently, it was reported that the increase in TSS led to not only decreased struvite recovery but also lower struvite purity40.
Our previous work27 has shown the effect of lignin as the seed material which can enhance phosphorus recovery and improve the struvite quality from the synthetical biosolid digestion supernatant. The findings that TSS and alkalinity generally had a negative effect on phosphorus recovery from struvite formation were all reported in wastewater in the absence of any seed material. The objective of this study was to investigate how wastewater characteristics influence struvite precipitation in the presence of lignin as the seed. The wastewater characteristics including PO4–P and NH4–N concentrations, TSS and alkalinity will be evaluated regarding their impact on the performance of lignin-induced struvite precipitation in terms of phosphorus recovery efficiency and purity of formed struvite. Our study further confirms the effect of lignin in promoting phosphorus recovery from synthetic wastewaters with different characteristics, which widens the scope of using lignin as the seed for promoting phosphorus recovery and recycling from various wastewaters.
Materials and methods
Materials
The synthetic wastewaters with different phosphate and ammonium concentrations were prepared using sodium dihydrogen phosphate (NaH2PO4) and ammonium chloride (NH4C1). The alkalinity was adjusted by adding sodium bicarbonate (NaHCO3). Magnesium chloride hexahydrate (MgCl2·6H2O) was added as the magnesium source for struvite. All the reagents were of analytical grade and were purchased from Sigma Aldrich. In this study, the suspended solids were separated from the raw biosolid digestate supernatant by centrifuging at 5000 rpm for 10 min, and then were added to the synthetic wastewater to mimic the different TSS concentrations. The Kraft lignin used in this study was obtained from West Fraser Hinton Pulp (Alberta, Canada), which contains low ash and low sulfur content (0 to 3 wt.%), with a specific gravity of 1.02–1.60. The properties of this Kraft lignin including its particle size distribution at different pH and dosage, X-ray powder diffraction analysis, elemental composition and zeta potential at different pH have been reported in our previous work27.
Struvite precipitation experiments
The struvite precipitation from synthetic wastewaters or the real wastewater was conducted in a beaker with a magnetic stir bar at 200 rpm for 1 min at room temperature, then decreased to 60 rpm for 45 min, and settled for 1 h to precipitate. The supernatant was filtered through a 0.45 μm glass fiber filter to measure the residual PO4–P concentration. After centrifuging the suspension samples at 5000 rpm for 10 min, the precipitate was collected by decanting the supernatant, then the precipitate was washed with distilled water by repeated dispersing in water, centrifugation and decanting the supernatant for three times, and finally dried at 40 °C overnight for characterizations. Each experiment was conducted at least in triplicate.
Based on our lignin-induced struvite precipitation study27, the optimal conditions are at pH of 7.9 with the Mg/P molar ratio of 1.5 and the lignin dosage of 6 g/L. These conditions enabled the formation of maximum amount and highest purity of struvite. Therefore, 1 M MgCl2 was added to adjust the Mg/P molar ratio to 1.5 and 1 M NaOH was used to adjust the pH to 7.9 for this study.
Chemical analysis and calculation
The PO4–P concentration in the solution was measured by Hach methods (TNT844, Hach, USA) before and after the reaction. To measure TSS, water samples were filtered through a 0.45 μm pre-weighted filter. The filter was dried in an oven at 105 °C until the weight of the filter no longer changed and the increase in filter weight representing the mass of the TSS was used to calculate the TSS concentration. The precipitate was analyzed via X-ray diffraction (XRD; Rigaku Ultimate IV, Japan) and scanning electron microscopy (SEM; Zeiss Sigma 300 VP-FESEM, USA) configured with energy dispersive X-ray spectroscopy (EDS; Bruker EDS System, USA). The peaks of the XRD spectra were compared to the Inorganic Crystal Structure Database (ICSD) for struvite confirmation using the reference card PDF #97-006-0626.
Mineral precipitation modelling
Supersaturation index (SI) of various minerals that might be formed together with struvite was calculated by the equilibrium speciation model Visual MINTEQ (ver. 3.0) to predict the potential co-precipitates. The different ion concentrations in feed synthetic wastewater were utilized as model input. SI was calculated using Eq. (1):
where IAP is the ion activity product and Ksp represents the solubility product of the precipitation phase. The minerals only precipitate when SI > 041.
Results and discussion
The influencing factors, including concentrations of PO4–P, NH4–N, TSS and alkalinity were studied to examine their impact on the performance of lignin-induced struvite precipitation from synthetic wastewater.
Struvite precipitation from synthetic wastewaters
Impact of PO4–P concentration on struvite crystallization
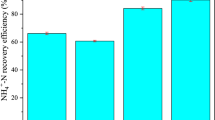
The PO4–P recovery efficiencies at different initial PO4–P concentrations were examined at the lignin dosage of 0 and 6 g/L. As shown in Fig. 1a, when the initial PO4–P concentration is 100 mg/L, the PO4–P recovery efficiency is 37.33% at the lignin dosage of 0 g/L, and it reaches 54.51% at the lignin dosage of 6 g/L (two-tailed t-test, p < 0.05, statistically significantly different). At a higher initial PO4–P concentration of 250 mg/L, the PO4–P recovery efficiency increases to 43.93% without the lignin addition and 63.13% at the lignin dosage of 6 g/L (two-tailed t-test, p < 0.05, statistically significantly different), respectively. The PO4–P recovery increment as a result of lignin addition from 0 to 6 g/L is all around 45% within the PO4–P concentration range studied. Therefore, the use of lignin is effective to enhance phosphorus recovery at different PO4–P concentrations.
The influence of initial PO4–P concentration on PO4–P recovery efficiency is consistent with the previous result that the decrease in PO4–P concentration could decrease the phosphorus removal37. The struvite crystallization is mainly attributed to the supersaturation index (SI) degree of the initial conditions determined by the concentration of all the three struvite constituent ions (Mg2+, PO43−, NH4+)42. As the concentration of PO4–P increases, the degree of supersaturation increases as well, leading to a faster struvite growth rate and consequently, a higher PO4–P recovery efficiency.
Impact of NH4–N concentration on struvite crystallization
As Fig. 1b shows, the PO4–P recovery efficiencies at different initial NH4–N concentrations were also tested at the lignin dosage of 0 and 6 g/L. The result is similar to that obtained under different initial PO4–P concentrations. As the NH4–N concentration increases from 250 to 1000 mg/L, the PO4–P recovery efficiency increases gradually under both lignin dosages of 0 and 6 g/L (two-tailed t-test, p < 0.05, statistically significantly different). The addition of lignin is still effective at various NH4–N concentrations with the maximal increment of PO4–P recovery of 44.6%.
It has been found that higher NH4–N concentration not only promotes the PO4–P recovery but also increases the purity of the precipitate43,44. It is because that the NH4–N concentration could influence the supersaturation of the solution and affect the struvite crystallization. Moreover, except for the effect on increasing ionic strength, another possible reason is that excess NH4–N is capable of maintaining the pH of the solution because of its buffering capacity, which is conducive to the struvite formation44.
Therefore, at a relatively lower pH of 7.9, the effect of lignin on enhancing PO4–P recovery efficiency is still significant at different PO4–P and NH4–N concentrations, which indicates the effect-stability and availability of lignin in promoting struvite crystallization.
Impact of alkalinity on struvite crystallization
Afterwards, we prepared the synthetic biosolid digestate supernatant with the different alkalinity to investigate the effect of alkalinity on PO4–P recovery.
Phosphorus recovery efficiency
According to Wei et al.39, alkalinity is another important parameter affecting PO4–P recovery efficiency. Without lignin addition, as Fig. 2a shows, the PO4–P recovery enhances significantly as the alkalinity of synthetic wastewater increases gradually. When the alkalinity increases from 232 to 1590 mg/L as CaCO3 at the initial pH of 7.9, the PO4–P recovery efficiency increases by 40.28% (p < 0.05, statistically significantly different). The PO4–P recovery efficiency of the synthetic supernatant under 3000 mg/L alkalinity as CaCO3 is higher than 95%, which is similar to the PO4–P recovery efficiency of the real supernatant (with 3080 mg/L alkalinity), so alkalinity may be the main reason for the high PO4–P recovery efficiency of the real lagoon supernatant. The pH of the supernatant after the reaction was measured and the results are shown in Fig. 2a. The pH after the reaction is 6.43 and 7.63, respectively, when the alkalinity is 232 and 3000 mg/L as CaCO3. As the alkalinity increases, a stronger buffering capacity prevents the pH from dropping. Therefore, under higher alkalinity, struvite crystallization and aggregation processes can last longer and lead to a higher PO4–P recovery efficiency.
While at a pH of 9.0, it shows a decreasing trend of phosphate removal with the increase of alkalinity in the wastewater in the previous study39, because the co-existing carbonate can react with the Mg2+ and form more magnesite at a higher pH.
The effect of lignin addition under different alkalinity was also investigated and the result is shown in Fig. 2b. Although the PO4–P recovery efficiency is enhanced with the lignin addition at different alkalinity, the increment of PO4–P recovery efficiency decreases as alkalinity increases. Since the PO4–P recovery efficiency is already very high at higher alkalinity without lignin addition, the effect of lignin becomes insignificant. Therefore, in this study, lignin is more efficient in enhancing the PO4–P recovery efficiency at a lower alkalinity.
Compositions of precipitate
The PO4–P recovery efficiency increases with the increasing alkalinity, while the composition of the collected precipitation may also change at the higher alkalinity. The prediction of possible precipitates under different alkalinity (1590 and 3000 mg/L as CaCO3) by Visual MINTEQ is shown in Fig. 3. As alkalinity increases, the SI values of struvite and MgCO3 increase simultaneously while the SI of Mg3(PO4)2 and MgHPO4·3H2O decreases, which indicates that struvite is more likely to crystalize under higher alkalinity. Moreover, as pH rises gradually from 8.5 to 9.5 when alkalinity is 3000 mg/L as CaCO3 (Fig. 3b), more impurities start to appear such as artinite (Mg2(OH)2CO3·3H2O) and hydromagnesite (Mg5(CO3)4(OH)2·4H2O), reducing the reduction of the produced struvite purity.
At pH 7.9, the dominant precipitate may still be struvite for both alkalinity of 1590 and 3000 mg/L as CaCO3, as the SI of struvite is the highest among all the potential precipitates. It is also proved by XRD analysis of the precipitates at the alkalinity of 3000 mg/L as CaCO3, as shown in Fig. 4. Struvite crystal is examined and confirmed by comparison with the struvite reference card from the Inorganic Crystal Structure Database (ICSD). The other precipitates are not detected in this XRD analysis, which is likely due to their small amounts. The other co-precipitates may be determined by the following SEM and EDS analysis.
Morphology and elemental analysis
The morphology characteristics of the precipitate under various alkalinities were obtained by SEM. Different magnification views for the samples at alkalinity 1590 and 3000 mg/L as CaCO3 are shown in Fig. 5.
Without lignin addition, under higher alkalinity, the struvite still remains its typical orthorhombic morphology while some irregular cluster crystals are attached to the struvite surface. The EDS results shown in Fig. 6a indicate that the cluster crystals are likely to be carbonate compounds, such as magnesium carbonate (MgCO3) simultaneously co-precipitated with struvite, which is consistent with the precipitation tendency predicted by Visual MINTEQ (Fig. 3).
Compared to struvite at the alkalinity of 232 mg/L as CaCO3, the size of the struvite produced under higher alkalinity 3000 mg/L as CaCO3 is much smaller (decreasing from 30 to around 10 μm), which is consistent with the previous study38. A possible reason may be that higher alkalinity stabilizes the pH at a relatively high level which leads to a higher supersaturation, thus the nucleation rate is higher than the growth rate and more fine particles form45,46. Therefore, although higher alkalinity results in higher PO4–P recovery, more fine crystals and co-precipitates are likely to form, which reduce the purity of the struvite collected.
Compared to the precipitate without lignin addition at the alkalinity of 3000 mg/L as CaCO3 (Fig. 5b), with 6 g/L lignin addition (Fig. 5c) the lignin-struvite clusters are observed and nearly all the crystals are orthorhombic, which indicates that the purity of struvite is higher. The EDS (Fig. 6b) also shows that Mg/P atomic ratio of the crystals are all around 1.0, which confirmed that lignin still reduces the potential of co-precipitates under higher alkalinity.
Struvite precipitation from the real wastewater
The phosphorus-rich biosolid digestate supernatant, which was collected from a digester sludge thickening lagoon in the City of Edmonton, was used to evaluate the performance of phosphorus recovery efficiency. The lagoon supernatant was pretreated on-site in an Ostara facility for phosphorus removal. The characteristics of this kind of wastewater are shown in Table 147,48.
When the real biosolid digestate supernatant under its origin pH 7.9 is used, the PO4–P recovery efficiency with or without lignin addition is 98.67% ± 0.83% and 95.55% ± 1.25% (lignin = 6 g/L or 0 g/L, Mg/P molar ratio = 1.5), respectively, which is much higher than the PO4–P recovery efficiency when using the synthetic wastewater. The composition of the real biosolid digestate supernatant is relatively complex compared to the synthetic wastewater. Therefore, the content of the total suspended solids (TSS) might also be a possible factor resulting in the higher PO4–P recovery efficiency.
To figure out if the content of TSS might contribute to a high phosphorus recovery from the real wastewater, different concentrations of TSS were added to the real biosolid digestate supernatant and the phosphorus recovery from these mixtures were tested. Different dewatering methods and operation procedures may cause variable TSS concentrations in municipal wastewater, which could influence struvite crystallization37. Figure 7a shows that under the real biosolid digestate supernatant with different TSS, there are nearly no differences of the PO4–P recovery efficiency (p > 0.05, statistically no difference). Figure 7b also depicts the relationship between the TSS and phosphorus recovery efficiency with or without the lignin addition at pH 7.9 using the synthetic biosolid digestate supernatant. When the TSS was increased from 0 to 205 mg/L, the change of PO4–P recovery efficiency was not significant (p > 0.05, statistically no difference), which indicates that in this study under the tested pH range, the PO4–P recovery efficiency for both lignin- and non-lignin- struvite precipitation is not interfered by the suspended solids. Moreover, TSS is not the related reason for the higher PO4–P recovery efficiency of real pre-Ostara biosolid digestate supernatant.
Conclusions
The lignin-induced struvite crystallization was investigated from the synthetic wastewaters with different initial PO4–P and NH4–N concentrations, TSS and alkalinity. The phosphorus recovery of lignin-induced struvite precipitation from the synthetic wastewaters increases with increasing concentrations of PO4–P, NH4–N or alkalinity. At a relatively lower pH of 7.9, the effect of lignin on enhancing struvite precipitation and PO4–P recovery from the synthetic wastewaters is still significant under different PO4–P and NH4–N concentrations. Moreover, lignin is more efficient to promote PO4–P recovery under relatively lower alkalinity and still effective to reduce the potential of co-precipitates formation under higher alkalinity. Also, in this study, the result indicates that increasing TSS concentration to 205 mg/L in the synthetic wastewater does not show the significant effect on phosphorus recovery efficiency. Therefore, the effect of lignin in promoting phosphorus recovery from synthetic wastewaters with different characteristics is confirmed in this study, which widens the scope of using lignin as the seed for promoting phosphorus recovery and recycling from various wastewaters. Compared with the synthetic wastewaters, adding lignin only slightly increases PO4–P recovery efficiency from the real biosolid digestate supernatant, which can be ascribed to its high alkalinity (> 3000 mg/L as CaCO3). It should be noted that natural wastewaters are relatively more complex than synthetic wastewaters, so the findings observed with synthetic wastewaters may not be directly applicable to natural wastewaters, and more studies on lignin-induced struvite crystallization from different natural wastewaters will be conducted.
References
Cordell, D., Rosemarin, A., Schröder, J. J. & Smit, A. L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 84, 747–758 (2011).
Rittmann, B. E., Mayer, B., Westerhoff, P. & Edwards, M. Capturing the lost phosphorus. Chemosphere 84, 846–853 (2011).
Abeysiriwardana-Arachchige, I. S. A. et al. Maximizing phosphorus recovery as biofertilizer in an algal wastewater treatment system. Resour. Conserv. Recycl. 170, 105552 (2021).
Amann, A. et al. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 130, 127–139 (2018).
Liu, X., Wen, G., Hu, Z. & Wang, J. Coupling effects of pH and Mg/P ratio on P recovery from anaerobic digester supernatant by struvite formation. J. Clean. Prod. 198, 633–641 (2018).
Al-Mallahi, J., Sürmeli, R. Ö. & Çalli, B. Recovery of phosphorus from liquid digestate using waste magnesite dust. J. Clean. Prod. 272, 122616 (2020).
Mavhungu, A. et al. Advocating circular economy in wastewater treatment: Struvite formation and drinking water reclamation from real municipal effluents. J. Environ. Chem. Eng. 8, 103957 (2020).
Krishnamoorthy, N. et al. Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. J. Environ. Chem. Eng. 9, 105579 (2021).
Ryu, H. D., Lim, C. S., Kang, M. K. & Lee, S. I. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J. Hazard. Mater. 221–222, 248–255 (2012).
Mayer, B. K. et al. Total value of phosphorus recovery. Environ. Sci. Technol. 50, 6606–6620 (2016).
Silva, M. & Baltrusaitis, J. A review of phosphate adsorption on Mg containing materials: kinetics, equilibrium, and mechanistic insights. Environ. Sci. Water Res. Technol. 6, 3178–3194 (2020).
Sena, M. & Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 139, 194–204 (2018).
Wu, B., Wan, J., Zhang, Y., Pan, B. & Lo, I. M. C. Selective phosphate removal from water and wastewater using sorption: process fundamentals and removal mechanisms. Environ. Sci. Technol. 54, 50–66 (2020).
Sena, M., Seib, M., Noguera, D. R. & Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 280, 124222 (2021).
Wang, J., Burken, J. G., Zhang, X. & Surampalli, R. Engineered struvite precipitation: Impacts of component-ion molar ratios and pH. J. Environ. Eng. 131, 1433–1440 (2005).
Bacelo, H., Pintor, A. M. A., Santos, S. C. R., Boavetura, R. A. R. & Botelho, C. M. S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 381, 122566 (2020).
Hasan, M. N. et al. Recent technologies for nutrient removal and recovery from wastewaters: A review. Chemosphere 277, 130328 (2021).
Oleszkiewicz, J., Kruk, D. J., Devlin, T., Lashkarizadeh, M. & Yuan, Q. Options for improved nutrient removal and recovery from municipal wastewater in Canadian context. Environ. Technol. 20, 681–695 (2015).
Bunce, J. T., Ndam, E., Ofiteru, I. D., Moore, A. & Graham, D. W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 6, 1–15 (2018).
Brown, N. & Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: Current understanding and future direction. Rev. Environ. Sci. Biotechnol. 13, 321–328 (2014).
Goel, S. & Kansal, A. Phosphorous recovery from septic tank liquor: Optimal conditions and effect of tapered velocity gradient. J. Clean. Prod. 275, 124056 (2020).
van der Kooij, S. et al. Phosphorus recovered from human excreta: A socio-ecological-technical approach to phosphorus recylcing. Resour. Conserv. Recycl. 157, 104744 (2020).
Desmidt, E. et al. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 45, 336–384 (2015).
Sathiasivan, K., Ramaswamy, J. & Rajesh, M. Struvite recovery from human urine in inverse fluidized bed reactor and evaluation of its fertilizing potential on the growth of Arachis hypogaea. J. Environ. Chem. Eng. 9, 104965 (2021).
Tansel, B., Lunn, G. & Monje, O. Struvite formation and decomposition characteristics for ammonia and phophorous recovery: A review of magnesium-ammonia–phosphate interactions. Chemosphere 194, 504–514 (2018).
Adnan, A., Dastur, M., Mavinic, D. S. & Koch, F. A. Preliminary invetigation into factors affecting ontrolled struvite crystallization at the bench scale. J. Environ. Eng. Sci. 3, 195–202 (2004).
Li, M. et al. Phosphorus recovery from synthetic biosolid digestion supernatant through lignin-induced struvite precipitation. J. Clean. Prod. 276, 124–235 (2020).
Ali, M. I. & Schneider, P. A. A fed-batch design approach of struvite system in controlled supersaturation. Chem. Eng. Sci. 61, 3951–3961 (2006).
Le Corre, K. S., Valsami-Jones, E., Hobbs, P., Jefferson, B. & Parsons, S. A. Struvite crystallisation and recovery using a stainless steel structure as a seed material. Water Res. 41, 2449–2456 (2007).
Pakdil, N. B. & Filibeli, A. The evaluation of pumice stone applicability at struvite crystallization by using Box–Benhken experimental design. J. Residuals Sci. Technol. 5, 95–102 (2008).
Muhmood, A. et al. Biochar seeding promotes struvite formation, but accelerates heavy metal accumulation. Sci. Total Environ. 652, 623–632 (2019).
Gul, S., Yanni, S. F., & Whalen, J. K. Lignin controls on soil ecosystem services: Implications for biotechnological advances in biofuel crops in Lignin: Structural Analysis, Applications in Biomaterials and Ecological Significance (ed. Lu, F.) 375–416 (Nova Science Publishers, 2014).
Ye, Z. et al. Phosphorus recovery from wastewater by struvite crystallization: Property of aggregates. J. Environ. Sci. 26, 991–1000 (2014).
Kataki, S., West, H., Clarke, M. & Baruah, D. C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 107, 142–156 (2016).
Barnes, D., Li, X. & Chen, J. Determination of suitable pretreatment method for old-intermediate landfill leachate. Environ. Technol. 28, 195–203 (2007).
Desmidt, E. et al. Factors influencing urease driven struvite precipitation. Sep. Purif. Technol. 110, 150–157 (2013).
Ping, Q., Li, Y., Wu, X., Yang, L. & Wang, L. Characterization of morphology and component of struvite pellets crystallized from sludge dewatering liquor: Effects of total suspended solid and phosphate concentrations. J. Hazard. Mater. 310, 261–269 (2016).
Liu, X., Xiang, L., Song, Y., Qian, F. & Meng, X. The effects and mechanism of alkalinity on the phosphate recovery from anaerobic digester effluent using dolomite lime. Environ. Earth Sci. 73, 5067–5073 (2015).
Wei, J. et al. Phosphorus recovery from wastewater using light calcined magnesite, effects of alkalinity and organic acids. J. Environ. Chem. Eng. 7, 103334 (2019).
Le, V.-G. et al. Struvite recovery from swine wastewater using fluidized-bed homogeneous granulation process. J. Environ. Chem. Eng. 9, 105019 (2021).
Desmidt, E., Ghyselbrecht, K., Monballiu, A., Verstraete, W. & Meesschaert, B. D. Evaluation and thermodynamic calculation of ureolytic magnesium ammonium phosphate precipitation from UASB effluent at pilot scale. Water Sci. Technol. 65, 1954–1962 (2012).
Adnan, A., Koch, F. A. & Mavinic, D. S. Pilot-scale study of phosphorus recovery through struvite crystallization – II: Applying in-reactor supersaturation ratio as a process control parameter. J. Environ. Eng. Sci. 2, 473–483 (2003).
Stratful, I., Scrimshaw, M. D. & Lester, J. N. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 35, 4191–4199 (2001).
Wu, J., Qian, P. & Li, Y. A pilot-scale study on struvite pellet crystallization for phosphorus recovery from sludge liquor. China Environ. Sci. 37, 941–947 (2017).
Liu, X. et al. Influence of process parameters on phosphorus recovery by struvite formation from urine. Water Sci. Technol. 68, 2434–2440 (2013).
Ghosh, S., Lobanov, S. & Lo, V. K. Impact of supersaturation ratio on phosphorus recovery from synthetic anaerobic digester supernatant through a struvite crystallization fluidized bed reactor. Environ. Technol. 40, 2000–2010 (2019).
Yang, S. et al. The value of floc and biofilm bacteria for anammox stability when treating ammonia-rich digester sludge thickening lagoon supernatant. Chemosphere 233, 472–481 (2019).
Shao, Y., Florentino, A. P., Buchanan, I., Mohammed, A. & Liu, Y. Microbial population dynamics in a partial nitrification reactor treating high ammonia strength supernatant from anaerobically digested sludge: Role of the feed water characteristics. Int. Biodeterior. Biodegrad 137, 109–117 (2019).
Acknowledgements
This work was supported by the Alberta Innovates-Alberta Bio Future Lignin Challenge 1.0 Program and Alberta Bio Future Lignin Pursuit Program, Canada Research Chair (CRC) in Future Community Water Services (Liu, Y.), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (Lu, Q.) and the Start-up Fund from the University of Calgary (Lu, Q.).
Author information
Authors and Affiliations
Contributions
M.L. conducted the experiments and wrote the original manuscript. H.S. and H.Z. conducted the data analysis using the software Visual MINTEQ. A.M. supplied water samples and supervised students. Y.L. and Q.L. designed the project, supervised the students and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, M., Zhang, H., Sun, H. et al. Effect of phosphate and ammonium concentrations, total suspended solids and alkalinity on lignin-induced struvite precipitation. Sci Rep 12, 2901 (2022). https://doi.org/10.1038/s41598-022-06930-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06930-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.