Abstract
Food waste is becoming more prevalent, and managing it is one of the most important issues in terms of food safety. In this study, functional proteins and bioactive peptides produced from the enzymatic digestion of black soldier fly (Hermetia illucens L., BSF) fed with food wastes were characterized and quantified using proteomics-based analysis. The results revealed approximately 78 peptides and 57 proteins, including 40S ribosomal protein S4, 60S ribosomal protein L8, ATP synthase subunit alpha, ribosomal protein S3, Histone H2A, NADP-glutamate dehydrogenase, Fumarate hydratase, RNA helicase, Chitin binding Peritrophin-A, Lectin C-type protein, etc. were found in BSF. Furthermore, functional analysis of the proteins revealed that the 60S ribosomal protein L5 (RpL5) in BSF interacted with a variety of ribosomal proteins and played a key role in the glycolytic process (AT14039p). Higher antioxidant activity was found in peptide sequences such as GYGFGGGAGCLSMDTGAHLNR, VVPSANRAMVGIVAGGGRIDKPILK, AGLQFPVGR, GFKDQIQDVFK, and GFKDQIQDVFK. It was concluded that the bioconversion of food wastes by BSF brought about the generation of a variety of functional proteins and bioactive peptides with strong antioxidant activity. However, more studies are required to exploit BSF's potential in the value addition of food wastes.
Similar content being viewed by others
Introduction
Food waste management is a major issue hampering the efficiency and sustainability of food supply chains. Improper food waste disposal can contribute to greenhouse gas (GHG) emissions, water depletion and threaten land use and biodiversity1,2. In most developed and developing countries, food waste is the most significant component of municipal solid waste3. Annually, around 100 million tons of food waste is generated in the European Union4. Currently, microbial technologies (e.g. anaerobic digestion, composting) treat food waste, converting organic matter into methane and fertilizer5. However, inadequate cellulose degradation, low purity of high-value products, and the tendency for fermentation systems to acidify are the major hindrances to the scaling up of these technologies6. Therefore, developing new technologies for treating and recycling food waste increases researchers' interest. Recently, transforming food waste into high-value products by feeding food waste to insects emerged as a beneficial strategy7,8. Moreover, insects' high food intake and low nutritional requirements help to dissipate food waste and convert it into high-value products such as oils and proteins9,10,11.
Hermetia illucens known as the Black Soldier Fly (BSF), is an insect belonging to the order of Dipterans and family of Stratiomyidae with a life cycle consisting of five stages; egg, larva, prepupa, pupa, and adult12. In treating solid waste, black soldier fly (Hermetia illucens L.) larvae (BSFL) offer benefits such as increased food waste reduction efficiency and reproductive capacity and reduced greenhouse gas emissions such as methane and carbon dioxide13. Studies have confirmed that treating pig manure with BSF reduces nitrogen by 55.2%, potassium by 52.8% and phosphorus by 44.3%14. Due to their simple feeding and high protein properties, insects were proposed by FAO/WHO in 2013 as sustainable protein substitutes15. BSF can be used as a feed additive for fish and poultry feeds because of its higher protein content (40–48%)16. In addition, BSF contained various bioactive peptides with antihypertensive, antimicrobial, antioxidant, anti-diabetic, immunomodulatory and mineral binding properties and could develop high-value products17,18.
Various functional components have been identified in insects, including fatty acids, flavonoids, polyphenols, proteins, antioxidant enzymes19,20,21. Enzymatic hydrolysis methods were found to have higher extraction efficiency22. Similarly, mass spectrometry is a powerful tool for identifying and quantifying immunoreactive proteins in a short time23. Likewise, it has a carefully curated database of all possible proteins present in an organism, a considerable advantage for peptide sequence identification. Some work has been conducted previously to identify bioactive peptides in different insects24,25,26,27; however, work on characterization and quantification of bioactive peptides in BSF feeding on food waste is relatively scarce.
Therefore, to address this knowledge gap, in this study, an integrated approach containing enzymatic hydrolysis and mass spectroscopy was employed for the efficient protein extraction and characterization of bioactive peptides from BSF feeding on food waste. Moreover, bioactive peptides functions, antioxidant properties, safety, and application prospects were also analyzed.
Results and discussion
Antioxidant activity of protein hydrolysate in BSF
In this study, the antioxidant properties of peptides in BSF extracts were determined by analyzing DPPH radical scavenging, ABTS radical scavenging and hydroxyl radical scavenging activities.
DPPH radical scavenging activity
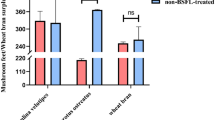
DPPH radical scavenging activity is widely used to evaluate the antioxidant properties of natural extracts28. When encountering free radical scavengers, the lone pair of DPPH electrons are paired, making its absorbance at the maximum absorption wavelength smaller. The DPPH radical scavenging activity of BLPHs was 31.06%, while the radical scavenging activities of B-1 (< 3 kDa), B-2 (3–10 kDa) and B-3 (> 10 kDa) were 42.29%, 72.23% and 0, respectively (Fig. 1a). The DPPH radical scavenging ability of BSF was enhanced by ultrafiltration treatment. The low molecular weight B-1 and B-2 exhibited the highest DPPH radical scavenging properties. However, the protein hydrolysate of B-3 failed to remove the DPPH radical. Arise et al.29 reported that peptide size was inversely related to DPPH radical scavenging activity. Similar results, with a lower molecular weight peptide exhibiting a higher DPPH radical scavenging activity, have also been reported for protein hydrolysates of rapeseed29 and milk30. Liu et al.31 reported a higher DPPH scavenging activity of low molecular weight proteins (< < 3 kDa) from the extracts of male silkworm moths.
Free radical scavenging activities of BSF protein hydrolysates (BLPHs) and ultrafiltration of different fractions B1 (< 3 kDa), B2 (3–10 KDa), B3 (> 10 kDa). (a) DPPH radical scavenging activity; (b) ABTS radical scavenging activity; (c) hydroxyl radical scavenging activity (n = 3, p < 0.05, ANOVA).
ABTS radical scavenging activity
ABTS radical scavenging activity can be applied to both lipophilic and hydrophilic compounds, and it is excellent in assessing the radical scavenging activity of protein hydrolysates and peptides32. As shown in Fig. 1b, the ABTS scavenging capacity of BLPHs was 17.34%, while the radical scavenging activities of B-1 (< 3 kDa), B-2 (3-10 kDa) and B-3 (> 10 kDa) were 89.99%, 29.00% and 0, respectively. B-1 showed the highest scavenging of ABTS, which may be due to smaller amino acids, including cysteine, tryptophan and tyrosine, amino acids with increased activity in removing ABTS33,34. Consistent with the results of the DPPH radical scavenging assay, protein hydrolysates of B-3 (MW > 10 kDa) failed to remove ABTS radicals.
Hydroxyl radicals scavenging activity
Hydroxyl radicals are the most reactive radicals and can react with most biomolecules, including peptides, proteins, lipids and DNA35. Hydroxyl radicals have the highest electron reduction potential36. Therefore, some antioxidants with strong electron transfer properties may be hydroxyl radical scavengers37. As shown in Fig. 1c, the hydroxyl radical scavenging capacity of BLPHs was 29.85%, and the radical scavenging activities of B-1 (< 3 kDa), B-2 (3–10 kDa) and B-3 (> 10 kDa) were 77.11%, 91.67% and 83.13%, respectively. The results were consistent with the free radical scavenging activities of DPPH and ABTS, with lower weight hydrolytic peptides showing better scavenging of hydroxyl radicals38,39.
Identification and annotation of peptides in BSF extracts
Based on HPLC–ESI–MS/MS proteomics analysis, a total of 78 peptides were identified from the BSF extracts and classified into 57 proteins, as shown in Table S1. Gene ontology (GO) classification is commonly used to analyze the potential function of proteins40. The potential role of functional proteins in BSF was evaluated for GO functional classification based on their biological processes, cellular composition and molecular function. As shown in Fig. 2, the most significant clusters of biological processes include 40S ribosomal protein S4 (17.65%), 60S ribosomal protein L8 (17.65%) and 40S ribosomal protein S7 (11.77%) proteins, which are mainly composed of RNA and protein aggregation binding and contribute to the formation of large ribosomal subunits. Among the functional peptides, the most abundant clusters were ATP synthase subunit alpha (17.24%), Ribosomal protein S3 (17.24%), whose functions are to regulate ATP citrate lyase activity and contribute to the structural integrity of the ribosome, respectively41,42. The more abundant cluster in the cellular component was Elongation factor-1 alpha (40%), a eukaryotic translation elongation factor substance43.
Proteomics functional analysis
To better understand the interaction of insect-derived proteins generated during the food waste treatment by BSF, we constructed a PPI network through the STRING following a previously reported method44. KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway database (https://www.kegg.jp/kegg/kegg1.html) was used to perform metabolic pathway analysis. As shown in Fig. 3 and Table 1, these interactions were divided into seven KEGG metabolic pathways: Glycolysis/Gluconeogenesis, Citrate cycle (TCA cycle), Pyruvate metabolism, Metabolic pathways, Carbon metabolism, Biosynthesis of amino acids, Ribosome. Figure 3 shows a global view of the proteome and metabolic pathways in BSF. Each circle represents a phosphorylated protein, and the straight line represents the interaction between proteins; the more substantial the interaction between two proteins, the thicker the linkage. Pyruvate kinase (CG12229) was involved in Pyruvate metabolism, Carbon metabolism, Glycolysis/Gluconeogenesis, Biosynthesis of amino acids. 60S ribosomal protein L5 (RpL5) interacts with various ribosomal proteins and directs the glycolytic process of CG1229 (AT14039p), promoting potassium and magnesium ions and pyruvate kinase activity. It has been shown that 60S ribosomal protein L5 (RpL5) is a nucleoplasmic shuttle protein that plays a vital role in assembling large ribosomal subunits and in the intracellular transport of 5S rRNA45. Teng et al.46 found that deletion of RPL5 strongly inhibited cell cycle progression with effects on cell cycle progression arising from reduced ribosome content and translational capacity, which inhibited the accumulation of cell cycle proteins at the translational level.
The biological process involved in CG4434 (Glutamate dehydrogenase [NAD(P) +]) is described as a redox process, the catabolism of glutamate to 2-ketoglutarate, which interacts with CG10749 (L-malate dehydrogenase) to promote the life activities of the BSF. The two enzymatic reactions catalyzed by phosphofructokinase and glutamate dehydrogenase have activated glycolysis47. It is involved in the biological process described with: tricarboxylic acid cycle; malate metabolic process; cellular carbohydrate metabolic process.
Antioxidant activity prediction analysis
The Peptide Ranker was used to score the peptide sequences identified by the mass spectrometry to predict the biological activity of BSF (Table 2). The amino acid sequence of the peptide was used to obtain a specific Peptide Ranker score between 0 and 1, with higher scores indicating a higher probability of being a biologically active peptide48. The Peptide Ranker prediction of BSF extracts identified 28 bioactive peptides with a probability higher than 0.30. The bioactive peptides were derived from 17 identified precursor proteins, including LIM domain protein, Elongation factor-1 alpha (Fragment), biological process protein (Histone H2A 60S ribosomal protein L8, 40S ribosomal protein S4, 60S ribosomal protein L14, Lectin C-type protein), molecular function proteins (Fumarate hydratase, RNA helicase, Ribosomal protein S3, Chitin-binding Peritrophic-A, Aconitate hydratase, mRNA helicase. Aconitate hydratase, mitochondrial, Arginine kinase).
According to the BIOPEP database49, the antioxidant fragments in the BSF peptides were predicted and found to contain mainly glycine (G), proline (P), phenylalanine (F), arginine (R) basic amino acids, histidine (H), lysine (K) basic amino acids, tyrosine (Y), valine (V) and leucine (L). H, F, Y are aromatic amino acids. V and L are hydrophobic amino acids. G is an aliphatic amino acid. P is a heterocyclic amino acid. R and K are basic amino acids.
There have been numerous reports on the relationship between amino acids and antioxidant activity. The hydrophobic amino acids in peptides can enhance the antioxidant activity of peptides by increasing their solubility in lipids and by interacting with free radicals50. Overall, the peptides GYGFGGGAGCLSMDTGAHLNR and AGLQFPVGR were identified in this experiment with high activity scores, which may be attributed to the presence of the hydrophobic amino acids glycine (G) and arginine (R) at the N-terminal end of the peptide (Table 2). The single hydrogen atom of glycine (G) contributes to the flexibility of the peptide backbone and thus to the antioxidant properties. Arginine (R) has been reported to act as a free radical scavenger, inhibiting pro-oxidant active enzymes and indirectly acting as an antioxidant to scavenge oxygen-containing free radicals with high antioxidant activity. Lysine helps to remove reactive oxygen species (hydrogen oxides, superoxide and hydroxyl radicals.) in a Maillard reaction with other reducing sugars51. Thus VVPSANRAMVGIVAGGGRIDKPILK, GFKDQIQDVFK, GFKDQIQDVFK exhibit higher antioxidant properties because of the presence of K. Among the free amino acids tryptophan, Trp (W) is known to have the strongest uptake of oxygen radicals and is thought to be responsible for the high antioxidant activity of the peptide52.
Safety evaluation of potentially active peptides
To further evaluate the safety of peptides with potential antioxidant activity extracted from BSF, an online software, ToxinPred and AllergenFP, were used to predict the peptides' allergenicity and toxicity (Table 3)53. The peptide sequences, including GYGFGGGAGCLSMDTGAHLNR, VVPSANRAMVGIVAGGGRIDKPILK, HFQAPSHIR, TIRYPDPLIK and IGGIGTVPVGR, with protein LIM domain protein, KOW motif protein, 60S ribosomal protein L8, 40S ribosomal protein S4, and Elongation factor-1 alpha (Fragment), were observed to have allergenic properties. These allergenic risk peptides need to be removed when developing active peptide products for BSF. In contrast, the antioxidant peptides AGLQFPVGR, VGIKAPGIIPR, GFIGPGVDVPAPDMGTGER, SQINFPIGGPTER, AVDSLVPIGR, GFKDQIQDVFK, TQLEPPISTPHCAR, SKIPFNVTPGSEQAR, SKIPFNVTPGSEQAR, SKIPFNVTPGSEQAR, SKIPFNVTPGSEQAR, SKIPFNVTPGSEQAR. SKIPFNVTPGSEQIR, VLVDGPLTGVPR, GVEEDWLSAR are non-toxic and non-allergenic. These peptides belong to Histone H2A, ATP synthase subunit alpha, NADP-glutamate dehydrogenase, Fumarate hydratase, RNA helicase, Chitin binding Peritrophin-A Aconitate hydratase, mitochondrial, 60S ribosomal protein L14, Lectin C-type protein.
Histone H2A variants have also been reported to have anti-microbial activity in vertebrates and invertebrates by acting as antimicrobial peptides (AMP) in host immune responses54. Arockiaraj et al. identified Histone H2A with high immune activity in freshwater shrimp55. Fumarate hydratase is an enzyme of the tricarboxylic acid cycle (TCA cycle) that catalyzes the hydration of fumaric acid to malic acid56. Fumarate hydratase was detected as a major component of the antimicrobial peptide in juniper extracts57. RNA helicase can have a direct stimulatory effect on viruses58.
In contrast, chitin-binding Peritrophin-A is widely used in packaging, agriculture and cosmetics59. Aconitate hydratase Lectin C-type protein promotes wound healing and skin protection and has been detected in sea cucumbers60,61. Therefore, BSF feeding on food waste has high antioxidant activity and application potential in food processing, cosmetics, and pharmaceuticals.
Conclusion
This study characterised the high-value antioxidant functional proteins and peptides of BSF based on HPLC–ESI–MS/MS proteomics analysis. Seventy-eight peptides and 57 proteins were identified from the BSF extracts. The most significant clusters of biological processes included 40S ribosomal protein S4 (17.65%), 60S ribosomal protein L8 (17.65%) and 40S ribosomal protein S7 (11.77%). Proteomic functional analysis revealed that the 60S ribosomal protein L5 (RpL5) in BSF could interact with the glycolytic process of CG1229 (AT14039p), thus facilitating the breakdown of glutamate to 2-ketoglutarate and other activities. Moreover, about 28 bioactive peptides with 17 precursor proteins were identified from peptide ranker prediction. Safety evaluation of bioactive peptides depicted some peptides with allergic risk, while most of the identified peptides were non-toxic and non-allergenic. Therefore, functional proteins and bioactive peptides in BSF feeding on food waste can be used in food processing, cosmetics, and medicine. Likewise, the use of food waste as feed for BSF is a promising value-addition strategy for better management of food wastes.
Materials and methods
Materials
Eggs of BSF were obtained from Changzhou Wiley Food Waste Treatment Plant (Jiangsu, China). Food waste was taken from the cafeteria of Beijing Industrial and Commercial University (Beijing, China) and used to culture BSF. Consecutive food waste collections were mixed, crushed and stored at − 20 °C. The incubation material consisted of wheat bran, peanut bran and water in a mass ratio of 1:1:13 with a moisture content of 67%.
BSF sample preparation
Incubation of BSF
Freshly qualified eggs (the eggs having ability to be hatched) of BSF were obtained and incubated for three days at 30–32 °C. 0.10 g BSFL were carefully weighed using Mettler RT1200 electronic precision scales after incubation and placed in 150 g of incubation material at 30–32 °C for five days. To keep the material soft and wet, a little amount of water is sprayed over it. The BSF was collected after the BSFL had hatched for roughly seven days and had been raised on food waste for 14 days.
Preparation of BSF samples
To eliminate any residual food from the gastrointestinal system, the BSF were fasting for around 48 h. Freeze-drying was performed to eliminate water from the insects and keep the peptides functional in the BSF. For further investigation, approximately 100 g of BSF were freeze-dried, crushed, and kept at − 18 °C. Fats were extracted using the Folch technique [19], which involved combining the extractant (chloroform and methanol 4:1 v/v) with the BSFL powder in a 5:1 ratio, sonicating for 15 min at 100 w, centrifuging at 5000 r/min at 4 °C for 5 min and collecting the residue. The defatted BSF powder was dried at 50 °C to remove the extractant.
Enzymatic extraction of BSF protein
Preparation of the enzymatic hydrolysate of BSF
At pH 8.0 and 55 °C, alkaline protease purchased from Beijing Biotopped Technology Co., LTD (Beijing, China) was used to hydrolyze the proteins. The enzyme/substrate ratio was 1.5:100 (w/v) for all hydrolysis processes, while the substrate/solvent ratio was 1:20 (w/v). The mixture was incubated for 2 h before heated for 10 min at 100 °C. The supernatant, which included BSFL protein hydrolysate solution (BLPHs), was freeze-dried and kept at − 20 °C after centrifugation for 10 min. The protein content of BLPHs was determined using the Bradford technique.
Ultrafiltration of hydrolysate
The hydrolysate was ultrafiltered using filters with a cut-off molecular weight of 3 kDa and 10 kDa, respectively, to obtain three ultrafiltration fractions, which were classified according to their molecular weight: < 3 kDa (B1), 3–10 kDa (B2) and > 10 kDa (B3)62,63,64.
Determination of the antioxidant activity of BSF
Assay of 2,2′-diphenyl-1-picrylhydrazyl(DPPH)radical scavenging activity
The capacity of BLPHs to scavenge DPPH radicals was measured according to the method described by Chung et al.65. 1 mL of sample (initial protein content is 0.2 mg/mL) was mixed with 1 mL of DPPH-95% methanol solution, and the reaction was carried out for 30 min, then absorbance was measured at 515 nm. The cleaning effect was calculated using the formula:
Asample is the absorbance of the DPPH solution with the sample; Acontrol is the absorbance of the DPPH solution without the sample.
2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate) (ABTS) radical scavenging activity
The capacity of BLPHs to scavenge ABTS radicals was performed following the already reported procedure66. Briefly, equal volumes of ABTS solution and K2S2O8 were reacted in the dark for 12 h at 25 °C, then diluted with phosphate buffer (PBS) until their absorbance at 734 nm reached 0.7 ± 0.02. Then 0.2 mL of sample (1 mg⋅mL−1) was added to 0.8 mL of working solution held at 25 °C for 5 min. The absorbance was measured at 734 nm to calculate the total antioxidant capacity.
Asample is the absorbance of the ABTs solution with the sample; Acontrol is the absorbance of the ABTs solution with 95% ethanol.
Hydroxyl radicals scavenging activity
The capacity of BLPHs to scavenge hydroxyl radicals was evaluated according to the previous method67. The samples, FeSO4 and salicylic acid–ethanol solution were mixed at a ratio of 1:1:1. Then 1.0 mL of H2O2 was added and incubated in a water bath at 37 °C for 30 min. Absorbance was measured at 510 nm using a spectrophotometer. Ascorbic acid was used as a positive control. The hydroxyl radical scavenging activity was calculated using the formula
A2 is the absorbance of the sample; A1 is the absorbance of the solution without the sample.
Peptide identification by LC–MS/MS
The UHPLC-QTOF-MS/MS (1290 Infinity Series, Agilent Technologies, Santa Clara, CA, USA) was used to determine proteins in BSF extracts. The chromatograph consisted of a binary pump, a thermostat and an autosampler coupled with a 6550 UHD iFunnel Q-TOF liquid mass spectrometry system. Compounds were ionized by electrospray ionization (ESI) using a Jet Stream Technology ion source. Chromatographic separation was performed on a 2.1 × 150 mm, 1.8 µm particle size Agilent RRHD Eclipse Plus C18 column. Instrument control and data acquisition were performed using Agilent Mass Hunter workstation software. LC parameters were set as follows: 10 µL injection volume, 0.3 mL⋅minˉ1 mobile phase flow. The mobile phase consisted of 0.1% formic acid aqueous solution (solvent A) and 0.1% formic acid acetonitrile solution (solvent B). The gradient was applied as follows: 0–4 min at 3% B; 4–42 min at 35% B; 42–47 min at 40% B; 47–52 min at 90% B; 52–55 min at 90% B and run at 3% B Run for 5 min. The ion source gas (nitrogen) was set to 250 °C at a flow rate of 14 L min−1, the nebulizer pressure was 37 psi, the sheath gas temperature was 250 °C, and the sheath gas flow rate was 11 L min−1. The capillary voltage was set to 3500 V while the nozzle voltage was set to 1000 V and the voltage of fragmentor to 400 V. Positive ions were collected in MS scan mode and automated MS/MS mode at a scan rate of 9 scan/s for MS and 7 scan/s for MS/MS. Internal mass calibration was enabled, using two reference ions at m/z 121.0509 and 922.0098.
UniProt KB/Swiss-Prot database searches were performed for protein and peptide identification using the Spectrum Mill MS Proteomics Workbench (Agilent Technologies) with a 50 ppm mass tolerance. MS/MS search results were validated using the Spectrum Mill automated threshold strategy and peptide model to optimize fractions and R1 automatically-species and protein specificity of selected peptides in FASTA format were searched against the NCBInr database using the Protein BLAST search tool and the Blastp algorithm (US National Library of Medicine).
Proteomics functional and Antioxidant activity prediction analysis
To better understand the interaction of insect-derived proteins generated during the food waste treatment by BSF, we constructed a PPI network through the STRING following a previously reported method44. While the Peptide Ranker was used to score the peptide sequences identified by the mass spectrometry to predict the biological activity of BSF.
Safety evaluation of potentially active peptides
To further evaluate the safety of peptides with potential antioxidant activity extracted from BSF, an online software, ToxinPred and AllergenFP were used to predict the peptides' allergenicity and toxicity.
Statistical analysis
One way analysis of variance (ANOVA) was used to evaluate significant differences between various parameters. The significant differences in all comparisons were set at p < 0.05. GraphPad Prism 8 was used for plotting and data analyses.
References
Ebner, J., Babbitt, C., Winer, M., Hilton, B. & Williamson, A. Life cycle greenhouse gas (GHG) impacts of a novel process for converting food waste to ethanol and co-products. Appl. Energy 130, 86–93 (2014).
Tonini, D., Albizzati, P. F. & Astrup, T. F. Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manag. 76, 744 (2018).
Sze, E., Yau, Y. H. & Wu, K. C. Application of anaerobic bacterial ammonification pretreatment to microalgal food waste leachate cultivation and biofuel production. Mar. Pollut. Bull. 153, 111007 (2020).
Winkel, T. D., Wahlen, S. & Jensen, T. in Nordic Conference on Consumer Research.
Wang, P. et al. Effects of graphite, graphene, and graphene oxide on the anaerobic co-digestion of sewage sludge and food waste: attention to methane production and the fate of antibiotic resistance genes. Bioresour. Technol. 339, 125585 (2021).
Gianico, A., Gallipoli, A., Pagliaccia, P. & Braguglia, C. M. Anaerobic bioconversion of food waste into energy: A critical review (2013).
Smetana, S., Ites, S., Parniakov, O., Aganovic, K. & Heinz, V. in 71st Annual Meeting of the European Federation of Animal Science.
Ojha, S., Buler, S. & Schlüter, O. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 118, 600–609 (2020).
Scala, A. et al. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 10, 1–8 (2020).
McDonald, C., Campbell, K. A., Benson, C., Davis, M. J. & Frost, C. J. Workforce development and multiagency collaborations: a presentation of two case studies in child welfare. Sustainability 13, 10190 (2021).
Kim, C.-H. et al. Use of black soldier fly larvae for food waste treatment and energy production in asian countries: a review. Processes 9, 161 (2021).
Julita, U., Fitri, L., Putra, R. & Permana, A. Mating success and reproductive behavior of black soldier fly Hermetia illucens L. (diptera, stra-tiomyidae) in tropics. J. Ento-mol. 17, 117–127 (2020).
Rehman, K. U. et al. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 154, 366–373 (2017).
Li, Q., Zheng, L., Hao, C., Garza, E. & Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 90, 1545–1548 (2011).
Köhler, R., Kariuki, L., Lambert, C. & Biesalski, H. K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia Pac. Entomol. 22, 372–378 (2019).
Belghit, I. et al. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture (2018).
Moretta, A. et al. Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front. Cell. Infect. Microbiol. 11, 453 (2021).
Manniello, M. et al. Insect antimicrobial peptides: potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 89, 1–24 (2021).
Henriques, B. S., Garcia, E. S., Azambuja, P. & Genta, F. A. Determination of chitin content in insects: an alternate method based on calcofluor staining. Front. Physiol. 11, ARTN 11710.3389/fphys.2020.00117 (2020).
Hbl, M., Mráz, P., Ipo, J., Hotiková, I. & Kopec, T. Polyphenols as food supplement improved food consumption and longevity of honey bees (Apis mellifera) intoxicated by pesticide thiacloprid. Insects 12 (2021).
Li, H., Dai, C., Zhu, Y. & Hu, Y. Larvae crowding increases development rate, improves disease resistance, and induces expression of antioxidant enzymes and heat shock proteins in Mythimna separata (Lepidoptera: Noetuidae). J. Econ. Entomol. 4 (2021).
Hao, et al. Effects of enzymatic hydrolysis assisted by high hydrostatic pressure processing on the hydrolysis and allergenicity of proteins from ginkgo seeds. Food Bioprocess Technol. 9, 839–848 (2016).
Nadeem, M., Mumtaz, M. W., Danish, M., Rashid, U. & Raza, S. A. Calotropis procera: UHPLC-QTOF-MS/MS based profiling of bioactives, antioxidant and anti-diabetic potential of leaf extracts and an insight into molecular docking. J. Food Meas. Charact. 13, 3206–3220 (2019).
Altomare, A. A., Baron, G., Aldini, G., Carini, M. & D’Amato, A. Silkworm pupae as source of high-value edible proteins and of bioactive peptides. Food Sci. Nutr. 8, 2652–2661 (2020).
Zielińska, E., Baraniak, B. & Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 53, 2542–2551 (2018).
Sousa, P., Borges, S. & Pintado, M. Enzymatic hydrolysis of insect Alphitobius diaperinus towards the development of bioactive peptide hydrolysates. Food Funct. 11, 3539–3548 (2020).
Jakubczyk, A., Karaś, M., Rybczyńska-Tkaczyk, K., Zielińska, E. & Zieliński, D. Current trends of bioactive peptides—New sources and therapeutic effect. Foods 9, 846 (2020).
Li, K., Li, X.-M., Ji, N.-Y. & Wang, B.-G. Natural bromophenols from the marine red alga Polysiphonia urceolata (Rhodomelaceae): structural elucidation and DPPH radical-scavenging activity. Bioorg. Med. Chem. 15, 6627–6631 (2007).
He, R., Girgih, A. T., Malomo, S. A., Ju, X. R. & Aluko, R. E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 5, 219–227. https://doi.org/10.1016/j.jff.2012.10.008 (2013).
Cui, Q., Sun, Y. X., Cheng, J. J. & Guo, M. R. Effect of two-step enzymatic hydrolysis on the antioxidant properties and proteomics of hydrolysates of milk protein concentrate. Food Chem. 366, 10. https://doi.org/10.1016/j.foodchem.2021.130711 (2022).
Liu, Y., Wan, S., Liu, J., Zou, Y. & Liao, S. Antioxidant activity and stability study of peptides from enzymatically hydrolyzed male silkmoth. J. Food Process. Preserv. 41 (2017).
Carrasco-Castilla, J. et al. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 135, 1789–1795 (2012).
Phongthai, S., D’Amico, S., Schoenlechner, R., Homthawornchoo, W. & Rawdkuen, S. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem. 240, 156 (2018).
Lee, S. J. et al. Antioxidant activity of a novel synthetic hexa-peptide derived from an enzymatic hydrolysate of duck skin by-products. Food Chem. Toxicol. 62, 276–280 (2013).
Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 20, 2407 (2019).
Xiang, Q., Yu, J. & Wong, P. K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 357, 163–167 (2011).
Wang, Y. et al. Optimizing oxygen functional groups in graphene quantum dots for improved antioxidant mechanism. Phys. Chem. Chem. Phys. 21, 1336–1343 (2019).
Arise, A. K. et al. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 7, 2431–2437. https://doi.org/10.1039/c6fo00057f (2016).
Ren, J. et al. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 108, 727–736. https://doi.org/10.1016/j.foodchem.2007.11.010 (2008).
Zhang, C., Wei, X., Omenn, G. S. & Zhang, Y. Structure and protein interaction-based gene ontology annotations reveal likely functions of uncharacterized proteins on human chromosome 17. 17 (2018).
Long, C. N. et al. High-level production of Monascus pigments in Monascus ruber CICC41233 through ATP-citrate lyase overexpression. Biochem. Eng. J. 146, 160–169. https://doi.org/10.1016/j.bej.2019.03.007 (2019).
Brito Querido, J. et al. The cryo-EM structure of a novel 40S kinetoplastid-specific ribosomal protein. Structure 25, 1785–1794 e1783. https://doi.org/10.1016/j.str.2017.09.014 (2017).
Hamey, J. J. & Wilkins, M. R. Methylation of elongation factor 1A: Where, who, and why?. Trends Biochem. Sci. 43, 211–223. https://doi.org/10.1016/j.tibs.2018.01.004 (2018).
Kuo, C. P. et al. Analysis of the immune response of human dendritic cells to Mycobacterium tuberculosis by quantitative proteomics. Proteome Sci. 14, 1–11 (2016).
Zhu, J. et al. Expression and RNA interference of ribosomal protein L5 gene in Nilaparvata lugens (Hemiptera: Delphacidae). J. Insect. Sci. 3 (2017).
Teng, T., Mercer, C. A., Hexley, P., Thomas, G. & Fumagalli, S. Loss of tumor suppressor RPL5/RPL11 does not induce cell cycle arrest but impedes proliferation due to reduced ribosome content and translation capacity. Mol. Cell. Biol. 33, 4660–4671. https://doi.org/10.1128/mcb.01174-13 (2013).
Rittschof, C. C. & Schirmeier, S. Insect models of central nervous system energy metabolism and its links to behavior. Glia (2017).
Alar, A. F., Akr, B. & Gülseren, I. LC-Q-TOF/MS based identification and in silico verification of ACE-inhibitory peptides in Giresun (Turkey) hazelnut cakes. Eur. Food Res. Technol. (2021).
Shang, W. H. et al. In silico assessment and structural characterization of antioxidant peptides from major yolk protein of sea urchin Strongylocentrotus nudus. Food Funct. 9, 6435–6443 (2018).
Ajibola, C. F., Fashakin, J. B., Fagbemi, T. N. & Aluko, R. E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 12, 6685–6702 (2011).
Liu, H. et al. Enhancing the antioxidative effects of foods containing rutin and α-amino acids via the Maillard reaction: A model study focusing on rutin-lysine system. J. Food Biochem. 44, e13086 (2020).
Tsopmo, A. et al. Tryptophan released from mother’s milk has antioxidant properties. Pediatr. Res. 66, 614–618 (2009).
Yu, Z. et al. Identification and molecular docking study of fish roe-derived peptides as potent BACE 1, AChE, and BChE inhibitors. Food Funct. 11 (2020).
Li, C. et al. Preliminary study on a potential antibacterial peptide derived from histone H2A in hemocytes of scallop Chlamys farreri. Fish Shellf. Immunol. 22, 663–672 (2007).
Arockiaraj, J. et al. An unconventional antimicrobial protein histone from freshwater prawn Macrobrachium rosenbergii: analysis of immune properties. Fish Shellfish Immunol. 35, 1511–1522 (2013).
Ju, J. et al. Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crops Products 149, 112349 (2020).
Al-Dhafri, K., Chai, L. C. & Karsani, S. A. Purification and characterization of antimicrobial peptide fractions of Junipers seravschanica. Biocatal. Agric. Biotechnol. 28, 101554 (2020).
Ratnakomala, S., Ridwan, R., Lisdiyanti, P., Abinawanto, A. & Andi, U. Screening of actinomycetes producing an ATPase inhibitor of japanese encephalitis virus RNA helicase from soil and leaf litter samples. Microbiol. Indonesia 5, 15–20 (2011).
Zhao, X., Zhang, J. & Zhu, K. Y. Chito-protein matrices in arthropod exoskeletons and peritrophic matrices (2019).
Pustylnikov, S., Sagar, D., Jain, P. & Khan, Z. K. Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J. Pharm. Pharm. Sci. 17 (2014).
Kiew, P. L. & Don, M. M. Jewel of the seabed: sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 63, 616–636 (2012).
Liu, Z., Su, Y. & Zeng, M. Amino acid composition and functional properties of giant red sea cucumber (Parastichopus californicus) collagen hydrolysates. J. Ocean Univ. China 10, 80–84 (2011).
Zaky, A. A., Liu, Y., Han, P., Chen, Z. & Jia, Y. Effect of pepsin–trypsin in vitro gastro-intestinal digestion on the antioxidant capacities of ultra-filtrated rice bran protein hydrolysates (molecular weight > 10 kDa; 3–10 kDa, and< 3 kDa). Int. J. Pept. Res. Ther. 1–7 (2019).
Kim, S.-B., Yoon, N. Y., Shim, K.-B. & Lim, C.-W. Antioxidant and angiotensin I-converting enzyme inhibitory activities of northern shrimp (Pandalus borealis) by-products hydrolysate by enzymatic hydrolysis. Fish. Aquat. Sci. 19, 1–6 (2016).
Chung, Y. C., Chang, C. T., Chao, W. W., Lin, C. F. & Chou, S. T. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 50, 2454–2458. https://doi.org/10.1021/jf011369q (2002).
Zielińska, E., BaRaniak, B. & Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 9, 1–14 (2017).
Rahman, M. M., Byanju, B., Grewell, D. & Lamsal, B. P. High-power sonication of soy proteins: Hydroxyl radicals and their effects on protein structure. Ultrason. Sonochem. 64, 105019 (2020).
Acknowledgements
This research was funded by the National Key Research and Development Project of China (2019YFC1906303), National Natural Science Foundation of China (42007350), Beijing Municipal Natural Science Foundation (8202010), and the Research Foundation for Youth Scholars of Beijing Technology and Business University (QNJJ2021-31).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.L. and P.W.; methodology, J.L.; software, J.L.; validation, Y.G., B.Z. and Y.Q.; formal analysis, Y.G.; investigation, B.Z.; resources, Y.Q.; data curation, A.M.; writing—original draft preparation, J.L.; writing—review and editing, A.M.; visualization, J.L.; supervision, L.R. and P.W.; project administration, L.R.; funding acquisition, L.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, J., Guo, Y., Muhmood, A. et al. Probing the antioxidant activity of functional proteins and bioactive peptides in Hermetia illucens larvae fed with food wastes. Sci Rep 12, 2799 (2022). https://doi.org/10.1038/s41598-022-06668-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06668-9
This article is cited by
-
Functional and biological activities of Edible Bird’s Nest (EBN) protein by proteomic and bioinformatic analyses
Journal of Food Measurement and Characterization (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.