Abstract
The sterile insect technique is a promising environmentally friendly method for mosquito control. This technique involves releasing laboratory-produced sterile males into a target field site, and its effectiveness may be affected by the extent of adult mosquito predation. Sterile males undergo several treatments. Therefore, it is vital to understand which treatments are essential in minimizing risks to predation once released. The present study investigates the predation propensity of four mantis species (Phyllocrania paradoxa, Hymenopus coronatus, Blepharopsis mendica, Deroplatys desiccata) and two gecko species (Phelsuma standingi, P. laticauda) on adult Aedes aegypti, Ae. albopictus and Anopheles arabiensis mosquitoes in a laboratory setting. First, any inherent predation preferences regarding mosquito species and sex were evaluated. Subsequently, the effects of chilling, marking, and irradiation, on predation rates were assessed. The selected predators effectively preyed on all mosquito species regardless of the treatment. Predation propensity varied over days for the same individuals and between predator individuals. Overall, there was no impact of laboratory treatments of sterile males on the relative risk of predation by the test predators, unless purposely exposed to double the required sterilizing irradiation dose. Further investigations on standardized predation trials may lead to additional quality control tools for irradiated mosquitoes.
Similar content being viewed by others
Introduction
Mosquito-borne diseases such as malaria and dengue are a significant threat to global health. Malaria, a parasitic infection transmitted by Anopheline mosquitoes, is responsible for more than 409,000 deaths in 20191 while dengue fever, a viral infection transmitted by Aedes mosquitoes, causes around 40,000 deaths every year2. With the absence of reliable vaccines for effective prevention, the management of the main vectors appears to be the best strategy of control3. Intervention efforts such as environmental sanitation, indoor residual spray, and long-lasting insecticide-treated bed nets have contributed to limiting the incidence of these diseases in the last decades3. However, their burden on public health is still substantial. The weakness of the available control strategies is partly due to the lack of comprehensive understanding of biological interactions, including co-evolution mechanisms between mosquito vectors, pathogens and environment, and the role of mosquito vectors in the food chain of living organisms4,5. Therefore, a good knowledge of such interactions in general or regarding a specific control strategy would contribute to developing more effective alternative or complementary vector control methods including biological and genetic techniques.
Natural enemies are well known in biocontrol for their beneficial actions of reducing the density of insect pests6. Early mosquito control approaches included the use of natural predators. Mainly, predators of the mosquito aquatic life stages were considered7,8,9. Predators with high predation rates on mosquito larvae and pupae include fish10,11, odonate young instars12, other mosquito species13, amphibians14, and copepods15. Although attention was mainly given to mosquito pre-imago stage predation, recent studies demonstrated that predators of adult mosquitoes, such as spiders and geckos, have a high potential to reduce mosquito populations16,17,18,19,20. Adult mosquito predators also include bats21, dragon flies22, frogs23, birds24. More broadly, adult mosquitoes may be an essential part of the food resources of terrestrial predators of arthropods such as mantises, known to prey on small flying insects25,26. Therefore, investigating adult mosquito predation would offer more biological control options and is also vital to define its potential interactions with genetic control.
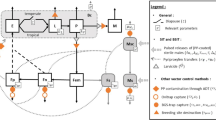
Genetic control techniques, such as the sterile insect technique (SIT), are promising tools as components of area-wide integrated management of mosquitoes. However, these techniques, involve releasing laboratory-produced (sterile) mosquitoes into a target field site, and their effectiveness may be affected by the extent of adult mosquito predation. The SIT is an environment-friendly, species-specific technique releases sterile male insects into a target area which will compete with their wild counterparts to mate with wild females, resulting in a decline of the target insect pest population27. Its success relies on the capacity of sterile males to mate with wild females and therefore requires a sufficient number of sterile males of good quality to compete with wild males28,29. This requires mastering all components of the technique, from the laboratory to the field, that can critically affect the quality of the produced mosquito. Aedes aegypti, Ae. albopictus and Anopheles arabiensis are among the species currently targeted by SIT programmes30. Significant advances have been made (and are being optimised) in developing SIT package against these mosquitoes, including colonisation, mosquito mass-rearing31,32,33,34,35,36, sex-separation37,38,39,40, handling41, radiation42,43, quality control44,45, and release46. In addition, field investigations such as the ecology of reproduction of mosquitoes47 and the sterile male behaviour48,49 have been carried out to determine the technique’s feasibility. Recent pilot trials on Aedes mosquitoes showed promising results46,50. However, much remains to be discovered about biological interactions and the fate of released males in the wild, for instance, the risk of predation by abundant predators. It has been reported that colonisation and mass rearing conditions adversely affect the ability of males of the Mexican fruit fly, Anastrepha ludens, to escape spider predation51. In addition, Rathnayake et al.52 found that sterile and fertile males of the Queensland fruit fly, Bactrocera tryoni, were similarly vulnerable to predation. Both groups were more prone to predation than females. A good understanding of the impact of adult mosquito predation on laboratory-produced males would therefore contribute to defining the SIT requirements better, especially the quality and the number of mosquitoes to release in a target area.
The present study aims to determine whether the dead leaf mantis species, Phyllocrania paradoxa, Hymenopus coronatus, Blepharopsis mendica, Deroplatys desiccata and the day geckos, Phelsuma standingi, and P. laticauda, prey on adult mosquitoes, Ae. aegypti, Ae. albopictus and An. arabiensis under laboratory settings. Furthermore, the study assessed mosquito chilling, marking and irradiation on their vulnerability to predation. Finally, the predators’ preferences between mosquito species and sexes were evaluated. The predators were selected based on the following hypotheses: they share their distribution areas with at least one of the assessed mosquito species; the mantises are leaf-flower mantis species that eat pollinators and may have interactions with mosquitoes. The selected gecko species are diurnal species which are more likely to interact with Aedes species, currently the main targets of SIT programs.
Results
Mantis predation propensity on Aedes and Anopheles mosquitoes
Dynamics of mantis predation on Aedes mosquitoes
Phyllocrania paradoxa readily preyed on Ae. aegypti mosquitoes. This mantis used sit-and-wait and active hunting modes to capture its preys (Videos 1 and 2). All individuals caught about five mosquitoes (50%) within 60 min and around ten mosquitoes in 210 min (Fig. 1). All the mosquitoes (10) were caught within 24 h. A similar capacity of preying was observed between the predators. Indeed, the risk of death of mosquitoes was similar with all P. paradoxa individuals (Cox model: χ2 = 1.601, df = 2, p = 0.4491; Supplementary Table S1).
Dynamics of mantis predation on marked Aedes albopictus mosquitoes
Phyllocrania paradoxa preyed on both marked and unmarked male Ae. albopictus. These mosquito treatments showed similar vulnerability to mantis predation (Cox model: χ2 = 1.3071 df = 2, p = 0.5202, Supplementary Table S2). Fifty per cent of mosquitoes were caught within 150 min (Supplementary Fig. S1) and all were caught within 24 h.
Effect of marking on male Aedes vulnerability to mantis predation
Hymenopus coronatus 1, P. paradoxa 1 and P. paradaoxa 2 preyed similarly on unmarked and marked male Ae. aegypti (GLMM, Mantis: χ2 = 3.1155, df = 2, p = 0.2106, Mosquito: χ2 = 0.1308, df = 1, p = 0.7176). Overall, less than 60% of each mosquito treatment were caught within one hour (Supplementary Fig. S2).
Relative vulnerabilities of Aedes aegypti, Aedes albopictus and Anopheles arabiensis species to mantis predation
Within each sex, Ae. aegypti and Ae. albopictus were similarly vulnerable to P. paradoxa predation (p > 0.05, Supplementary Table S3; Supplementary Fig. S3a,b). For female mosquitoes, no difference was observed between mantis individuals in the number of mosquitoes caught (p > 0.05, Supplementary Table S3). However, for males, P. paradoxa 3 caught more mosquitoes than P. paradoxa 2 (Supplementary Fig. S3a; Odds ratio ± se = 0.274 ± 0.116; z. ratio = − 3.060; p = 0.0022).
Females of Ae. aegypti and An. arabiensis were similarly predated by both B. mendica and H. coronatus 2 (χ2 = 0.0308, df = 1, p = 0.8607; Supplementary Table S3). However, B. mendica caught more mosquitoes than H. coronatus 2 overall (Odds ratio ± se = 3.67 ± 1.36; z. ratio = 3.506, p = 0.0005; Supplementary Fig. S4a).
Mosquito species was found to affect the predation rate in the comparison of Ae. albopictus and An. arabiensis (χ2 = 5.2805, df = 1, p = 0.02157; Supplementary Fig. S4b). Female Ae. albopictus were significantly more caught than female An. arabiensis (Odds ratio ± se = 2.06 ± 0.633, z. ratio = 2.362; p = 0.0182). No difference was observed between mantis individuals (χ2 = 2.8374, df = 2, p = 0.24203; Supplementary Table S3).
Relative vulnerabilities of male and female mosquitoes to mantis predation
Blepharopsis mendica and H. coronatus 2 and P. paradoxa 3 similarly predated male and female mosquitoes within Ae. albopictus species (Supplementary Fig. S5a; GLMM, Mantis: χ2 = 3.5950, df = 2, p = 0.1657, Mosquito sex: χ2 = 1.3470, df = 1, p = 0.2458). Within Ae. aegypti species, these mantises caught more males than females (Odds ratio ± se = 2.71 ± 0.776, z. ratio = 3.493, p = 0.0005; Supplementary Fig. S5b). Overall, B. mendica and H. coronatus 2 showed similar predation rates (Odds ratio ± se = 1.12 ± 0.37; z. ratio = 0.334, p = 0.9404) and both were statistically higher than that of P. paradoxa 3 (B. mendica /P. paradoxa 3: Odds ratio ± se = 5.28 ± 1.91; z. ratio = 4.609, p < 0.001; H. coronatus 2 /P. paradoxa 3: Odds ratio ± se = 4.73 ± 1.70; z. ratio = 4.331, p < 0.001). Within An. arabiensis (Supplementary Fig. S5c), predation rate was not affected by mosquito sex (χ2 = 1.6551, df = 1, p = 0.1983) and only B. mendica significantly caught more mosquitoes than P. paradoxa 3 (Odds ratio ± se = 3.27 ± 1.35; z. ratio = 2.863, p = 0.0117; Supplementary Table S4).
Effect of chilling on male Aedes albopictus vulnerability to mantis predation
All the mantises preyed on chilled and non-chilled Ae. albopictus males with up to 60% mosquitoes caught per treatment within 60 min (Supplementary Fig. S6). No significant difference was observed between mosquito treatments (χ2 = 2.1668, df = 1, p = 0.1410). Only B. mendica showed a slightly higher predation rate than H. coronatus 1 (Odds ratio ± se = 4.49 ± 2.48; z. ratio = 2.478, p = 0.0503; Supplementary Table S5).
Effect of irradiation on male Aedes vulnerability to mantis predation
The dosimetry confirmed that all doses received lay within a 5% error range. All the mantises preyed on irradiated and unirradiated males of both Aedes species. For Ae. aegypti, except B. mendica, all mantises mostly caught less than 50% mosquitoes per treatment within 60 min (Fig. 2). Significant effect was observed on factor mantis and on its interaction with mosquito treatment but not on the mosquito treatment (Supplementary Table S6). Blepharopsis mendica caught more mosquitoes, fertile or sterile ones, than the other predators (Supplementary Table S7). However, P. paradoxa 3 caught marginally more sterile males than fertile ones (GLMM, Emmeans, p = 0.0499; Supplementary Table S7) whereas D. desiccata caught more fertile males (GLMM, Emmeans, p = 0.0355; Supplementary Table S7) and no difference was observed with the other mantises (GLMM, Emmeans, p > 0.05; Supplementary Table S7).
For Ae. albopictus, predation rate was less than 50%. When males were irradiated with 65 Gy, a significant effect was only observed in the interaction between the mantis factor and the mosquito treatment (Supplementary Table S8). Phyllocraniaparadoxa marginally caught more fertile than sterile males (Odds ratio ± se = 4.215 ± 2.44, z. ratio = 2.480, p = 0.0131) and H. coronatus 1 similarly caught fertile and sterile males (Odds ratio ± se = 0.568 ± 0.273, z. ratio = − 1.179, p = 0.2386; Supplementary Fig. S7). In contrast, when males were irradiated with 100 Gy, no significant difference was observed between predators (χ2 = 5.8281, df = 4, p = 0.2124) and all of them caught more sterile males than fertile ones (Odds ratio ± se = 0.392 ± 0.124; z. ratio = − 2.967, p = 0.0030; Fig. 3).
Gecko predation propensity on Aedes and Anopheles mosquitoes
Predatorial behavior of Phelsuma standingi on Aedes and Anopheles mosquitoes
Phelsuma standingi showed high predation rate on mosquitoes (Video 3), up to 100% (40 mosquitoes) within an hour. Similar vulnerability to gecko predation was observed for the following compared mosquito treatments: marked vs unmarked male Ae. aegypti (GLMM, χ2 = 2.8607, df = 1, p = 0.0908; Fig. 4a), chilled vs non chilled male Ae. albopictus (χ2 = 1.0886, df = 1, p = 0.2968; Fig. 4b), male vs female Ae. aegypti (χ2 = 3.1709, df = 1, p = 0.0750; Supplementary Fig. S8a), male vs female An. arabiensis (χ2 = 0.9445, df = 1, p = 0.3311; Supplementary Fig. S8b), fertile vs sterile male Ae. aegypti (χ2 = 0.0904, df = 1, p = 0.7637; Fig. 5a), fertile vs sterile male Ae. albopictus irradiated at 65 Gy (χ2 = 1.4262df = 1, p = 0.2324; Fig. 5b) or at 100 Gy (χ2 = 1.0763, df = 1, p = 0.2995; Fig. 5c). However, P. standingi showed a marked preference for An. arabiensis as compared to Ae. aegypti (GLMM, Emmeans, Odds ratio ± se = 62.8 ± 38.6; z. ratio = 6.743, p < 0.0001; Fig. 6a), and to Ae. albopictus (Odds ratio ± se = 0.0643 ± 0.0238; z. ratio = 4.498, p < 0.0001; Fig. 6b).
Considering the total number of mosquitoes caught, P. standingi 1 was significantly more effective than P. standingi 2 in the tests “marked vs unmarked male Ae. aegypti (Odds ratio ± se = 641 ± 833; z. ratio = 4.977, p < 0.001; Fig. 4a), “chilled vs non-chilled male Ae. albopictus (Odds ratio ± se = 592 ± 646; z. ratio = 5.844, p < 0.001: Fig. 4b), fertile vs sterile male Ae. aegypti (Odds ratio ± se = 1343 ± 962; z. ratio = 10.058, p < 0.001; Fig. 5a), “fertile vs sterile male Ae. albopictus irradiated at 65 Gy” (Odds ratio ± se = 11.400 ± 0.068; z. ratio = 406.955, p < 0.001; Fig. 5b). In contrast, P. standingi 2 showed a higher predation rate than P. standingi 1 in the test “fertile vs sterile male Ae. albopictus irradiated at 100 Gy” (Odds ratio ± se = 447 ± 343; z. ratio = 7.948, p < 0.001; Fig. 5c).
Relative vulnerabilities of different mosquito species to Phelsuma laticauda predation
When Ae. aegypti and An. arabiensis were offered to P. laticauda together, predation rates were less than 40% for An. arabiensis and ranged between 0 and 90% for Ae. aegypti (Fig. 7a). No difference was observed in the total number of mosquitoes caught between P. laticauda 1 and P. laticauda 2 (GLMM: χ2 = 3.288, df = 1, p = 0.070). However, P. laticauda 2 caught more Ae. aegypti than An. arabiensis (GLMM, Emmeans, Odds ratio ± se = 8.15 ± 3.803; z. ratio = 4.498, p < 0.001) while no difference was observed with P. laticauda 1 (Odds ratio ± se = 1.60 ± 0.697; z. ratio = 1.078, p = 0.2811).
When Ae. aegypti and Ae. albopictus were offered together, predation rates were less than 50% for gecko individuals (Fig. 7b). No difference was observed in the predation rate between P. laticauda 1 and P. laticauda 2 (χ2 = 2.598, df = 1, p = 0.1070), and between mosquito species (χ2 = 2.598, df = 1, p = 0.1070).
Discussion
The results of the study showed that the predation propensity and prey preferences of the tested predators are diverse, likely mirroring their naturally encountered prey according to their native environment and behavioural activities. The mantises effectively preyed on Aedes and Anopheles mosquito species, indicating that mosquitoes are part of these mantis species diet (Fig. 8).
All the mantises were able to eat more than ten mosquitoes within an hour. Neither mosquito species and sex, nor marking and chilling affected the mosquito vulnerability to mantis predation under our study conditions. However, the effect of irradiation depended on the dose. Indeed, irradiation with 100 Gy led to more vulnerable mosquitoes as compared to unirradiated mosquitoes, whereas the doses of 65 Gy or 70 Gy mainly had no impact or even led to less vulnerable mosquitoes. It is known that prey speed influences susceptibility to predation. Since mantises are mainly sit-and-wait predators53, chances for a mosquito to escape from predation may rely on its flight speed. Adverse effects of high irradiation doses on male mosquito quality including competitiveness and flight ability have previously been reported44,45,54,55. The vulnerability of males irradiated with 100 Gy could therefore be related to reducing of their flight ability. This result indicates that high irradiation doses can expose sterile males to increased predation and then compromise the success of the SIT hence the necessity to adjust the optimal irradiation dose. However, 100 Gy is a very high dose for this species and nearly double that is needed for full sterility. The 100 Gy irradiation treatment served as verification that highly negatively affected mosquitoes, with debilitating treatment consequences are indeed more prone to predation, meaning that the actual dose applied in this species for near full sterility does not increase risk of predation- at least in artificial settings.
Mantis predation may be considered as a potential tool for quality control for laboratory-produced mosquitoes. Furthermore, our study showed that mantis predation propensity on mosquitoes is driven by complex factors that need to be elucidated. Indeed, predation rate varied between mantis species, individuals from the same mantis species, and over days of predation. In some tests, the mantises presented similar feeding rates while in others, significant differences were observed. The development stage (age) and especially molting periods and the sex of the predators may play an essential role in this variation as they were of different ages and may have different food requirements at a given time. Indeed, low predation rates (0% or nearly) were mainly recorded during molting periods. Overall and regardless of the treatment, B. mendica showed more predatory capacities as compared to the other mantis species. The use of this species could be further investigated as a quality control tool for sterile male mosquitoes.
It is has been reported that most predatory mantises exhibit a sexual cannibalism with females selectively eating the smaller males56,57. Although our study did not aim to investigate this question, cannibalism was noticed with P. paradoxa species with the loss of P. paradoxa 1 when the three individuals were maintained together in the same cage.
In general, the food web of geckos and mantises includes various species of insects including mosquitoes. Many studies carried out on house gecko feeding behavior, Hemidactylus frenatus and H. platyurus, showed that they feed mostly on Diptera with a preference for Lepidoptera and Culicidae18,58,59. However, literature research about the day geckos P. standingi and P. laticauda predation on mosquitoes was not found. Here, our study clearly showed that these geckos feed on Ae. aegypti, Ae. albopictus and An. arabiensis adult mosquitoes. Moreover, mosquito chilling, marking and irradiation procedures did not impact the laboratory-reared mosquito vulnerability to the standing’s day gecko predation. Phelsuma standingi demonstrated a relatively high predation rate, since it caught all or almost all of the mosquitoes provided (40 mosquitoes), like the Australian gecko Gehydra dubia and the exotic Asian house gecko H. frenatus which were found to consume 63–109 Aedes aegypti within 24 h in a similar study16. However, in contrast to these studies, no difference was observed between mosquito sexes in their vulnerability to P. standingi predation. The geckos used sit-and-wait for prey and active hunting predation modes to capture the mosquitoes and this may partly explain the high predation rates observed. However, like with mantises, we found that the predation rate significantly varied over time and between the two gecko individuals: at the beginning of the study, P. standingi 1 caught almost all of the mosquitoes provided (40 mosquitoes) while P. standingi 2 caught only a few, regardless of the treatment but the inverse was later observed in some tests. Critical factors such as the development stage (age) and the molting period and the sex of the gecko may also play an important role in the predation propensity. For example, Dor et al.60 found that females of the lizard, Norops serranoi, exhibited higher predation pressure and were more efficient at capturing the Mexican fruit fly, Anastrepha ludens, than males. Furthermore, our study showed that P. standingi predation propensity was affected by mosquito prey species. Indeed, when only one mosquito species was offered to this gecko, similar predation rates were observed between species. But when Anopheles and Aedes were offered together, the standing’s day gecko clearly showed preference for An. arabiensis than Ae. aegypti (80–95% vs 5–20%), and Ae. albopictus (55–100% vs 0–55%). However, the preference of P. laticauda for Aedes or Anopheles mosquito species is not clear since one individual showed similar predation rate on Ae. aegypti and An. arabiensis while another individual presented a preference for Ae. aegypti. This gecko was found to catch Ae. albopictus and Ae. aegypti similarly. The determinants of the gecko preference for a given mosquito species remains to be investigated. Like any generalist predator, gecko prey selection may be influenced by a wide range of factors including prey size, softness, taste, nutritive value, energy requirement to capture the prey and adaptive traits61,62. Indeed, gecko’s preference between mosquito species relying on their adaptive traits was previously reported by Canyon and Hii16 who observed that G. dubia preferred eating photophilic species, Ae. vigilax, An. annulipes, Coquillettidia xanthogaster, Culex annulirostris, Cx. sitiens (78–100%) compared to non-photophilic species Ae. aegypti, Ae. notoscriptus, Ae.vittiger and Cx. quinquefasciatus (33–53%).
Overall, our study has increased the cohort of known adult mosquito predators, which were not considered in the review of Collins et al.63. In addition, it has provided basic information about predation and factors that may influence predation risk of laboratory-produced sterile males. However, our results are not sufficient to predict their relationships with wild predators in the field since the study was carried out under laboratory conditions using laboratory-maintained preys and predators which are not accustomed to prey-predator interactions. Indeed, in our settings, mosquitoes had less chance to escape from predation since they were caged (30 × 30 × 30 cm for geckos and 15 × 15 × 15 cm for mantises) and could not undertake long-distance flights. In addition, the assessed predators are known to be polyphagous and their predation propensity on mosquitoes in field conditions may be influenced by other sources of food (nectar and other preys) and competitors and environmental factors. Therefore, investigations need to be enhanced by comparing, at least under semi-field conditions, the vulnerability of laboratory-produced mosquitoes and their field-collected counterparts to wild predators.
Material and methods
Biological material
Mosquito species used as preys
Aedes aegypti, Ae. albopictus and An. arabiensis mosquitoes were used as preys in this study. The strains used were maintained at the Insect Pest Control Laboratory (IPCL) of the joint FAO/IAEA Centre of Nuclear Techniques in Food and Agriculture, Seibersdorf, Austria under laboratory settings following the rearing guidelines developed at the IPCL (FAO/IAEA, 2017a, FAO/IAEA, 2017b). The Ae. aegypti strain originated from Juazeiro, Brazil and was provided to the IPCL in 2012 by Biofabrica Moscamed, a collaborative centre of the IAEA on the development of the SIT against mosquitoes. The Ae. albopictus colony originated from Rimini in Italy and was provided to the IPCL in 2018 by Centro Agricoltura Ambiente, a collaborative centre of the IAEA on the development of the Sterile Insect Technique against mosquitoes. The An. arabiensis strain originated from Dongola in the Northern State of Sudan and was provided to the IPCL by the Tropical Medicine Research Institute, Khartoum, in 2005.
Assessed predators
The predators under investigation in this study included four mantis species, Phyllocrania paradoxa, Hymenopus coronatus, Blepharopsis mendica, Deroplatys desiccata, and two gecko species, the standing’s day gecko, Phelsuma standingi, and P. laticauda. All mantises were used at their larval stage.
The characteristics of the predators are presented in Table 1. Preys and predators used in this study were kept in the same laboratory, where predation experiments were carried out. The environmental conditions in the laboratory were 26 ± 2 °C, 60 ± 10%RH, 14 h:10 h light: dark, including 1 h dawn and 1 h dusk. During the whole study period, the predators were fed only with adult mosquitoes. Predation tests were carried out on adult mosquitoes only. Predators were starved 3 days before every test. Tests performed on different days on a same predator individual were considered as replicates. Individuals from the same predator species were taken as different since they might be of different age or sex.
Investigation on mantis predation propensity on Aedes and Anopheles mosquitoes
All the tests were performed using BugDorm cages (15 × 15 × 15 cm), (MegaView Science Co. Ltd., Taichung 40762, Taiwan). Every cage contained a single predator.
Preliminary tests were carried out to determine whether mantises prey on both marked, and unmarked mosquitoes, and determine the predation rate over time. Thereafter, predation preference between different mosquito species and characteristics was evaluated.
Assessment of the dynamics of mantis predation on Aedes mosquitoes
Ten females Ae. aegypti were offered to every P. paradoxa in individual BugDorm cages. The number of remaining mosquitoes was recorded every 30 min for 210 min and after 24 h. Three individuals of P. paradoxa, named P. paradoxa 1, P. paradoxa 2, and P. paradoxa 3, were involved in this study. Two replicates were performed per individual.
Assessment of the dynamic of mantis predation on marked Aedes mosquitoes
Ten marked male Ae. albopictus were offered only to P. paradoxa 1. Both P. paradoxa 2 and P. paradoxa 3 were kept as controls with 10 unmarked male Ae. albopictus each. The number of remaining mosquitoes was recorded every 30 min for 210 min and after 24 h. Three replicates were performed. To mark, the mosquitoes were chilled for 5 min at 4 °C in a fridge and marked with pink fluorescent pigment (RADGLO JST, Radiant NV, Houthalen, Belgium or DayGlo Color Corp., Cleveland, USA) at the dose of 5 mg/1000 mosquitoes64. The control mosquitoes were chilled simultaneously for the same duration but not marked.
In the following experiments on mantis predation, we assessed their predation preference between different treatments of mosquitoes. Mosquitoes were offered to the mantises for one hour. Then, the mantises were removed from the cages, and the remaining mosquitoes were killed by freezing, identified and counted by treatment under a stereomicroscope.
Relative vulnerabilities of Aedes aegypti, Aedes albopictus and Anopheles arabiensis species to mantis predation
Relative vulnerability of Aedes species to mantis predation was evaluated by sex. Ten Ae. albopictus and 10 Ae. aegypti of the same sex were provided at once to every individual mantis. For female mosquitoes, three replicates were performed with P. paradoxa 1, P. paradoxa 2, and P. paradoxa 3. For male mosquitoes, three replicates were performed with P. paradoxa 2, and P. paradoxa 3.
Relative vulnerability of Aedes and Anopheles species to mantis predation were assessed; first, between Ae. albopictus and An. arabiensis, and second, between Ae. aegypti and An. arabiensis. For each comparison, 10 females Aedes (Ae. albopictus or Ae. aegypti) and 10 female An. arabiensis were offered at once to every individual mantis in a cage. Four replicates were performed with P. paradoxa 3, H. coronatus 2 and B. mendica in both part of the study.
Effect of marking on male Aedes vulnerability to mantis predation
Ten marked and 10 unmarked males Ae. aegypti were offered at once to every individual mantis. Four replicates were performed with P. paradoxa 2, P. paradoxa 3 and H. coronatus 1. Mosquitoes were marked as described above.
Effect of chilling on male Aedes vulnerability to mantis predation
Tests were carried out on male Ae. albopictus chilled at 4 °C for 2 h in a fridge. The chilled mosquitoes were marked with pink fluorescent pigments64 and kept in the laboratory for one hour before the test to allow recovery. The non-chilled mosquitoes were not marked. Ten chilled and 10 non-chilled males were offered at once to every individual mantis in a cage. Three replicates were performed with P. paradoxa 3, H. coronatus 1, H. coronatus 2, B. mendica, and D. desiccata.
Effect of irradiation on male Aedes vulnerability to mantis predation
A self-contained 60Co Gammacell 220 (Nordion Ltd, Kanata, Ontario, Canada) irradiator was used for mosquito irradiation. The dosimetry system used to verify the dose received by the batches was based on GafChromic HD-V2 (Ashland Advanced Materials, Bridgewater NJ, USA). Three films of HD film were packed in individual envelopes and placed directly below the adult samples. The temperature near the sample and films was measured before and after radiation exposure. Films were read with an optical density reader after 24 h of development.
Adult mosquitoes were chilled for 5 min in a refrigerator and then transferred to a plastic box for irradiation. Subsequently, the mosquitoes were kept in the laboratory for, at least, one hour before the tests. Control mosquitoes were taken from the batch of chilled mosquitoes. Tests were performed on both Ae. aegypti, and Ae. albopictus. For each mosquito species, 10 irradiated and 10 unirradiated males were offered at once to every individual mantis in separate cages. Irradiated and unirradiated males were alternatively marked in the different repetitions using pink, fluorescent dust pigment described previously to ease identification. For Ae. aegypti, four replicates were performed with P. paradoxa 3, and H. coronatus 1, and three replicates with B. mendica, H. coronatus 2, and D. desiccata. For Ae. albopictus, tests were performed four times with P. paradoxa 3and H. coronatus 1. Mosquitoes were irradiated at the adult stage with 70 Gy for Ae. aegypti and 65 Gy for Ae. albopictus. The doses induce a level of sterility exceeding 99% in these species (unpublished data from another study). To evaluate better the effect of irradiation on mosquito vulnerability to mantis predation, further tests were performed using male Ae. albopictus irradiated at 100 Gy, a very high dose expected to induce debilitating biological effects, against unirradiated males. Three replicates were performed with P. paradoxa 3, H. coronatus 1, H. coronatus 2, B. mendica, and D. desiccata.
Relative vulnerabilities of male and female mosquitoes to mantis predation
Tests were performed on Ae. albopictus, Ae. aegypti and An. arabiensis species. For every mosquito species, 10 males and 10 females were offered at once to every individual mantis in separate cages. For Ae. aegypti, five replicates were performed with P. paradoxa 3, H. coronatus 2 and B. mendica. For Ae. albopictus and for An. arabiensis, three replicates were performed with P. paradoxa 3, H. coronatus 2 and B. mendica.
Investigation on the predation propensity of the geckos, Phelsuma standingi and Phelsuma laticauda, on Aedes and Anopheles mosquitoes
Relative vulnerabilities of different mosquito species and treatments to Phelsuma standingi predation
Predation propensity of the standing’s day gecko, Phelsuma standingi, on different mosquito species and treatments was evaluated. Two individuals of P. standingi, hereafter named P. standingi 1, and P. standingi 2, were involved in this study. The gecko’s predation propensity was assessed for: (1) marked versus unmarked male Ae. aegypti: five replicates were done for both geckos; (2) chilled (at 4 °C for 2 h) versus non-chilled male Ae. albopictus: three replicates were done for both geckos; (3) fertile versus sterile male Ae. aegypti, irradiated at 70 Gy): four replicates were done with both geckos; (4) fertile versus sterile male Ae. albopictus, irradiated at 65 Gy (4 replicates) or 100 Gy (3 replicates) with both geckos; (5) male versus female Ae. aegypti: only P. standingi 2 was evaluated, and three replicated were performed; (6) female Ae. albopictus versus female An. arabiensis: only P. standingi 2 was evaluated, and six replicates were performed; (7) female Ae. aegypti versus female An. arabiensis: only P. standingi 2 was evaluated, and three replicates were performed; (8) female versus male An. arabiensis: only P. standingi 2 was evaluated, and three replicates were performed. All experiments were conducted using BugDorm cages (30 × 30 × 30 cm). Geckos were kept in separated cages. In all experiments, 20 mosquitoes from each treatment (except in the test 1 where 10 mosquitoes per treatment were used) were pooled and offered to each gecko in a cage for one hour. Subsequently, the geckos were removed from the cages, and the remaining mosquitoes were frozen, identified and counted by treatment. The procedures for mosquito chilling and marking mentioned in the experiments with mantises, apply in the ones with the geckos.
Relative vulnerabilities of different mosquito characteristics to Phelsuma laticauda predation
Predation propensity of the gold dust day gecko, P. laticauda, on different mosquito species was assessed for: (1) female Ae. aegypti versus female An. arabiensis and (2) female Ae. albopictus versus female Ae. aegypti. The two individuals of P. laticauda named P. laticauda 1, and P. laticauda 2 were used in both experiments. For each gecko, four replicates were performed for experiment 1, and three replicates for experiment 2. In each experiment, 20 females from each mosquito species were pooled and offered to each gecko in a BugDorm cage (30 × 30 × 30 cm) for one hour. Afterward, the geckos were removed from the cages, and the remaining mosquitoes were frozen, identified and counted by species.
Statistical analysis
Data were analyzed using R software (version 4.0 0.0). Mixed-effects cox regression models were used to analyze the risk of death of mosquitoes in the presence of predators. Predators were considered as fixed effect and the replicates as random effect. To compare the predation rate between predators and between different groups of mosquitoes, binomial generalised linear mixed models fit by maximum likelihood (Laplace Approximation) with the proportion of caught mosquitoes as response variable, predators (gecko or mantis), mosquito treatment as fixed effects and the replicates as random effect were used. When explanatory variables or their interaction presents a significant effect, pairwise comparisons were performed between the levels of the factors, using the “Emmeans” package and considering the best fit linear mixed model.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. World malaria report 2020: 20 years of global progress and challenges. 299 https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020 (2020).
Bhanot, K., Schroeder, D., Llewellyn, I., Luczak, N. & Munasinghe, T. Dengue spread information system (DSIS). In Proceedings of the 4th International Conference on Medical and Health Informatics 150–159 (Association for Computing Machinery, 2020). https://doi.org/10.1145/3418094.3418133.
Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831 (2020).
Carrasco, D. et al. Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 34, 48–54 (2019).
Sokhna, C., Ndiath, M. O. & Rogier, C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin. Microbiol. Infect. 19, 902–907 (2013).
Flint, M. L. & Dreistadt, S. H. Natural Enemies Handbook: The Illustrated Guide to Biological Pest Control Vol. 3386 (Univ of California Press, 1998).
Chandra, G., Bhattacharjee, I., Chatterjee, S. N. & Ghosh, A. Mosquito control by larvivorous fish. Indian J. Med. Res. 127, 13–27 (2008).
Dambach, P. The use of aquatic predators for larval control of mosquito disease vectors: Opportunities and limitations. Biol. Control 150, 104357 (2020).
Sebastian, A., Sein, M. M., Thu, M. M. & Corbet, P. S. Suppression of Aedes aegypti (Diptera: Culicidae) using augmentative release of dragonfly larvae (Odonata: Libellulidae) with community participation in Yangon, Myanmar1. Bull. Entomol. Res. 80, 223–232 (1990).
Harrington, R. W. & Harrington, E. S. Effects on fishes and their forage organisms of impounding a Florida salt marsh to prevent breeding by salt marsh mosquitoes. Bull. Mar. Sci. 32, 523–531 (1982).
Mk, D. & Rn, P. Evaluation of mosquito fish Gambusia affinis in the control of mosquito breeding in rice fields. Indian J. Malariol. 28, 171–177 (1991).
Rk, S., Rc, D. & Sp, S. Laboratory studies on the predatory potential of dragon-fly nymphs on mosquito larvae. J. Commun. Dis. 35, 96–101 (2003).
Focks, D. A., Sackett, S. R., Dame, D. A. & Bailey, D. L. Effect of weekly releases of Toxorhynchites amboinensis (Doleschall) on Aedes aegypti (L.) (Diptera: Culicidae) in New Orleans, Louisiana. J. Econ. Entomol. 78, 622–626 (1985).
Brodman, R. & Dorton, R. The effectiveness of pond-breeding salamanders as agents of larval mosquito control. J. Freshw. Ecol. 21, 467–474 (2006).
Vu, S. N., Nguyen, T. Y., Kay, B. H., Marten, G. G. & Reid, J. W. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am. J. Trop. Med. Hyg. 59, 657–660 (1998).
Canyon, D. V. & Hii, J. L. K. The gecko: An environmentally friendly biological agent for mosquito control. Med. Vet. Entomol. 11, 319–323 (1997).
Strickman, D., Sithiprasasna, R. & Southard, D. Bionomics of the spider, Crossopriza lyoni (Araneae, Pholcidae), a predator of dengue vectors in Thailand. J. Arachnol. 25, 194–201 (1997).
Tkaczenko, G., Fischer, A. & Weterings, R. Prey preference of the common house geckos Hemidactylus frenatus and Hemidactylus platyurus. Herpetol. Notes 7, 482–488 (2014).
Weterings, R., Umponstira, C. & Buckley, H. L. Landscape variation influences trophic cascades in dengue vector food webs. Sci. Adv. 4, eaap9534 (2018).
Weterings, R., Umponstira, C. & Buckley, H. L. Predation on mosquitoes by common Southeast Asian house-dwelling jumping spiders (Salticidae). Argy 16, 122–127 (2014).
Puig-Montserrat, X. et al. Bats actively prey on mosquitoes and other deleterious insects in rice paddies: Potential impact on human health and agriculture. Pest Manag. Sci. 76, 3759–3769 (2020).
May, M. L. Odonata: Who they are and what they have done for us lately: Classification and ecosystem services of dragonflies. Insects 10, 62 (2019).
Raghavendra, K., Sharma, P. & Dash, A. P. Biological control of mosquito populations through frogs: Opportunities & constrains. Indian J. Med. Res. 128, 22–25 (2008).
Poulin, B., Lefebvre, G. & Paz, L. Red flag for green spray: adverse trophic effects of Bti on breeding birds. Journal of Applied Ecology 47, 884–889 (2010).
Korichi, R. et al. Ecological impact of trophic diet of mantids in Ghardaïa (Algerian Sahara). Ponte Int. Sci. Res. J. 72, 94–106 (2016).
Prete, F. R. The Praying Mantids (Johns Hopkins University Press, 1999).
Dyck, V. A., Hendrichs, J. & Robinson, A. S. Sterile Insect Technique: Principles And Practice In Area-Wide Integrated Pest Management (CRC Press, 2021).
Bouyer, J. & Vreysen, M. J. B. Yes, irradiated sterile male mosquitoes can be sexually competitive!. Trends Parasitol. 36, 877–880 (2020).
Parker, A., Vreysen, M., Bouyer, J. & Calkins, C. Sterile insect quality control/assurance. In Sterile Insect Technique: Principles And Practice In Area-Wide Integrated Pest Management 399–440 (2021).
Lees, R., Carvalho, D. O. & Bouyer, J. Potential impact of integrating the sterile insect technique into the fight against disease-transmitting mosquitoes. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management 2nd edn (eds Dyck, A. V. et al.) 1082–1118 (CRC Press, 2021).
Bimbilé Somda, N. S. et al. Cost-effective larval diet mixtures for mass rearing of Anopheles arabiensis Patton (Diptera: Culicidae). Parasit. Vectors 10, 619 (2017).
Bimbilé Somda, N. S. B. et al. Insects to feed insects-feeding Aedes mosquitoes with flies for laboratory rearing. Sci. Rep. 9, 1–13 (2019).
Maïga, H. et al. Assessment of a novel adult mass-rearing cage for Aedes albopictus (Skuse) and Anopheles arabiensis (Patton). Insects 11, 801 (2020).
Maïga, H. et al. Reducing the cost and assessing the performance of a novel adult mass-rearing cage for the dengue, chikungunya, yellow fever and Zika vector, Aedes aegypti (Linnaeus). PLOS Negl. Trop. Dis. 13, e0007775 (2019).
Mamai, W. et al. Black soldier fly (Hermetia illucens) larvae powder as a larval diet ingredient for mass-rearing Aedes mosquitoes. Parasite 26, 57 (2019).
Mamai, W. et al. Optimization of mass-rearing methods for Anopheles arabiensis larval stages: Effects of rearing water temperature and larval density on mosquito life-history traits. J. Econ. Entomol. 111, 2383–2390 (2018).
Bellini, R., Puggioli, A., Balestrino, F., Carrieri, M. & Urbanelli, S. Exploring protandry and pupal size selection for Aedes albopictus sex separation. Parasites Vectors 11, 650 (2018).
Yamada, H. et al. Genetic sex separation of the malaria vector, Anopheles arabiensis, by exposing eggs to dieldrin. Malar J. 11, 208 (2012).
Yamana, T. K. & Eltahir, E. A. B. Projected impacts of climate change on environmental suitability for malaria transmission in West Africa. Environ. Health Perspect. 121, 1179–1186 (2013).
Zacarés, M. et al. Exploring the potential of computer vision analysis of pupae size dimorphism for adaptive sex sorting systems of various vector mosquito species. Parasites Vectors 11, 656 (2018).
Culbert, N. J., Gilles, J. R. L. & Bouyer, J. Investigating the impact of chilling temperature on male Aedes aegypti and Aedes albopictus survival. PLoS ONE 14, e0221822 (2019).
Helinski, M. E., Parker, A. G. & Knols, B. G. Radiation-induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis. Malar J. 5, 41 (2006).
Yamada, H. et al. Identification of critical factors that significantly affect the dose-response in mosquitoes irradiated as pupae. Parasit. Vectors 12, 1–13 (2019).
Culbert, N. J. et al. A rapid quality control test to foster the development of the sterile insect technique against Anopheles arabiensis. Malar. J. 19, 1–10 (2020).
Culbert, N. J. et al. A rapid quality control test to foster the development of genetic control in mosquitoes. Sci. Rep. 8, 1–9 (2018).
Bouyer, J. et al. Field performance of sterile male mosquitoes released from an uncrewed aerial vehicle. Sci. Robot. 5, eaba6251 (2020).
Somda, N. S. B. et al. Ecology of reproduction of Anopheles arabiensis in an urban area of Bobo-Dioulasso, Burkina Faso (West Africa): Monthly swarming and mating frequency and their relation to environmental factors. PLoS ONE 13, e0205966 (2018).
Bellini, R., Medici, A., Puggioli, A., Balestrino, F. & Carrieri, M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J. Med. Entomol. 50, 317–325 (2013).
Vavassori, L., Saddler, A. & Müller, P. Active dispersal of Aedes albopictus: A mark-release-recapture study using self-marking units. Parasites Vectors 12, 583 (2019).
Zheng, X. et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 (2019).
Dor, A. & Liedo, P. Survival ability of Mexican fruit fly males from different strains in presence of the predatory orb-weaving spider Argiope argentata (Araneae: Araneidae). Bull. Entomol. Res. 109, 279–286 (2019).
Rathnayake, D. N., Lowe, E. C., Rempoulakis, P. & Herberstein, M. E. Effect of natural predators on Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) control by sterile insect technique (SIT). Pest Manag. Sci. 75, 3356–3362 (2019).
Kral, K. The functional significance of mantis peering behaviour. Eur. J. Entomol. 109, 295–301 (2012).
Bond, J. G. et al. Optimization of irradiation dose to Aedes aegypti and Ae. albopictus in a sterile insect technique program. PLoS ONE 14, e0212520 (2019).
Helinski, M. E., Parker, A. G. & Knols, B. G. Radiation biology of mosquitoes. Malar. J. 8, S6 (2009).
Hurd, L. E. et al. Cannibalism reverses male-biased sex ratio in adult mantids: Female strategy against food limitation?. Oikos 69, 193–198 (1994).
Lawrence, S. E. Sexual cannibalism in the praying mantid, Mantis religiosa: A field study. Anim. Behav. 43, 569–583 (1992).
Trujillo-Jiménez, P., Castro-Franco, R., Zagal, M. & Corona, Y. The Asian house gecko Hemidactylus frenatus. (2018).
Tyler, M. J. On the diet and feeding habits of Hemidactylus frenatus (Dumeril and Bibron) (Reptilia:Gekkonidae) at Rangoon, Burma. Trans. R. Soc. S. Aust. 84, 45–49 (1961).
Dor, A., Valle-Mora, J., Rodríguez-Rodríguez, S. E. & Liedo, P. Predation of Anastrepha ludens (Diptera: Tephritidae) by Norops serranoi (Reptilia: Polychrotidae): Functional response and evasion ability. Environ. Entomol. 43, 706–715 (2014).
Schmidt, J. M., Sebastian, P., Wilder, S. M. & Rypstra, A. L. The nutritional content of prey affects the foraging of a generalist arthropod predator. PLoS ONE 7, e49223 (2012).
Turesson, H., Persson, A. & Brönmark, C. Prey size selection in piscivorous pikeperch (Stizostedion lucioperca) includes active prey choice. Ecol. Freshw. Fish 11, 223–233 (2002).
Collins, C. M., Bonds, J. A. S., Quinlan, M. M. & Mumford, J. D. Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae s.l., on interacting predators and competitors in local ecosystems. Med. Vet. Entomol. 33, 1 (2019).
FAO/IAEA. Guidelines for mark-release-recapture procedures of Aedes mosquitoes. Version 1.0. In (eds Bouyer, J. et al.) 22 (Food and Agriculture Organization of the United Nations International Atomic Energy Agency, 2020).
Funding
The research presented in this paper was partially funded by the United States of America under the grant to the IAEA entitled: Surge expansion for the sterile insect technique to control mosquito populations that transmit the Zika virus and by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 682387–REVOLINC).
Author information
Authors and Affiliations
Contributions
N.S.B.S. designed and performed the study, analysed the data, and drafted the manuscript, which was critically revised by H.M., W.M., T.B., B.S.P., H.Y., and J.B. T.B. and T.W. participated in the experiments. B.S.P. contributed to data analysis. J.B. conceived and supervised the study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bimbilé Somda, N.S., Maïga, H., Mamai, W. et al. Adult mosquito predation and potential impact on the sterile insect technique. Sci Rep 12, 2561 (2022). https://doi.org/10.1038/s41598-022-06565-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06565-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.