Abstract
Three different types of lactic acid bacteria (Lactobacillus helveticus, Lactobacillus rhamnosus and Streptococcus thermophilus S3855) were used to manufacture white soft cheese. The resultant white soft cheeses were pickled for 28 days at refrigerator temperatures and were fed to the experimental rats. The chemical and microbiological analyses of white soft cheese were conducted at different storage periods (fresh, 7 days, 14 days, 21 days, and 28 days). The pH values and protein content of white soft cheese gradually decreased during the storage peroid. Conversely, the moisture content, titratable acidity, and fat/DM % of white soft cheese were found to increase with of the increase in pickling periods of up to 28 days. Microbiologically, the total viable count of bacteria in the control samples was lower than that in the other treatments. Furthermore, the treatments containing the L. helveticus and L. rhamnosus strains had the highest lactoacilli counts whereas the treatment containing the S. thermophilus strain had the highest streptococci counts. Twenty-five male Albino rats were used for experiemntal technique. Rats were fed with 70% basal diet with addition of 30% white soft cheese. Several pathological findings were present in all experimental groups apart from the control rats, and the kidney samples exhibited renal vascular congestion especially in the cortical area. The changes of the glomeruli comprise atrophy, distortion, hypocellularity of the glomerular tuft, and focal lymphoid cell reactions. The renal tubular epithelium showed a series of degenerative changes ranging up to necrosis. The liver samples showed variable hepatic injury in the form of thickening in the Glisson capsule, as well as dissociation and disorganization of hepatic cords. Hepatocellular vacuolar degeneration, presence of focal areas of nodular hyperplasia, the hyperplastic cells mixed with lymphocytic infiltration, congestion in the portal vein, periportal fibrosis and edema with the presence of newly formed nonfunctional bile ductulus. Based on the histopathology scores, the severity of renal and hepatic changes was significantly increased (P ≤ 0.05) in all of the experimental groups compared with the control group. Generally, the chemical composition, microbiological analysis and vital organs were significantly affected by using cultured white soft cheese.
Similar content being viewed by others
Introduction
White cheese has been made in Egypt for a long time. Photographic evidence of Egyptian cheese making was found on the murals of ancient Egyptian tombs, which date back to nearly 20,000 years BC. In recent times, the production and consumption of white soft cheese have been increased because of it being an inexpensive source of protein and a rich source in minerals and vitamins as well as having a high nutritional value. Cheese is considered a good potential source of probiotic bacteria for the intestinal tracts of humans, which is related to its unique physical characteristics and chemical composition compared with fermented milk products (higher pH value and lower titratable acidity, higher total solid and fat content, and higher nutrient availability)1. The use of starter cultures containing lactic acid bacteria has become a requirement in the cheese industry, and its main function is the production of lactic acid and different flavor compounds, which results in different types of cheese2. The addition of a starter culture of lactic acid bacteria in cheese making has led to an improvement in the microbiological quality of the cheese besides a slight improvement in the physical, chemical and sensory properties of white cheese3.
The use of starters in the production of cheese leads to a relatively low pH and encourages the separation of whey. It also prevents the growth of pathogenic bacteria, besides the generation of some aroma compounds and increases the degree of maturity of cheese4. Lactobacilli possess several peptide-degrading enzymes, which can hydrolyze the peptides into oligo-peptides and amino acids which may alter the flavor, body, texture, and consequently the sensory properties of cheese5.
The kidneys and liver are considered vital organs involved in metabolism, detoxification, storage, and excretion and are particularly exposed to damage6,7,8. The kidneys and liver of rats fed with Domiati cheese showed several pathological effects9,10. No previous literature has studied the effect of cultured white soft cheese on the kidneys and liver of rats using L. helveticus, L. rhamnosus and S. thermophilus S3855.Thus, this study aims to investigate the possibility of making a cultured white soft cheese using L. helveticus, L. rhamnosus, and S. thermophilus S3855 and monitor the changes in the chemical composition and microbiological properties of cheese during the pickling period. Moreover, the impact of cultured white soft cheese on the histopathological changes in the kidneys and liver of experimental rats was evaluated.
Materials and methods
Fresh cow’s milk was obtained from the Herd of the Animal Production Department, Faculty of Agriculture, Al-Azhar University (Branch of Assiut). An enzyme source (microbial rennet powder) was obtained from DSM (France) with a commercial name (Fromase R 2200). Salt: commercial sodium chloride was obtained from El-Nasr Company (Alexandria, Egypt). Starter strains: Lactobacillus helveticus (ATCC15009) was obtained from Cairo Microbiological Resource Center (MIRCEN), Faculty of Agriculture, Ain Shams University. Lactobacillus rhamnosus was obtained from the Dairy Department, Facultyof Agriculture, Al-Azhar University (Cairo). The S. thermophilus S3855 encapsulated strain was obtained from the Department of Dairy Science, Faculty of Agriculture Minia University. The albino rats were obtained from the Farm of National Organization for Drug Control and Research, Giza, Egypt.
Manufacture of white soft cheese
White soft cheese was made by conventional method of making Domiati cheese according to the adopted method of11 with some modifications as follows:
Cow’s milk (3% fat) was divided into four equal portions. Every part was heated to 72 ± 1 °C for 15 min, and 4% salt (w/w) was added. It was then rapidly cooled to 40 °C and starters were added, followed by the addition of rennet for all portions:
Control cheese | Nonstarter cheese |
L. helveticus cheese | Adding 0.75% (w/w) L. helveticus starter |
L. rhamnosus cheese | Adding 0.75% (w/w) L. rhamnosus starter |
S. thermophilus S3855 cheese | Adding 0.75% (w/w) S. thermophilus (S3855) starter |
Approximately 750 g of the resultant cheese was immersed in plastic containers (1000 g) filled up with cheese whey which was previously heated to 90 °C. The cheese containers were stored in a refrigerator at 6 ± 1 °C and the cheese samples were taken fresh and after 7, 14, 21 and 28 days for analysis.
Chemical analysis
Moisture content and titratable acidity were determined using the IDF12 and AOAC13 methods, respectively. The pH values were measured using a pH meter (model 68 ESD 19713 USA). Fat content was determined using the AOAC methods13. Total nitrogen contents were determined as described using the IDF14.
Microbiological analyses
The total bacterial count was determined using Marshall’s methods15. Lactobacilli count was estimated on a selective medium for lactobacilli (MRS) and streptococci count on an M17 agar medium, respectively16. Coliform bacteria were enumerated using the method of IDF17. Molds and Yeasts were enumerated in accordance with the FDA criteria18.
Adaptation
The present study was performed according to the Egyptian laws and guidelines of the University for Animal Care. The Faculty of Veterinary Medicine National Ethical Committee, Assiut University, Egypt, has authorized all the steps in the present work. The study was reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
The rats (Albino Rattus norvegicus) were housed in screen- bottomed aluminum cages in rooms maintained at 25 ± 1 °C, with a relative humidity of approximately 55% and given access to tap water for 28 days. The rats were fed with a basal diet for 1 week before starting the experiment. The ingredients of the basal diet were as follows: crude protein (12%), corn oil (8%), salt mixture (4%), vitamin mixture (1%), maize starch (70%) and fiber (5%).
Experimental feeding techniques
Twenty-five male albino rats were randomly and equally divided into five groups, five rats each. After the acclimatization period for 7 days, each Group was fed with one of the following diets every day for 28 days.
Group no | Diet |
G1 | Basal diet |
G2 | Basal diet (70%) + control cheese (30%) |
G3 | Basal diet (70%) + cheese containing L. helveticus (30%) |
G4 | Basal diet (70%) + cheese containing L. rhamnosus (30%) |
G5 | Basal diet (70%) + cheese containing S. thermophilus S3855 (30%) |
Specimen processing and staining
At the end of the respective experimental periods, the animals from each Group were weighted and then anesthetized via the intraperitoneal injection of equithensin (1 mL/kg). After the loss of all reflexes, the animals were transcardially perfused with warm saline followed by Trump’s fixative (3.7% formaldehyde plus 1% glutaraldehyde in saline buffer)19. Afterward, the kidney and liver specimens were obtained dissected and immediately fixed in 10% neutral buffer formalin for 24 h to ensure perfect fixation, after which they were dehydrated in a graded alcohol series, cleared in xylene, and finally embedded in paraffin. The tissue was cut into 3 μm thick sections and then stained with hematoxylin and eosin20. The sections were examined for histopathological changes using an OLYMPUS BX51 microscope and photographed with an OLYMPUSDP72 camera adapted to the microscope (Department of Anatomy and Histology, Assiut University, Egypt).
Ordinal method for validating histopathologic scoring
Each animal was assigned a score on the basis of the tissue histopathological examination21. The samples were scored quantitatively and semiquantitatively, with an assessment based on the visual field inspection of a minimum of 10 sections from each Group. Histopathological lesions were scored by an experienced veterinary pathologist who was unaware of the experimental treatments or their information in advance. The photographs were taken at a magnification of 40 × and the cell numbers of the hepatocyte alterations were counted in 10 randomized areas (each 1 mm2)22,23.
Furthermore the severity of of each lesion was scored as follows: 0 = no lesions; 1 = minimal (1–10% of the tissue section affected); 2 = mild (11–25%); 3 = moderate (26–45%) and 4 = severe (> 45%)21,22,24.
Statistical analysis
Collected data were subjected to the analysis of variance (ANOVA) using the Statistical Analysis System at a 5% level of significance. The mean differences were separated using the least significant difference and expressed as means ± SE. The Shapiro -Wilk’s W test was conducted for the assumption of normality in which the test was insignificant.
Data on the histopathological results were expressed as mean ± S.D. The measurements obtained from the experimental groups were statistically estimated via GraphPad Prism (version 5) (San Diego, California, USA) using one‑way ANOVA with Tukey’s post hoc multiple comparison tests. A P value of P < 0.05 was considered statistically significant22,24.
Result and discussion
Table 1 shows the data on the chemical composition of white soft cheese made with different strains of L. helveticus, L. rhamnosus and S. thermophilus S3855 during pickling periods, which was stored in a refrigerator for up to 28 days.
pH values
The data in Table 1 indicated the significant difference in the pH values of white soft cheese (P < 0.05) among all cheese treatments. The pH values of the control cheese, L. helveticus cheese, L. rhamnosus cheese and S. thermophilus S3855 cheese ranged from 6.43 ± 0.03 to 5.21 ± 0.01, 6.46 ± 0.01 to 5.19 ± 0.00, 6.49 ± 0.02 to 5.08 ± 0.01 and 6.47 ± 0.01 to 5.28 ± 0.01, respectively. The pH values of all cheese samples gradually decreased during the storage peroid of up to 28 days because of the accumulation of lactic acid produced by the lactic acid bacteria. These results are in consistent those reported by Perotti et al.25, Mansour and Zaki26 and Zonoubi and Goli27.
Titratable acidity (TA %)
The data in the same table indicated that the acidity of white soft cheese increased with the increase in storage periods at refrigerator temperatures up to 28 days. The control samples had lower TA than those of the other treatments at the end of the pickling period (28 days) as the control samples doesn’t inoculated with any strain. Conversely, the treatment containing the L. rhamnosus strain had the highest TA% at the end of the storage period. The differences TA titratable acidity among the cheese treatments might be attributed to the varying growth rates of microorganisms and their ability to ferment lactose during the pickling period. The same trend was obtained by Mansour and Zaki26, Zonoubi and Goli27 and Naeim28.
Moisture content
The data in Table 1 indicated that the moisture content of white soft cheese increaed with the increase in pickling periods at refrigerator temperatures up to 28 days. The treatment containing the L. helveticus strain and stored for 28 days had a higher moisture content than the other treatments. This increase of moisture content could be attributed to the refrigerator temperature which helps to imbibe the whey into the curd. Similar results were reported by Kebary et al.5.
Protein content
White soft cheese showed a clearly significant (P < 0.05) decrease in protein content during the pickling period in all treatments. The decrease in protein content related to the increase in the moisture content of white soft cheese during the pickling period. However, the protein content of cheese samples was agreed with the results that found by29,30,31.
Fat/dry matter (F/DM)
The data in the same table indicated that, the F/DM of white soft cheese increased with the increase in pickling periods at refrigerator temperatures up to 28 days in all treatments. Moreover, the cheese samples containing the S. thermophilus S3855 strain had a lower F/DM than the other cheese tsamples at at the end of the pickling period (28 days). Conversely, the treatment containing the L. helveticus strain had the highest F/DM at the end of the pickling period. Similar results were obtained by Kebary et al.5 and El-Sayed and El-Sayed31.
Table 2 shows the microbiological analyses of white soft cheese made with deferent strains of lactic acid bacteria (L. helveticus, L. rhamnosus and S. thermophilus S3855) and pickled at refrigerator temperatures up to 28 days.
Streptococci counts
Significant differences (P < 0.05) in streptococci count (Tale 2) were observed, and the count ranged from 3.65 ± 0.03 to 8.67 ± 0.01 log cfu/g-1. Moreover, it was indicated that the control samples had lower streptococci counts than other cheese samples. Conversely, the samples of S. thermophilus S3855 cheese had the highest streptococci counts (8.67 ± 0.01 log cfu g-1) at 7 days of the pickling period. The streptococci counts of L. helveticus cheese and L. rhamnosus cheese were higher than that of the control cheese. This may be related to the synergistic effect between lactobacilli and streptococci during ripening. Moreover, the streptococci counts of were increased in the beginning days during the storage period and then decreased up to the end of pickling period. These results are consistent with those obtained by Papademas et al.32 and Dafalla and Abdel Razig33.
Lactobacilli count
The obtained data in the same table indicated that the treatment of L. helveticus cheese and L. rhamnosus cheese had higher lactobacilli counts than the control and S. thermophilus S3855 cheese. Moreover, the lactobacilli counts were increased with the increase in storage periods to reach the highest count in 14 days of the pickling period in all cheeses. For the except S3855 cheese, if the highest count was reached in 7 days, then the counts were decreased in all treatments up to the end of the pickling period. Moreover, the data of lactobacilli counts indicated significant differences (P < 0.05) among all treatments, the count ranging from 3.70 ± 0.10 to 8.67 ± 0.01 log cfu g−134,35,36.
Total bacterial count
Table 2 showed significant differences (P < 0.05) and indicated that the control samples had lower total bacterial counts than the other cheeses. Moreover, the highest counts were obtained from the S. thermophilus S3855 cheese samples that were pickled for 7 days. By contrast the lowest total counts were obtained from the fresh control samples. Furthermore, the total bacterial counts were increased at the beginning of the pickling period and then decrease up to 28 days in all treatments. These results are agreed with those of Kebary et al.5 and Dafalla and Abdel Razig33.
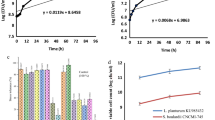
Figure 1 shows the bacteriological properties (streptococci count, lactobacilli count, total bacterial count and coliform count) of rat feces before and after feeding with white soft cheese.
Streptococci count
The obtained data in Fig. 1A indicated that, the streptococci counts before and after feeding with white soft cheese did not show any significant difference (P > 0.5) . In all rat groupd, it is clear that the streptococci counts after feeding with white soft cheese were higher than those before feeding due to the increase in the number of streptococci in inoculated and uninoculated cheese. These results are similar with those obtained by37.
Lactobacilli count
The obtained data in Fig. 1B indicated that, the lactobacilli counts before and after feeding with white soft cheese among all rat groups showed significant difference (P < 0.05). Also, lactobacilli counts after feeding with white soft cheese were higher than those before feeding in all rat groups. Furthermore, Group 2 had the highest lactobacilli count before feeding with white soft cheese, whereas Group 3 had the highest lactobacilli counts after feeding.
Total bacterial count
The obtained data in Fig. 1C showed significant differences (P < 0.05) and indicated that the treatment of Group 1 initialy had lower total bacterial count than that of the other expermintal groups, whereas , the treatment of Group 4 had the highest count. Conversly, the treatment of Group 1 after feeding had lower total bacterial counts than that of the other treatments whereas the treatment of Group 3 had the highest count.
Coliform count
The obtained data in Fig. 1D indicated that the in treatment Group 3 initialy had the lowest coliform counts than that of the other treatments, whereas the treatment of Group 1 had the highest counts. Conversely, the treatment of Group 4 after 4 weeks of feeding had the lowest counts of coliform groups than that of the other treatments, whereas the treatment of Group 1 had the highest count. The data also indicated that the coliform count decreased after the feeding period in all groups, which could be attributed to the presence of lactic acid bacteria.These results are in agreement with those obtained by38, who found that the probiotic strain L. plantarum as a single species or in combination with oil (Lini oleum virginale) decreased the total coliform count of and increased the lactobacilli in the feces of rats.
Histopathological observations
Renal histopathological changes
The histopathological changes in the liver tissue have been observed in all experimental groups apart from the control rats on the basis of the typical histological architecture of the normal renal parenchyma (Fig. 2) as observed previously by Brzóska et al.39.
Photomicrograph of the rat kidneys of Group I showed normal histology architectures. (A, B) Renal cortex contains normal glomerulus (red arrows) and Bowman’s space (BS). Proximal convoluted tubules (B, thin arrows) and distal convoluted tubules (B, thick arrowheads), magnified in the selected square. (C) Normal renal medulla, thick ascending, Henle’s loop (black thin arrows), thick descending, Henle’s loop (stars), thin segment, Henle’s loop (red arrows). H&E stain, the bar size was indicated under pictures.
However, the sections taken from the rat kidneys of Group II (treated with basal diet 70% + control cheese 30%) showed several pathological findings: the cortical area showed glomerular atrophy, and the glomerular tuft was condensed and BS appeared to be wider than normal (Fig. 3A–C). Proximal and distal convoluted tubules exhibited degenerated epithelial lining. The vasculatures at the cortical area showed severe congestion; the blood vessels were dilated widely and engorged with blood (Fig. 3B,C). The tubules in the renal medulla showed vacuolation, degeneration and desquamation of its lining epithelium (Fig. 3D), and intense congestion was oserved in the blood vessels between the renal tubules (Fig. 3D).
Photomicrograph of the rat kidneys from Group II (treated with basal diet 70% + control cheese 30%). (A–C) Glomerular atrophy (thick arrows, maximized in the selected square), degeneration and desquamation of the renal tubular epithelium (thin arrows), and cortical vessels widely dilated and congested (star), (D) Renal medulla showing the degeneration of renal tubules (Henle’s loop) (red arrowheads), vascular congestion (stars), and hemorrhage between the renal tubules. H&E stain, the bar size was indicated under pictures.
The renal sections of Group III rats (treated with basal diet 70% + cheese containning L. helveticus 30%) showed glomerular atrophy, and some renal corpuscles revealed a variable degree of glomerular loss (Figs. 4A,B). Degeneration of proximal and distal convoluted tubules was observed and the epithelium was desquamated inside the lumen of some tubules and appeared as epithelial casts (Fig. 4B,C). The arcuate artery located at the corticomedullary junction was dilated and congested with thickening of its wall due to fibroses. Perivascular edema and hemorrhage around the arcuate artery (Fig. 4D,E). Henle’s loop showed dilatation with desquamated epithelium inside its lumen (Fig. 4F).
Photomicrograph of the rat kidneys of Group III (treated with basal diet 70% + cheese containing L. helveticus 30%). (A) Glomerular atrophy (red arrows). (B) Some areas showing severe atrophy and loss of glomerulus with degenerated renal corpuscle (red arrow). (B, C) Renal tubular degeneration and desquamation of its epithelial lining (blue arrows). (D) The arcuate artery at corticomedullary junction showing congestion (arrows) with perivascular edema (stars). (E) The arcuate artery showing severe congestion (black star), thick degenerated wall (scales), perivascular hemorrhage and edema (blue star). (F) Renal medulla tubules showing severe degeneration and desquamation of its lining epithelium inside its lumen (arrows) with interstitial hemorrhage between the renal tubules. H&E stain, the bar size was indicated under pictures.
The histopathological examination of the kidneys of Group IV (treated with basal diet 70% + cheese containing L. rhamnosus 30%) showed that the changes of the glomeruli comprise atrophy, distortion, and hypocellularity of the glomerular tuft. BS was widened and the nucleus of the remaining capillary tuft was condensed (Fig. 5A). The renal tubular epithelium showed a series of degenerative changes up to necrosis; both proximal and distal convoluted tubules were affected. The tubular epithelium sometimes showed the lysis of the cytoplasm and pyknosis of the nucleus. Sometimes the necrotic epithelium sloughed and the tubules were dilated. Changes in the tubules were diffuse and involved a massive area of the both cortex (Fig. 5B) and medulla (Fig. 5F). The most prominent histopathologic changes were present mainly in the renal vasculature of the glomeruli and renal tubules. The primary changes were present in the renal vasculature, especially those in the cortical area (Fig. 5C). The interstitial capillaries were dilated and engorged with blood. In some cases, the blood vessels at the corticomedullary junction were congested with a very mild perivascular edema (Fig. 5D,E). Focal small and multiple areas of lymphoid cell reactions were oserved in the interstitial areas and around the vasculatures (Fig. 5D,E). Our results are consistent with those of Al-Mathkhury and Baraaj40 who found that the renal tissues of the rats that were injected intraperitoneally with Lactobacillus bulgaricus, Lactobacillu plantarum and Lactobacillu acidophilus showed congestion of the vessels with blood and hemorrhage.
Photomicrograph of the rat kidneys of Group IV (basal diet 70% + cheese containing L. rhamnosus 30%). (A) Glomerular atrophy; BS widened and glomerulus showing necrobiotic changes (white arrows), renal tubules dilated with degeneration and necrosis of its epithelial lining (black arrow), interstitial mononuclear cellular infiltration (red arrows). (B) Disorganization and degeneration of renal tubules and vacuolation of the lining epithelium (black arrows). (C,D) Severe vascular dilatation and congestion (stars). Focal areas of mononuclear cellular aggregations (c, red head arrows). (E, F) Severe vascular dilatation and congestion in the corticomedullary junction (stars), fibrinoid degeneration of vascular wall (black arrow), severe perivascular focal mononuclear cellular aggregations (red arrow). (F) Degeneration in the renal medullary epithelium (arrows). H&E stain, the bar size was indicated under pictures.
The kidneys of Group V (treated with basal diet 70% + cheese containing S. thermophilus S3855 30%) showed prominently glomerular atrophy and hypocellularity, which were associated with the widening the BS (Fig. 6A). Sever cortical vascular congestion (Fig. 6B), Severe necrotic changes of renal tubular epithelium in both cortex (Fig. 6C) and medulla (Fig. 6D); the necrotic changes were severe and diffuse , which resulted in the loss of the entire epithelium. Hemorrhage at the corticomedullary junction (Fig. 6C). The intertubular blood vessels were congested (Fig. 6D).
Photomicrograph of the rat kidneys of Group V (basal diet 70% + cheese containing S. thermophilus S3855 30%), (A) glomerular atrophy (red arrows), interstitial hemorrhage (star). (B) Vascular congestion (stars). (C) Renal tubular necrosis (selected square, arrows), severe hemorrhage at the cortico-medullary junction (star). (D) Loop of Henle’s showing dilatation and desquamation of its lining epithelium (arrows) hemorrhage between the renal tubules (stars). H&E stain, the bar size was indicated under pictures.
The kidneys of rats that were fed with Domiati cheese stored in a refrigerator exhibited regions of hemorrhage and edema in the tubules and in the interstitial areas. Moreover, it showed the degeneration of renal tubules; and partly the degeneration of renal corpuscles9,10.
Based on the histopathology scores, the severity of glomerular atrophy (Fig. 7A), renal tubular degeneration (Fig. 7B) and vascular congestion (Fig. 7C) were significantly increased (P ≤ 0.05) in all treated experimental groups compared with the control Group.
Histomorphometry graph showing the semiquantitaive measurements of rats kidney alterations between the experimental groups. Plots showing (A) glomerular atrophy, (B) renal tubular degeneration and (C) vascular congestion among the experimental groups. Data were represented as mean ± standard error. Significant differences versus the control groups determined through one-way ANOVA with Tukey’s post hoc test are marked by different asterisks: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Hepatic histopathological changes
The microscopic examination of the rat liver of Group I revealed normal hepatic architectures which comprised, normal central vein, hepatic cords, hepatocytes and portal area contents (bile duct, hepatic artery, and vein) (Fig. 8).
Photomicrograph of the rat liver Group I showed normal histological architectures. (A) Intact parenchymal hepatic architecture. (B) Normal central vein (C.V), hepatic sinusoids between hepatic lobules (S), hepatocytes (selected square, arrowheads). (C) Portal area containing normal portal veins (PV), hepatic artery, and bile duct. H&E stain, the bar size was indicated under pictures.
Liver sections taken from the rats kidneys of Group II (treated with basal diet 70% + control cheese 30%) showed variable hepatic injuries such asthickening in the Glisson capsule surrounding the liver with extensive hemorrhage and edema (Fig. 9A). Central vein dilated and engorged with blood (Fig. 9B), dissociation, and disorganization of hepatic cords, and vacuolation of hepatocytes (Fig. 9C). The portal areas involved wide areas in hepatic parenchyma as the portal veins extensively were dilated and filled with blood with periportal edema mixed with fine threads of fibrosis which branched in-between hepatic tissue (Fig. 9D).
Photomicrograph of rat liver of Group II (treated with basal diet 70% + control cheese 30%). (A) Glisson capsule surrounding the liver was thickened and exhibits severe hemorrhage and edema (arrows). (B) Central vein was dilated and engorged with blood (arrows). (C) Dissociation of hepatic cords and vacuolation of hepatocytes (red arrows). (D) The portal areas showing dilated and congested portal veins (black arrowheads), periportal edema mixed with fine threads of fibrosis (stars). H&E stain, the bar size was indicated under pictures.
The liver sections of Group III (treated with basal diet 70% + cheese containing L. helveticus 30%) showed congested central veins with degeneration and desquamation of its endothelium (Fig. 10A). Hepatocellular vacuolar degeneration, hepatic cells appearing as empty spaces with a marginally located flattened nucleus which similar to the appearance of signet rings (Fig. 10B). The portal areas showed congestionin the portal vein with severe periportal fibroses which extends as tracts between hepatic lobules (Fig. 10C,D).
Photomicrograph of the rat livers of Group III (treated with basal diet 70% + cheese containing L. helveticus 30%). (A) Degeneration of the endothelium lining the central vein (star). (B) Vacuolar degeneration of hepatocytes (white arrows). (C, D) Portal area showing congestion in the portal veins with mononuclear inflammatory cellular infiltration (star), periportal fibroses (red arrows). H&E stain, the bar size was indicated under pictures.
The histopathological examination of the rat liver of Group IV(treated with basal diet 70% + cheese containg L. rhamnosus 30%) showed dilated central vein with degenerated endothelial lining (Fig. 11A) and vacuolar degeneration of hepatocytes (steatotic like cells) (Fig. 11B,C). The presence of focal areas of nodular hyperplasia, the hyperplastic cells mixed with lymphocytic infiltration (Fig. 11D,E), other focal nodular areas exhibiting stellate scar with abnormal blood vessels, the vessels showing a marked thickening of the wall without lumen (Fig. 11A,F). Wide portal areas due to extensive widening and congestion in the portal veins with periportal fibrosis and edema, and the presence of newly formed nonfunctional bile ductulus (Fig. 11G–I). Similar results were obtained by39 who found that the liver tissues of the rats that were injected intraperitoneally with L. bulgaricus, L. plantarum and L. acidophilus showed inflammatory cells infiltration and also necrosis.
Photomicrograph of the rat livers of Group IV (basal diet 70% + cheese containing L. rhamnosus 30%). (A) Dilated central vein with degenerated endothelial lining (star), focal nodular hyperplasia (selected square). (B,C) Vacuolar degeneration of hepatocytes (red arrows). (D) Focal nodular hyperplasia (selected square) magnified in E. hyperplastic cells mixed with lymphocytic infiltration (arrows). (F) Focal nodular hyperplasia of the liver parenchyma and stellate scar with abnormal blood vessels. Part of the vessels showing a marked thickening of the wall without lumen (arrows). (G,H) Wide portal areas due to extensive widening and congestion in the portal veins (red star) and periportal edema (black star). I: periportal fibrosis and edema (stars) and newly formed bile ductulus (arrows). H&E stain, the bar size was indicated under pictures.
The liver sections of Group V (basal diet 70% + cheese contaning S. thermophilus S3855 30%) showing congested central vein (Fig. 12A), and vacuolation in hepatocytes (Fig. 12B). The focal areas of fibrosis with mild mononuclear lymphocytic infiltration (Fig. 12C). The focal areas of coagulative necrosis were also found (Fig. 12D). Wide portal areas showed extensively wide portal veins filled with blood with severe periportal edema (Fig. 12E,F).
Photomicrograph of the rat livers of Group V (basal diet 70% + cheese containing S. thermophilus S3855 30%). (A) Dilated central vein engorged with blood (star). (B) Vacuolation in hepatocytes (arrows). (C) Focal areas of fibroses (red arrows), mononuclear lymphocytic infiltration (black arrowheads). (D) Focal areas of coagulative necrosis (white arrows). (E) Wide portal areas showing extensively wide portal veins filled with blood (stars). (F) Severe periportal edema (star). H&E stain, the bar size was indicated under pictures.
The liver of rats fed with Domiati cheese stored in a refrigerator revealed vacuolation in the cytoplasm of the hepatocytes. Otherwise, the liver of the other rats showed the normal structure of the hepatic lobules. The histopathological changes that happened in the liver of rats were perhaps due to an increase in the levels of the biogenic amines of Domiati cheese9,10.
Based on histopathology scoring, the severity of vascular congestion (Fig. 13A), hepatocellular changes (Fig. 13B) and periportal fibrosis (Fig. 13C) were significantly increased (P ≤ 0.05) in all treated experimental groups compared with the control Group.
Histomorphometry graph showing the semiquantitative measurements of rats liver alterations between the experimental groups. Plots showing (A) vascular congestion, (B) hepatocellular changes, and (C) periportal fibrosis among the experimental groups. Data were represented as mean ± standard error. Significant differences versus the control Group determined through one-way ANOVA with Tukey’s post hoc test are marked by different asterisks: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Probiotics may theoretically be implicated in inducing different side effects as:
Systemic infections, excessive immune stimulation in susceptible individuals, gene transfer and deleterious metabolic activities; those in according to a 2002 report jointly released by the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations (http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0282-tab-03-ref-19-joint-faowhovol219.pdf).
Our experimental results showing several systemic disturbances in liver and kidney cellular metabolism which alter its morphology in experimental groups, in addition to several vascular and inflammatory changes. Our findings were in agreement with previously recorded studies, which found many side effects after probiotic administration41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62.
Lactobacillus bacteremia and overt sepsis have previously been observed in association with S. boulardii [cerevisiae], Lactobacillus GG, Bacillus subtilis, Bifidobacterium breve, or combination probiotics41,42,43,44,45,46,47. Endocarditis has also been observed as a result of both Lactobacillus and Streptococcus probiotics48,49. Lactobacillus rhamnosus has also been linked to the formation of an abscess on two occasions50,51.
Because probiotics have been found to alter both the innate and adaptive immune systems, including cytokine release and dendritic cell function52,55, there has been some worry regarding the risk of overstimulating the immune system in some individuals, possibly leading to autoimmune phenomena or inflammation.
It was confirmed that, plasmids carrying genes giving resistance to tetracycline, erythromycin, chloramphenicol or lincosamide, macrolide, streptomycin, and streptogrammin are found in lactic acid bacteria56,58. Transfer of conjugation from enterococci to lactobacilli and lactococci can occur in animals' guts as well as in vitro; however, transfer to lactobacilli is uncommon 59.
One clinical trial raised serious concerns about the safety of probiotics60. The subjects in the probiotic arm of the trial had a greater mortality rate, which was ascribed to intestinal ischemia. The scientists speculated that probiotic bacteria delivery raised the oxygen demand in the gut mucosa, despite the fact that blood flow was already modest. Alternatively, the probiotics could have caused an inflammatory response in the small bowel, resulting in decreased capillary blood flow. In patients with pancreatitis, two previous smaller-scale investigations (later combined to boost power)61,62 showed a reduction in septic sequelae, surgical intervention, and infected necrosis in patients with pancreatitis given a symbiotic containing lactic acid bacteria and fiber.
As a result, many investigators have engaged in discussions with regulators concerning the challenges these policies pose to those seeking to advance scientific knowledge regarding the efficacy and safety of these products. It is critical to validate the identity of the organism through molecular testing at a reference laboratory if an infection appears to be caused by a probiotic strain and this aspect will be taken in our consideration in the following research work.
Conclusions
White soft cheese is the most consumed dairy product in Egypt and North Africa. Adding low levels of salt when making this type of cheese may threaten the safety of the cheese during storage, so many manufacturers resort to storing cheese under refrigeration. In this study, some strains of lactic acid bacteria were used to improve the chemical composition and physical properties of the cheese as well as its capability to produce lactic acid that may help in enhancing the preserving ability of the cheese. Where, the cheese treatments fulfilled the purpose of adding lactic acid bacteria, as L. rhamnosus cheese had the highest values of titratable acidity. Multiple histopathological alterations were present in all experimental groups aside from the control rats. The rat kidneys of the experimental groups showed renal vascular congestion particularly in the cortical region. The glomerular changes consist of atrophy, distortion, hypocellularity of the glomerular tuft, and focal lymphoid cell reactions. The renal tubular epithelium showed a series of degenerative changes up to necrosis. The hepatic tissues exhibited variable hepatic injury as a thickening in the Glisson capsule, dissociation, and disorganization of hepatic cords. Hepatocellular vacuolar degeneration, presence of focal areas of nodular hyperplasia, the hyperplastic cells mixed with lymphocytic infiltration, congestion in the portal vein, periportal fibrosis, and edema with the presence of newly formed nonfunctional bile ductulus.
On the basis of histopathology scores, the severity of renal and hepatic changes was significantly increased in all treated experimental groups compared with the control Group.
Change history
14 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-08515-3
References
Karimi, R., Mortazavian, A. M. & da Cruz, A. G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Tech. 91(3), 283–308 (2011).
Vapur, U. E., Ersan, L. Y. & Özcan, T. Effects of starter culture combination on the characteristic of white cheese. Int. J. Agric. Res. Innov. Technol. 3(2), 277–286 (2019).
Al-Ghamdi, A. Y., Salih, H. M. A. & Abdalla, M. O. M. Microbiological, physicochemical and sensory characteristics of traditional white soft cheese (Gibnabayda) supplemented with commercial starter culture. GSC Adv. Res. Rev. 7(2), 6–15 (2021).
Mudawi, H. A., Khairalla, L. M. & El-Tinay, A. H. Evaluation of the effect of starter culture on the quality of white soft cheese (Gibna Beyda). Univ. Khartoum J. Vet. Med. Anim. Prod. 7(2), 104–110 (2016).
Kebary, K. K., El-Shazly, H. A. & Youssef, I. T. Quality of probiotic UF domiati cheese made by Lactobacillus rhamnosus. Int. J. Curr. Microbiol. App. Sci. 4(7), 647–656 (2015).
Bunout, D. Nutritional and metabolic effects of alcoholism, their relationship with alcoholic liver disease. Nutrition 7, 583–589 (1999).
Thurman, R. G. et al. Mechanisms of ethanol-induced hepatotoxicity: Studies in rats. Front. Biosci. 4, 42–46 (1999).
Ozougwu, J. C. & Eyo, J. E. Hepatoprotective effects of Allium cepa extracts on paracetamol-induced liver damage in rat. Afr. J. Biotech. 13(26), 2679–2688 (2014).
El-Zahar, K. M. Biogenic amines and microbiological profile of egyptian cheeses. Univ. J. Food Nutr. Sci. 2, 18–26 (2014).
Ali, H. M., Deabes, M. M., Soliman, A. S. & EL kholy, A. Effect of feeding the experimental rats with domiati cheese on their histopathological alterations. J. Food Dairy Sci. Mansoura Univ. 8, 425–430 (2017).
Metwalli, N. H., Shalabi, S. I., Zahran, A. S. & El-Demerdash, O. The use of Soybean milk in soft cheese making: Organoleptic and chemical properties of Domiati cheese made from a mixture of Soybean milk and whole milk. J. Food Tech. 17, 297–305 (1982).
IDF. Milk, cream, fermented milks, and evaporated milk–total solids. FIL-IDF 21B, International Dairy Federation Brussels, Belgium (1987).
AOAC. Official methods of analysis association of official analytical chemists. (17th Wisconsin: George Banta Co. Inc, 2000).
IDF. Milk. Protein determination, determination of nitrogen content. Kjeldahl method and calculation of crude protein content. Standard FIL-IDF 20B. International Dairy Federation Brussels, Belgium (1993).
Marshall, R. T. Standard methods for the examination of dairy products 16th edn. (American Public Health Association, 1992).
IDF. Yoghurt. Enumeration of characteristic microorganisms. Colony count technique at 37°C. FIL-IDF 117B International Dairy Federation Brussels, Belgium. Int. Dairy J. 12, 923–931 (1997).
IDF. Milk and milk products. Enumeration of coliforms- colony counts technique and most probable number technique at 30°C. FIL-IDF 73A International Dairy Federation Brussels, Belgium (1985).
FDA. Bacteriological analytical manual. 9th Ed., AOAC International (2002).
Sayed, R. K. et al. Lack of NLRP3 inflammasome activation reduces age-dependent sarcopenia and mitochondrial dysfunction, favoring the prophylactic effect of melatonin. J. Gerontol. Ser. A 74, 1699–1708 (2019).
Bancroft, J. D., Layton, C. & Suvarna, S. K. Bancroft’s theory and practice of histological techniques 7th edn. (Churchill Livingstone, 2013).
Gibson-Corley, K. N., Olivier, A. K. & Meyerholz, D. K. Principles for valid histopathologic scoting in research. Vet. Pathol. 50, 1007–1015 (2013).
Ali, F. A. Z. M., Abdel-Maksoud, F., Abd Elaziz, H. O., Al-Brakati, A. & Elmahallawy, E. K. Descriptive histopathological and ultrastructural study of hepatocellular alternations induced by aflatoxin B1 in rats. Animals 11, 509 (2021).
Monmeesil, P., Fungfuang, W., Tulayakul, P. & Pongchairerk, U. The effects of astaxanthin on liver histopathology and expression of superoxide dismutase in rat aflatoxicosis. J. Vet. Med. Sci. 81(8), 1162–1172 (2019).
Mahmoud, U. T. et al. Zinc oxide nanoparticles prevents multidrug resistant staphylococcus- induced footpad dermatitis in broilers. Avain Pathol. 29, 1–55 (2021).
Perotti, M. et al. Incorporation of probiotic bacteria (Lactobacillus acidophilus and Bifidobacterium spp.) in Argentinean ovine cheese. Dairy Sci. Tech. 94, 255–267 (2014).
Mansour, A. I. A. & Zaki, K. G. Studies on the use of Lactobacillus rhamnosus in white soft cheese manufacture. Arch. Agric. Sci. J. 1(1), 82–95 (2018).
Zonoubi, R. & Goli, M. The effect of complete replacing sodium with potassium, calcium, and magnesium brine on sodium-free ultrafiltration Feta cheese at the end of the 60-day ripening period: Physicochemical, proteolysis–lipolysis indices, microbial, colorimetric, and sensory evaluation. Food Sci Nutr. 9, 866–874 (2021).
Naeim, M. A. Impact of using metabolites of some lactic acid bacteria on properties and quality of UF white soft cheese. J. Food Dairy Sci. 3(3), 185–192 (2012).
Dafalla, A., Abdel Razig, K. A. & Elrofaei, N. A. Effect of types of probiotic bacteria on physiochemical properties of sudanese white soft cheese. Am. Sci. Res. J. Eng. Technol. Sci. (ASRJETS) 78(1), 83–97 (2021).
Çetinkaya, A. & Öz, F. The effect of wheat germ on the chemical properties and fatty acids of white cheese during the storage time. Food Sci. Nutr. 8, 915–920 (2020).
El-Sayed, H. S. & El-Sayed, S. M. A modern trend to preserve white soft cheese using nano-emulsified solutions containing cumin essential oil. Environ. Nanotechnol. Monitor. Manag. 16, 100499 (2021).
Papademas, P. et al. Conventional and omics approaches shed light on Halitzia cheese, a long-forgotten white-brined cheese from Cyprus. Int. Dairy J. 98, 72–83 (2019).
Dafalla, A. I. & Abdel-Razig, K. A. The role of probiotic bacteria on microbiological and acceptability of sudanese white soft cheese. Int. J. Multidiscip. Res. Anal. 4, 496–505 (2021).
Candioti, M. C. et al. ReggianitoArgentino cheese: Influence of Lactobacillus helveticus strains isolated from natural whey cultures on cheese-making and ripening processes. Int. Dairy J. 12(11), 923–993 (2002).
Mannu, L., Comunian, R. & Francesca Scintu, M. Mesophilic lactobacilli in fioresardo cheese: PCR-identification and evolution during cheese ripening. Int. Dairy J. 10, 383–389 (2000).
Giménez, P. Preventing undesired eye formation in soft cheese. Int. Dairy J. 116, 104958 (2021).
Zaki, K. G. Studies on using of probiotic bacteria on soft cheese making. Ph.D. Thesis, Fac. Agric., Al-Azhar Univ (2018).
Cokasova, D. et al. The effect of new probiotic strain Lactobacillus plantarum on counts of coliforms, lactobacilli and bacterial enzyme activities in rats exposed to N, N-dimethylhydrazine (chemical carcinogen). Acta Vet. 81, 189–194 (2012).
Brzóska, M., Moniuszko-Jakoniuk, J., Pilat-Marcinkiewicz, B. & Sawicki, B. Liver and kidney function and histology in rats exposed to cadmium and ethanolm. Alcohol. Alcoholism 38, 2–10 (2003).
Al-Mathkhury, H. J. F. & Baraaj, A. H. Pathological effect of lactobacilli on rat liver and kidney. J. Babylon Univ. Pure Appl. Sci. 20, 1428–1433 (2012).
Burkhardt, O. et al. Saccharomyces boulardii induced sepsis: Successful therapy with voriconazole after treatment failure with fluconazole. Scand J. Infect. Dis. 37, 69–72 (2005).
Kunz, A. N., Noel, J. M. & Fairchok, M. P. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J. Pediatr. Gastroenterol. Nutr. 38, 457–458 (2004).
Land, M. H. et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115, 178–181 (2005).
Lestin, F., Pertschy, A. & Rimek, D. Fungemia after oral treatment with Saccharomyces boulardii in a patient with multiple comorbidities. Dtsch. Med. Wochenschr. 128, 2531–2533 (2003).
Oggioni, M. R. et al. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J. Clin. Microbiol. 36, 325–326 (1998).
Ohishi, A. et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 156, 679–681 (2010).
Zein, E. F. et al. Lactobacillus rhamnosus septicemia in a diabetic patient associated with probiotic use: A case report. Ann Biol Clin (Paris) 66, 195–198 (2008).
Mackay, A. D. et al. Lactobacillus endocarditis caused by a probiotic organism. Clin. Microbiol. Infect. 5, 290–292 (1999).
Presterl, E. et al. Endocarditis by Lactobacillus rhamnosus due to yogurt ingestion?. Scand J. Infect. Dis. 33, 710–714 (2001).
Conen, A. et al. A pain in the neck: Probiotics for ulcerative colitis. Ann. Intern. Med. 151, 895–897 (2009).
Rautio, M. et al. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin. Infect. Dis. 28, 1159–1160 (1999).
Vaarala, O. Immunological effects of probiotics with special reference to lactobacilli. Clin. Exp. Allergy 33, 634–640 (2003).
Veckman, V. et al. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J. Leukoc. Biol. 75, 764–771 (2004).
Braat, H. et al. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J. Mol. Med. 82, 197–205 (2004).
Drakes, M., Blanchard, T. & Czinn, S. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 72, 3299–3309 (2004).
Lin, C. F. et al. Molecular characterization of a plasmidborne (pTC82) chloramphenicol resistance determinant (cat-TC) from Lactobacillus reuteri G4. Plasmid 36, 116–124 (1996).
Gevers, D. et al. Molecular characterization of tet (M) genes in Lactobacillus isolates from different types of fermented dry sausage. Appl. Environ. Microbiol. 69, 1270–1275 (2003).
Tannock, G. W. et al. Molecular characterization of a plasmid-borne (pGT633) erythromycin resistance determinant (ermGT) from Lactobacillus reuteri 100–63. Plasmid 31, 60–71 (1994).
Morelli, L., Sarra, P. G. & Bottazzi, V. In vivo transfer of pAM beta 1 from Lactobacillus reuteri to Enterococcus faecalis. J. Appl. Bacteriol. 65, 371–375 (1988).
Besselink, M. G. et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 371, 651–659 (2008).
Olah, A. et al. Early enternal nutrition with specific Lactobacillus and fibre reduces sepsis in severe acute pancreatitis. Br. J. Surg. 89, 1103–1107 (2002).
Olah, A. et al. Synbiotic control of inflammation and infection in severe acute pancreatitis: A prospective, randomized, double blind study. Hepatogastroenterology 54, 594–598 (2007).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors wrote the main manuscript text. K.H.S. prepared tables (1–2) and Fig. (1). F.A.A. and R.E. prepared Figs. (2 to 13). All authors performed the statistical analysis and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the legends of Figures 10, 11 and 12. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salman, K.H., Ali, F.A. & Elhanbaly, R. Effect of cultured white soft cheese on the histopathological changes in the kidneys and liver of albino rats. Sci Rep 12, 2564 (2022). https://doi.org/10.1038/s41598-022-06522-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06522-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.