Abstract
The aim of this study was to identify the antimicrobial effect and mechanism of whey protein and blueberry juice mixed systems fermented with Lactobacillus against Escherichia coli during storage. The whey protein and blueberry juice mixed systems were fermented with L. casei M54, L. plantarum 67, S. thermophiles 99 and L. bulgaricus 134 and had different antibacterial activities against E. coli during storage. The antimicrobial activity of the mixed whey protein and blueberry juice mixture systems was the highest, with an inhibition zone diameter of approximately 230 mm, compared with the whey protein or blueberry juice systems alone. There were no viable E. coli cells 7 h after treatment with of the whey protein and blueberry juice mixed systems as determined by survival curve analysis. Analysis of the inhibitory mechanism showed that the release of alkaline phosphatase, electrical conductivity, protein and pyruvic acid contents, and aspartic acid transaminase and alanine aminotransferase activity in E. coli increased. These results demonstrated that these mixed systems fermented with Lactobacillus, especially those containing blueberries, could inhibit the growth of E. coli and even cause cell death by destroying the cell membrane and cell wall.
Similar content being viewed by others
Introduction
Escherichia coli (E. coli), which usually resides in the intestinal tracts of mammals, was previously regarded as nonpathogenic bacteria. Most E. coli strains in food are nonpathogenic to humans, but some unique strains of E. coli, such as Shiga toxin-producing E. coli O157:H7, are pathogenic to both humans and animals, especially infants and young animals, and can cause intestinal infections, including abdominal pain, bloody diarrhoea, haemorrhagic colitis, haemolytic uraemic syndrome and even death1. E. coli often attaches to raw and unpasteurized meat and dairy products causing food contamination and food-borne illnesses2. The reason why these bacteria are so difficult to remove is due to their ability to form biofilms. The biofilm matrix consists of proteins, polysaccharides and so on, and can protect the bacteria contained within the biofilm and allow the bacteria to adapt to environment3. In addition, another difficult problem is the emergence and spread of antibiotic-resistant bacterial strains4. Therefore, it is urgent that effective alternative approaches be developed to prevent and improve such chronic infections and diseases. In recent years, many studies have suggested that some natural ingredients in plants can inhibit the growth of various foodborne pathogens 4.
Blueberry have been recognized as one of the most valuable fruits that contain a large quantity of nutritious components including organic acids, vitamins, minerals and micronutrients5. Furthermore, blueberries are an excellent source of bioactive compounds, including anthocyanins, phenolic acids, and flavonols, which endow this fruit with a series of beneficial properties, such as cancer protection and antioxidant, anti-inflammatory, and antibacterial activities6. Previous studies indicated that the phytochemicals in lowbush wild blueberries inhibit the growth of E. coli O157:H7 and increased the membrane permeability of E. coli O157:H77. In particular, the phenolics and anthocyanins in blueberries not only exhibit strong scavenging of free radicals such as ·OH and DPPH, but also inhibit the growth of E. coli, Salmonella enteritidis, Staphylococcus aureus and so on. The antimicrobial activity of these blueberry compounds may disrupt the integrity of the cell membranes of foodborne pathogens8. A study reported that probiotic-fermented blueberry juices improved the reducing power and showed stronger antibacterial activity against E. coli than fresh blueberry juice9. Another study noted that lactic acid bacteria have antibacterial effects due to the key antibacterial compounds they produce, such as bacteriocin, hydrogen peroxide and organic acids10. In addition, lactoferrin found in the whey protein fraction of milk can interact with the lipopolysaccharide layer of gram-negative bacteria, disrupt their outer membrane and enhance their susceptibility to the lysozyme by increasing membrane permeability11. However, the antibacterial effect and mechanism of a Lactobacillus-fermented whey protein and blueberry juice mixed system on foodborne pathogens is unclear. Thus, the inhibitory mechanisms of the mixed fermentation systems against foodborne pathogens during storage deserve deep investigation.
The objectives of this study were: (1) to examine the antibacterial effect of a Lactobacillus-fermented whey protein and blueberry juice mixed system on E. coli during storage, and (2) to investigate the possible antibacterial mechanism of this system from the perspective of cells membrane integrity. The Lactobacillus-fermented whey protein and blueberry juice mixed system showed high antibacterial activity (inhibition zone diameters and survival curves) against E. coli. Then, the cell membrane integrity (as determined by evaluating the activities of AKP, AST and ALT, electrical conductivity, and protein and pyruvate contents) was studied according to the inhibition mode. Therefore, the Lactobacillus-fermented whey protein and blueberry juice mixed system has potential for becoming a novel antibacterial agent and provides a basis for its application as an antibacterial agent in foods and medicines.
Material and methods
Materials
Whey protein (protein content 80%) was obtained from Fonterra group (Auckland, New Zealand). Blueberries (Vaccinium angustifolium) were purchased from farmers in Daxinganling, China. The bacterial strain E. coli, Lacticaseibacillus casei M54 (L. casei M54), Lactiplantibacillus plantarum 67 (L. plantarum 67), Streptococcus thermophiles 99 (S. thermophiles 99) and Lactobacillus bulgaricus 134 (L. bulgaricus 134) were preserved in the Jiangsu Key Laboratory of Dairy Biotechnology and Safety Control, Yangzhou University. The kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Preparation of the blueberry and whey protein fermented beverages
The Lactobacillus strains were activated in 10 mL of Man-Rogosa-Sharpe (MRS) broth at 37 °C for 16 h. S. thermophiles was activated in 10 mL of M17 broth at 42 °C for 12 h. The mixed bacterial culture consisted of L. casei M54 and L. plantarum 67 at a ratio of 1:1, as well as S. thermophiles 99 and L. bulgaricus 134. The sample treatments of samples and Lactobacillus strains used were shown in Table 1. Blueberry juice was prepared from 17 g of blueberries by breaking the fruits with a crusher followed by filtering using a 200 mesh filter screen. Subsequently, the blueberry juice was mixed with distilled water to reach a blueberry juice content of 17% (w/v). One hundred millilitres of blueberry juice was added to 6 g (w/v) of sugar and 6 g (w/v) of whey protein powder. Then, the pH values of the blueberry and whey protein mixtures were adjusted to 6.5 and 7.0, and pasteurized in a water bath. After cooling to room temperature, 2 mL of the mixed bacterial culture was added to 100 mL of the blueberry and whey protein mixture to achieve an inoculum of 2% (v:v), respectively. The blueberry and whey protein mixtures fermented with L. casei M54 and L. plantarum 67 were incubated at 37 °C, and the other mixtures fermented with S. thermophiles 99 and L. bulgaricus 134 were incubated at 42 °C.
Determination of the inhibition zone diameters
etermination of the E. coli inhibition zone diameters for the whey protein and blueberry juice mixed systems fermented with Lactobacillus was performed according to the method of Zhong et al.9 with slight modification. Briefly, 100 μL of 1 × 106 CFU/mL E. coli suspensions was coated with glass coating rod uniformly on disposable sterilized solid agar plates. After 10 min, the medium was punched using a 10 mm diameter perforator that had been sterilized previously. Subsequently, 200 μL of the whey protein and blueberry juice mixtures fermented with Lactobacillus was placed into the holes with a pipette. Finally, the agar plates were placed in an electric 37 °C constant temperature incubator for 12 h.
Survival curve analysis
Survival curves were used to determine the mode of antibacterial action of the whey protein and blueberry juice mixed systems fermented with Lactobacillus against E. coli according to the method of Zhou et al.8. In brief, all whey protein and blueberry juice mixed systems fermented with Lactobacillus were added to E. coli suspensions (109 CFU/mL) to achieve a final concentration of 108 CFU/mL. E. coli incubated with LB medium was regarded as the control sample. Subsequently, the above mixture were incubated at 37 °C for 12 h, and the reduction in the number of viable calls was measured every two hours by the plate counting method.
Determination of the activity of AKP
The effects of the whey protein and blueberry juice mixed systems fermented with Lactobacillus on E. coli AKP activity were studied using an AKP assay kit according to the manufacturer's instructions. According to the method of Deng et al.12, the changes in absorbance at a wavelength of 520 nm represent the activity of AKP.
Determination of the electrical conductivity
Determination of the electrical conductivity of the leaked bacterial cell contents was determined by measuring electrolyte leakage into the incubation medium with a conductivity meter (DDS-307, Precision & Scientific Instrument Co. Ltd., Shanghai, China). After incubation in LB medium at 37 °C for 12 h, E. coli were separated by centrifugation at 7000 r/min for 20 min, washed three times with triple 10 mM PBS (pH 7.0) and diluted with the same buffer to approximately 106 CFU/mL. The samples fermented with Lactobacillus were then added to the bacterial cell suspensions. The mixtures were incubated with shaking (130 rpm) at 37 °C, and the conductivity was measured after 0, 4, 8 and 12 d13.
Determination of the released protein content
The content of protein released from E. coli was determined using the BSA method. E. coli in the logarithmic phase were treated with the whey protein and blueberry juice mixed systems fermented with Lactobacillus for 10 h. The protein contents in the supernatants were assessed using the Pierce BCA Protein Kit (Thermo Scientific) purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China)14.
Determination of the activity of AST and ALT
The activities of AST and ALT were determined as described previously for the determination of AKP activity. The activities of AST and ALT in the supernatants were assessed using the AST and ALT Kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions15.
Determination of the pyruvate content
The pyruvate content was determined from the measured AKP activity with some modifications. At first, the optical density of the supernatants at 600 nm was adjusted to 0.7. Then, the pyruvate content of E. coli was determined using a pyruvate kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions.
Statistical analysis
Collected data are expressed as the means ± standard deviations (SDs). Analysis of variance (ANOVA) was performed, and the means were compared by the Student–Newman–Keuls test. A value of p < 0.05 was considered to indicate statistical significance. Data were analysed by using a statistical software package (SPSS for Windows, 11.5,2002, SPSS Inc., USA).
Ethics approval and consent to participate
This experimental plant research (either cultivated or wild), including the collection of plant material, complied with institutional, national, or international guidelines. Field studies were conducted in accordance with local legislation. The plant materials have been widely consumed by local people and were used in our previous study.
Ethical approval
This article does not contain any studies with human or animal subjects.
Results and discussion
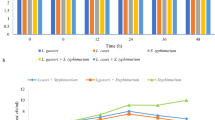
Antimicrobial properties
The antimicrobial properties of the whey protein and blueberry juice mixed systems fermented with Lactobacillus against E. coli during storage were determined by measuring the diameters of the zones of inhibition. As shown in Fig. 1, the antimicrobial activities of the blueberry and whey protein systems and the mixed systems fermented with L. casei M54 and L. plantarum 67 or S. thermophiles 99 and L. bulgaricus 134 were not significantly different. However, the blueberry and whey protein mixed fermentation systems showed the highest antimicrobial activity against E. coli after storage for 12 d, with inhibition zone diameters of approximately 230 mm and 225 mm, respectively. This result may be due to the increased release of polyphenols from the mixed blueberry and whey protein fermentation systems, as shown in our previous study16. It has been reported that polyphenols are the main antimicrobial agents affecting in vitro measurements17. Another report also suggested that the phenolics in blueberries are phytochemicals with strong antibacterial and antimycotic properties9. The antimicrobial activity of the blueberry and whey protein mixed system first increased and then decreased with prolonged storage time, which may be attributed to structural changes in the fermented antimicrobial products. In addition, the diameters of the antibacterial circles of the whey protein system fermented with S. thermophiles 99 and L. bulgaricus 134 against E. coli were 100 mm, 40 mm, 30 mm and 15 mm greater than those of the system fermented with L. casei M54 and L. plantarum 67, respectively. This effect may be closely related to the antimicrobial characteristics of lactic acid bacteria metabolites because different lactic acid bacteria have different metabolites including organic acids, fatty acids, short peptides and others18.
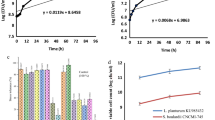
Effects of the mixed systems fermented with Lactobacillus on the survival of E. coli
To gain an insight into the effects of the mixed systems fermented with Lactobacillus on the growth of E. coli, survival curve analysis was carried out. As depicted in Fig. 2, the untreated control E. coli cells grew steadily for 12 h to more than 9 log CFU in the initial stationary growth phase. Compared with the control, all systems fermented with Lactobacillus inhibited E. coli at the initial phase. With prolonged incubation time, the number of viable cells treated with the blueberry system increased. This result suggested that the blueberry system has bacteriostatic effects against E. coli8. In particular, the viable E. coli cell count after the bacteria were treated with the blueberry and whey protein mixed system fermented with L. casei M54 and L. plantarum 67, whey protein and the mixed system fermented with S. thermophiles 99 and L. bulgaricus 134 decreased from approximately 7 log CFU to 0 log CFU, which indicated that these fermentation systems have bacteriostatic and bactericidal effects on E. coli. The above results demonstrated these samples have the potential to become antibacterial agents against E. coli.
Effect of the mixed fermentation systems on the activity of AKP
Cell membrane destruction has an unfavourable effect on the membrane-associated energy-transducing system. A study showed that the activity of AKP can regulate Ca2+ metabolism, which is related to calcium binding protein, the associated ion channels and energy metabolism based on phosphate. Once AKP is inactivated, cell differentiation is prevented by controlling the progress of dephosphorylation4. The effect of the blueberry and whey protein mixed system fermented with Lactobacillus on the AKP activity in E. coli was represented in Fig. 3. AKP activity of E. coli treated with blueberry system was higher than that of blueberry and whey protein mixed system and whey protein system. There was no significant difference between the systems fermented with L. casei M54, L. plantarum 67 and S. thermophiles 99, L. bulgaricus 134. Additionally, the activity of AKP was the highest when E. coli was treated with the blueberry system fermented with L. casei M54 and L. plantarum 67 (approximately 0.185 mg/ml) and the blueberry system fermented with S. thermophiles 99 and L. bulgaricus 134 (approximately 0.190 mg/ml) for 4 d. This result indicated that anthocyanins may enter the inner membrane of E. coli and stimulate AKP to flow out by disrupting the bacterial membrane and further inhibiting the growth of the bacteria4.
Effects of the mixed fermentation systems on the electrical conductivity of the cultures
The changes in the electrical conductivity of cell cultures treated with different samples were shown in Fig. 4, which were used to detect the permeability of the cell membrane. The electrical conductivity of E. coli cultures treated with all samples significantly (p < 0.05) increased during the first 4 days. Then, the electrical conductivity increased slowly. This result indicated that the E. coli cell membrane was disrupted and that the permeability of the cell membrane increased after treatment with all of whey protein and blueberry juice mixed systems fermented with Lactobacillus, which resulted in the leakage of intracellular ions such as Na+ and K+ that play a vital role in maintaining the balance of the organism and adjusting metabolic activity19. The results were in agreement with those that found an increase in relative electric conductivity of E. coli O157:H7 treated with different concentrations of natural antimicrobials for 2, 6 and 24 h14. A similar study suggested that treatment with Rosa rugosa tea polyphenol increased the permeability of the S. aureus cell membrane and caused ion leakage, which caused an increase in the electrical conductivity in the culture medium and finally cell death20. After storage for 12 days, the electrical conductivity of all treated E. coli decreased significantly (p < 0.05), which may be related to the reduction in phenolic compound contents in the samples. Previous study showed that the blueberry polyphenol content in fermented yogurt gradually decreased after storage for 28 days21. In particular, the electrical conductivities of the E. coli cultures treated with the systems fermented with L. casei M54 and L. plantarum 67 were relatively higher than those treated with S. thermophiles 99 and L. bulgaricus 134, which may be attributed to the production of different antibacterial substances. It was also suggested that the antibacterial substances of L. plantarum against E. coli are mainly organic acids and antimicrobial peptides22. The antibacterial effect of L. bulgaricus against E. coli has mainly been associated with lactic acid23.
Effects of the mixed fermentation systems on the content of protein released from E. coli
Whether the cell membrane is intact affects the growth and survival of bacterial cells. The bacterial cell membrane is a selective permeability barrier to the internal and external environment that controls the penetration of nutrients to maintain normal bacterial growth and drain metabolic waste. Therefore, to investigate the cell membrane integrity of E. coli treated with the blueberry and whey protein mixed systems fermented with Lactobacillus, the content of cellular proteins leaked from E. coli was studied.
Protein release from all samples remained stable, as shown in Fig. 5. The above results showed that the blueberry and whey protein mixed systems fermented with Lactobacillus have a significant inhibitory effect on E. coli. The content of leaked protein from the samples treated with the fermentation systems supplemented with blueberries were almost 33.33% higher than the contents leaked from the samples treated with the whey protein systems fermented with L. casei M54, L. plantarum 67 and S. thermophiles 99, L. bulgaricus 134 at 0 d. This result indicated that the main antibacterial compound may be associated with the components of blueberries. For example, the anthocyanin plus proanthocyanidin fraction plays a major role in damaging the integrity of the E. coli cell membrane8.
Effects of the mixed fermentation systems on AST and ALT concentrations in E. coli
AST and ALT are important intracellular enzymes in the metabolic pathways of organisms, participating in amino acid metabolism. With increasing cell wall and cell membrane permeability, intracellular enzymes can leak out. The concentrations of AST and ALT were measured to determine the integrality of cell wall and cell membrane, as shown in Fig. 6. The AST concentration was not significantly different among the blueberry systems, whey protein and blueberry systems. Nevertheless, the concentrations of AST in the bacterial cultures treated with the whey protein system were relatively lower during storage. Besides, the AST concentrations in E. coli treated with the whey protein system fermented with L. casei M54 and L. plantarum 67 were 20.83%, 142.86%, and 27.03% higher than those treated with the whey protein system fermented with S.thermophiles 99 and L. bulgaricus 134 after 8 days of storage. The ALT concentration results were roughly in agreement with those above. These results may be because antibacterial compounds such as bacteriocins that are produced by some Lactobacillus strains are more active at acidic pH than at neutral pH, which may have caused some of the antibacterial compounds to become inactive24.
Effects of the mixed fermentation systems on the pyruvate content
Pyruvate plays an essential role in the TCA cycle (tricarboxylic acid cycle) and glycolysis process, participating in the energy metabolism pathways in living organisms25. Therefore, the pyruvate content was measured to determine whether the cell membrane retained its integrity. As shown in Fig. 7, the pyruvate contents in E. coli cultures treated with systems fermented with L. casei M54 and L. plantarum 67 were approximately 54%, 140% and 50% higher than those in the systems fermented with S. thermophiles 99 and L. bulgaricus 134 at 0 d, respectively. Nevertheless, there were no significant differences after 12 days of storage. The results showed that the mixed systems fermented with L. casei M54 and L. plantarum 67 can disrupt E. coli membrane integrity, increase the permeability of the cell membrane and cause the leakage of pyruvate, affecting bacterial energy metabolism and finally leading to cell death. This may be related to the antimicrobial properties of probiotics. The result may be connected with the fact that the organic acids and fatty acids metabolized by L. plantarum, such as lactic acid and acetic acid, have strong antibacterial activity and can destroy the cell membrane integrity of E. coli and result in the leakage of its cellular contents, finally affecting normal bacterial growth18.
Conclusion
According to the above results, the fermentation systems that included blueberries were found to have the greatest antimicrobial effect against E. coli. For example, it was suggested that a whey protein and blueberry juice mixed system fermented with Lactobacillus displayed remarkable antibacterial characteristics against E. coli with antibacterial zone diameters of 230 nm and 225 mm at the end of storage, respectively. In particular, the blueberry systems had strong antibacterial effects against E. coli, as they disrupted the cell membrane integrity and affected the total content leakage, AKP activity, electrical conductivity and protein content. Additionally, the blueberry systems fermented with L. casei M54 and L. plantarum 67 also increased the pyruvate leakage, which interrupted normal growth of E. coli cells. Thus, the blueberry and whey protein mixed system fermented with Lactobacillus has great potential for future development as a natural antimicrobial agent to control pathogens. However, further studies are needed to explore what specific active components of the mixed systems fermented with Lactobacillus play a significant role in inhibiting the growth of E. coli and to investigate these antibacterial components against more microorganism strains. Furthermore, the results also indicated that the whey protein and blueberry juice mixed system fermented with Lactobacillus may become a promising natural preservative to prevent and control foodborne pathogens such as E. coli.
Data availability
The authors confirm that all data and materials as well as software applications to support their published claims and comply with field standards. All data can be provided upon request from the corresponding author.
References
Atnafie, B. et al. Occurrence of Escherichia coli O157:H7 in cattle feces and contamination of carcass and various contact surfaces in abattoir and butcher shops of Hawassa, Ethiopia. BMC Microbiol 17(1), 24 (2017).
Ahmed, A. M. & Shimamoto, T. Molecular analysis of multidrug resistance in Shiga toxin-producing Escherichia coli O157:H7 isolated from meat and dairy products. Int J Food Microbiol 193, 68–73 (2015).
Vestby, L.K., et al., Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics (Basel), 2020. 9(2).
Sun, X.-H. et al. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 94, 155–161 (2018).
Tobar-Bolanos, G. et al. Blueberry juice: Bioactive compounds, health impact, and concentration technologies: a review. J Food Sci 86(12), 5062–5077 (2021).
Li, S. et al. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: fermentation characteristics and evolution of phenolic profiles. Chemosphere 276, 130090 (2021).
Lacombe, A. et al. Phytochemicals in lowbush wild blueberry inactivate Escherichia coli O157:H7 by damaging its cell membrane. Foodborne Pathog Dis 10(11), 944–950 (2013).
Zhou, T. T. et al. The effect of Chinese wild blueberry fractions on the growth and membrane integrity of various foodborne pathogens. J Food Sci 85(5), 1513–1522 (2020).
Zhong, H. et al. Probiotics-fermented blueberry juices as potential antidiabetic product: antioxidant, antimicrobial and antidiabetic potentials. J Sci Food Agric 101(10), 4420–4427 (2021).
Desniar, et al., Organic acid produced by lactic acid bacteria from bekasam as food biopreservatives. IOP Conference Series: Earth and Environmental Science, 2020. 414.
Brumini, D. et al. Whey proteins and their antimicrobial properties in donkey milk: a brief review. Dairy Sci. Technol. 96(1), 1–14 (2015).
Deng, H. et al. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 150, 112018 (2021).
Li, Y.-Q. et al. Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 43, 22–27 (2014).
Stratakos, A. C. et al. The antimicrobial effect of a commercial mixture of natural antimicrobials against Escherichia coli O157:H7. Foodborne Pathog Dis 16(2), 119–129 (2019).
Eghbalpour, F. et al. Pathogenic features of urinary Escherichia coli strains causing asymptomatic bacteriuria during pregnancy. Gene Rep. 27, 101559 (2022).
Wen-qiong, W. et al. Structural and compositional changes of whey protein and blueberry juice fermented using Lactobacillus plantarum or Lactobacillus casei during fermentation. RSC Adv. 11(42), 26291–26302 (2021).
Chollakup, R., et al., Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. Lwt, 2020. 130.
Mao, Y., Zhang, X. & Xu, Z. Identification of antibacterial substances of Lactobacillus plantarum DY-6 for bacteriostatic action. Food Sci Nutr 8(6), 2854–2863 (2020).
Stratakos, A. C. et al. The in vitro effect of carvacrol, a food additive, on the pathogenicity of O157 and non-O157 Shiga-toxin producing Escherichia coli. Food Control 84, 290–296 (2018).
Zhang, Q., et al., Antimicrobial effect of tea polyphenols against foodborne pathogens. J. Food Prot. 2021.
Wei, X. et al. Study on the stability of blueberry polyphenols during storage in fermented yogurt. Food Industry 42(09), 167–171 (2021).
Jialin, Z. et al. Screening of Lactobacillus plantarum with antibacterial activity and research on the characteristics of antibacterial substances. Food Ferment Ind 42(09), 167–171 (2021).
Abedi, D. et al. In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res. Pharm. Sci. 8(4), 261–268 (2013).
Hati, S. et al. Influence of whey protein concentrate on the production of antibacterial peptides derived from fermented milk by lactic acid bacteria. Int. J. Pept. Res. Ther. 24(1), 87–98 (2017).
Saunier, E., Benelli, C. & Bortoli, S. The pyruvate dehydrogenase complex in cancer: an old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer 138(4), 809–817 (2016).
Acknowledgements
This study was conducted in the Jiangsu Key Laboratory of Dairy Biotechnology and Safety Control, Yangzhou University, Yangzhou, Jiangsu, China. The authors thank engineer Guo Sheng of Weiwei Food & Beverage Co., Ltd, Xuzhou, Jiangsu, China for the raw material supplement.
Funding
This work was supported by project of the National Science Foundation for Young Scientists of China (Grant No. 31901715), the Jiangsu Postdoctoral Foundation (2021K317C), the Jiangsu Provincial Practice and Innovation Plan Project (SJCX21_1630), a project funded by the China Postdoctoral Science Foundation (2022M712433), and a Science and Technology Innovation Platform of Yangzhou City and Yangzhou University jointly Builded (YZ2020265).
Author information
Authors and Affiliations
Contributions
Y.Q.: measurement analysis, writing-original draft, and methodology. L.G.: measurement analysis and resources of original materials. W.W.: writing-original draft, investigation, formal analysis, and funding support. T.C. measurement analysis. G.R. revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, Y., Gui-bing, L., Wen-qiong, W. et al. The mechanism of whey protein and blueberry juice mixed system fermented with Lactobacillus inhibiting Escherichia coli during storage. Sci Rep 13, 6614 (2023). https://doi.org/10.1038/s41598-023-33888-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33888-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.