Abstract
During two consecutive summer and winter seasons in Lahore, the health risk of air and dust-borne polycyclic aromatic hydrocarbons (PAHs) was evaluated. Gas chromatography/mass spectrometry (GS/MS) was used to determine air and dust samples from various functional areas across the city. The mean ∑16PAHs were higher in air 1035.8 ± 310.7 (pg m−3) and dust 963.4 ± 289.0 (ng g−1 d.w.) during winter seasons as compared to summer seasons in air 1010.9 ± 303.3 (pg m−3) and dust matrices 945.2 ± 283.6 (ng g−1 d.w.), respectively. PAHs ring profile recognized 3 and 4 rings PAHs as most dominant in air and dust samples. Estimated results of incremental lifetime cancer risk (ILCR) highlighted high carcinogenic risk among the residents of Lahore via ingestion and dermal contact on exposure to atmospheric PAHs. The total ILCR values in air among children (summer: 9.61E − 02, winter: 2.09E − 02) and adults (summer: 1.45E − 01, winter: 3.14E − 02) and in dust, children (summer: 9.16E − 03, winter: 8.80E − 03) and adults (summer: 1.38E − 02, winter: 1.33E − 02) during the study period. The isomeric ratios in the study area revealed mixed PAH sources, including vehicular emission, petroleum, diesel and biomass combustion. As a result, it is advised that atmospheric PAHs should be monitored throughout the year and the ecologically friendly fuels be used to prevent PAHs pollution and health concerns in the city. The findings of this study are beneficial to the local regulating bodies in terms of controlling the exposure and promoting steps to reduce PAHs pollution and manage health in Lahore.
Similar content being viewed by others
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are semi-volatile organic compounds (SVOCs) originating from various pyrogenic and petrogenic sources, including combustion and petroleum products1, 2. PAHs are arranged in 2–6 linear or cluster form rings3, 4, classified as low molecular weight (LMW) PAHs (less than four rings), and high molecular weight (HMW) PAHs (4–6 rings)5,6,7. In recent years, the PAHs have been detected in a number of media, including air, sediments, soils, and dust8,9,10. Air and dust are considered to be the major natural sinks and reservoirs of PAHs11. PAHs are acquiring more attention due to their carcinogenic and mutagenic properties causing adverse human health effects12.
United States Environmental Protection Agency (US EPA) recognized 16 polycyclic aromatic hydrocarbons as "Consent Decree" priority pollutants5, 13, of which 7 pollutants have been classified as potential human carcinogens: Benz(a)anthracene (BaA), Benzo(a)pyrene (BaP), Benzo(b)fluoranthene (BbF), Benzo(k)fluoranthene (BkF), Chrysene (Chry), Dibenz(ah)anthracene (DahA), and Indeno(1,2,3-cd) pyrene (IP)14. With the economic and industrial development of the urban environment, these pollutants' atmospheric concentrations have increased1, 2. A huge contribution of atmospheric PAHs includes vehicular emissions, power generation by oil and coal combustion, industrial plants, and residential heating15.
Absorption of contaminated foods, inhalation of polluted air and ingestion of dust are the most common routes of human exposure to PAHs16, 17. Among these exposure pathways, inhalation primarily refers to active and passive smoking and breathing in polluted indoor and outdoor environments18. Dermal exposure occurs when PAHs come into direct contact with the skin and eyes due to occupational or other environmental conditions19. Another major element of exposure in humans is dietary consumption of PAHs from various food categories (including fruits, vegetables, and meat)20. There is growing worried about the health effects of PAHs21. They cause severe respiratory and cardiovascular disorders, reduce lung capacity, myocardial infarction, asthma, and possibly cancer, as well as immune system failure10, 12, 22.
Pyrosynthesis and pyrolysis are two basic processes resulting in PAH formation in an oxygen-deficient environment from saturated hydrocarbons23. PAHs are present in both the gas and particulate phases of the atmosphere, where they are linked with particles24. Several studies have indicated that gas-phase PAHs concentrations are substantially higher than solid-phase PAHs concentrations25, 26. The PAHs distribution and persistence in the air are regulated by their physicochemical properties and climate conditions27, 28. Their toxicity depends on particulate size, molecular structure, chemical composition, and meteorology of the region29.
PAHs settle in dust because of dry and wet atmospheric deposition30 and function as natural sinks to store the organic chemicals in rural and urban environments31. In everyday life of residential and occupational settings, the pollutants are ingested by people through skin contact, dust absorption and inhalation32, 33. Much emphasis has recently been placed on the importance of polluted air and dust inhalation as a route to PAHs exposure in humans16, 17, 34. The size of street dust particle5 influences pAHs' structure and chemical composition. Larger dust particles often have less surface area for the deposition of PAHs. Therefore, smaller dust particles contain more PAHs than bigger ones35. Furthermore, due to their high hydrophobicity, PAHs with more molecular mass sink into the settled dust36, 37.
Pakistan, a developing South Asian nation with a population of 200 million and an annual growth rate of 2.4%, ranks seventh in the world regarding PAHs emissions38. Air pollution is an emerging issue in the big cities of Pakistan. The primary sources are growing urbanization, development of infrastructures, increasing industrial activities without appropriate air emission treatment or controls, transportation congestion, and vehicular exhaust22. Traffic pollution is one of the country's most significant man-made PAHs sources, accounting for 60% of the total PAHs released into the urban environment39.
Previously, only a few researches on PAHs toxicity in the environment and matrix of dust had been performed in Pakistan. Smith et al.40 carried out the first systematic survey in Lahore, Pakistan. Almost after 20 years, a study was conducted in Lahore, Gujranwala, and Rawalpindi showed that LMW PAHs were the most prevalent congeners, originating from the local petroleum refinery and vehicular emissions, respectively9. Furthermore, some epidemiological investigations on PAHs emissions in Pakistan from diverse occupational contexts like traffic wardens, brick kiln employees and workers from the petroleum industry were conducted10, 41, 42. However, no comprehensive research on air and dust PAHs with seasonal changes was performed in this PAHs pollution hotspot. Lahore has rapidly urbanized, with vehicle ownership and population density rising. On the other hand, the city has the initiative to monitor air quality for PAHs levels to manage air contamination5. To monitor the source and extent of PAHs concentrations to mitigate the environmental public health threats, a quantitative understanding of the possible sources of these pollutants in urban air and dust is needed12, 36. Therefore, the current study aimed to establish the distribution and levels of the profile of PAHs in air and dust samples of ten functional areas of Lahore city, assessing the seasonal variation in PAHs concentrations pattern and estimating the incremental lifetime cancer and non-cancer risks of PAHs through inhalation, ingestion and dermal routes of exposure.
Materials and methods
Study area
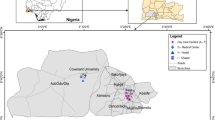
Lahore is Pakistan's second most populous city35. According to the 2017 census, Lahore's total population is 11.13 million, with a land area of about 1772.43 km2 36. It is situated in the Punjab province between latitudes 31° 20′ and 31° 50′ N and longitudes 74° 05′ and 74° 37′ E43, 44. Around 82% of the population resides in the city, and the remaining 18% lives in the rural areas around the city45. Lahore is situated at 63.0936 m in height above sea level. It has hot and semi-arid climate and is classified by the Köppen classification as a desert climate46. The average temperature is 24.1 °C, and 75.28 °F and 607–23.9 mm is annual rainfall recorded per year in Lahore47. The city has expanded due to a population movement and grown through a population shift for better sociocultural and economic reasons31. Figure 1 represents the map of the study area showing sampling sites of Lahore.
Sample collection
Air sampling
For PAHs monitoring in ambient air, ten sampling sites (Shah Jamal, UET, Ravi Road, Jail Road, Thokar Niaz Baig, Misri Shah, Manawa, Gulberg, Mohlanwal and Mughalpura) were selected in Lahore city, Pakistan and total 10 passive air samplers were deployed (one at each site) for consecutive 56-days during two sampling periods, winter (October–March) and summer (April–September) in year 2017 and 2018, respectively48. Based on population density, high traffic congestion and intensified anthropogenic activities, ten major hotspot areas of Lahore city were identified for collecting air and dust samples. The sampling locations were chosen by dividing the city into ten most inhabited residential and commercial sectors, going through heavy vehicular and industrial pollution, affecting the public health and environment of Lahore. Another criterion for selecting these areas was that they are fixed air monitoring sites of the Punjab Environment Protection Department (EPD), from where daily concentrations of major air pollutants such as carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), Ozone (O3), and particulate matter (PM2.5 and PM10) are monitored, considering these areas to be the most polluted in the city.
The Polyurethane Foam (PUF) discs (14 cm in diameter, 1.3 cm in thickness, and 0.02 g cm−3 in density) were sterile aluminum foil wrapped and sealed in zip-lock bags9 and installed between two stainless steel bowls of 20 cm and 30 cm diameter served as the exterior shelter with a 1.5 cm intra space between them that enable air to pass over the PUF discs15. Dichloromethane (DCM) and acetone were used to pre-extract all PUF discs for 48 h in the laboratory. Each Passive air sampler (PAS) was built at the deployment location to avoid contamination. PUF-PAS were installed on the rooftops of single-story buildings at the height of 5 m above ground level. Mean concentrations of gaseous-phase PAHs were collected by passive air samplers. Following the methodology stated in the previous calibration researches, the PUF standard passive air sampling rate was computed (Text S1)49, 50. After the sampling period, the PUFs were repaired, wrapped in aluminum foils and transferred to the laboratory and stored at − 20 °C till further analysis10, 51.
Dust sampling
Approximately 5 g of dust samples from each air sampling location were also collected after midday between 4 and 5 p.m. over the same time period as air samples. Each dust sample was made up of five separate subsamples collected from each sampling site and then combined to produce a composite sample52. To assemble the tiny particles, samples were gathered in stainless steel dustpans by using plastic brushes in a gentle sweeping movement. Each time new disposable dustpan and brush were used and covered with aluminum foil to minimize cross-contamination in samples53. Grits, hairs and organic materials were removed from the samples by subsequent sieving through 2 mm mesh (AASHTO classification). The samples were stored at − 20 °C until analysis54.
Sample preparation and extraction
Air and dust samples were spiked with 50 µL of deuterated PAHs as recovery standards (Nap-d8, Phe-d10, Chry-d12, 2, 4, 5, 6-T-m-x) and separately extracted with DCM for 24 h by using Soxhlet. The samples were then extracted (in triplicate) by 30 min ultra-sonication with dichloromethane and hexane solution (1:1 v/v), followed by 1 min of vortex agitation and centrifuge for 30 min at 3500–5000 rpm at room temperature55. Rotary evaporation (DIAHAN Scientific WEV-1001L) was performed for volume reduction before clean-up the samples. Alumina/silica column was used to purify the samples with 8 mm internal diameter, tightly packed with 3 cm neutral alumina (3% deactivated), 50% sulfuric acid–silica, 3 cm neutral silica gel (3% deactivated) and 1 cm anhydrous Na2SO4. Later, purified sample was extracted with 1:1 DCM and hexane (by volume), blown down to a final volume of 1 mL under a moderate nitrogen flow (0.2 mL)51. The samples were then placed in septa vials for further examination using gas chromatography–mass spectrometer (GC–MS).
Instrumental analysis
The samples were analyzed using GC/MS (QP2010, Shimadzu) for 16 priority PAHs (2–6 rings) in the Split Injection Mode (SIM). The injector and ion sources were both 200 °C. As the carrier gas, helium was used. The Column Flow was set to 1.6 mL/min. The oven temperature was held for 4 min at 50 °C, raised to 320 °C (held for 3 min)54, 55.
Quality assurance/quality control
Throughout sampling and analysis, strict quality assurance and control procedures were followed. All the chemicals and solvents used in the current study were analytical research-grade, acquired from Sigma-Aldrich now Merck KGaA (Germany), and checked for impurities prior to use. Na2SO4 was baked for 12 h at 450 °C and stored at 120 °C till use to eliminate any organic debris. The internal and recovery standards were purchased from Chem Service, USA. All the chemicals utilized in the laboratory procedures, for example, acetone (Ace), hexane (Hex), and dichloromethane (DCM) were of the GC analytical grade. Glassware used in sample preparation was heated at 400 °C overnight and stored at 100 °C before use. A set of PAHs standards was performed daily to maintain the instrument's stability, and the instrument's fluctuation was less than 10%. Method detection limits (MDLs) of target compounds were estimated as three times the standard deviation of the mean procedural blank concentrations. Recoveries of the native analytes tested for the reference material were greater than 72% for all PAHs samples. QA/QC was performed to identify any possible laboratory contamination by conducting method blanks, standard reference material recoveries, standard spiked recoveries, and GC/MS detection limits22. The dilutions for standards were ranged from 0.001 to 200 μg g−1.
PAHs diagnostic ratios
PAHs ratios such as Phen/Anth, Flan/Pyr, BaA/Chry and BaP/BghiP, IP/(IP + BghiP), Flu/(Flu + Pyr) and Anth/(Anth + Phen) are commonly used as tracers of PAHs emission sources54, 56,57,58. In the present study, these ratios were also determined for the source identification of PAHs.
Human health risk assessment
Cancer risk assessment
The carcinogenic potential of many PAHs, particularly High Molecular Weight (HMW) PAHs, is extensively documented in the literature59. The benzo(a)pyrene toxic equivalency factors (TEFs) were used to estimate benzo(a)pyrene equivalent (BaPeq) or benzo(a)pyrene toxicity equivalent (BaP-TEQ) concentrations to evaluate the incremental lifetime cancer risk (ILCR) of PAHs in air and dust samples.
Equation (1) was used to calculate ILCR.
BaP − TEQs was calculated by multiplying individual PAH concentration (Ci) by the WHO-recommended TEFs (toxic equivalency factors) values such as 0.001 (Nap, Ace, Acy, Fla, Phe, Flu and Pyr), 0.01 (Ant, Chr and B(ghi)P), 0.1 (B(a)a, B(b)F, B(k)F, and I(cd)P) and 1 (B(a)P and D(ah)A) established by Nisbet and LaGoy60 (Table 1). The computed BaP as TEQ values indicated a significant toxicity hazard linked with PAHs in air and dust samples61. Cancer risk from inhalation was estimated using WHO (2000) methods, and the unit risk (UR) of 8.7 × 10−5 (ng m−3) was used for a lifetime of 70 years exposure as one individual exposed to one unit BaP (1 ng m−3) on average. The potential cancer risk of human exposure via inhalation, ingestion, and dermal contact to air and dust-related PAHs was assessed in different age groups by an Incremental lifetime Cancer Risk (ILCR) model53, 61.
Equation (2)–(4) were employed to calculate cancer risk via various exposure routes.
where BaP − TEQ is the total of converted PAHs levels based on toxic equivalents of BaP calculated by multiplying each PAH concentration (ci) with the toxic equivalency factor (TEF). CSF is carcinogenic slope factor (mg kg−1 day−1), BW is body weight (kg), AT is the average life span (years), EF is the exposure frequency (day year−1), ED is the exposure duration (years), IRInhalation is the inhalation rate (m3 day−1), IRIngestion is the soil intake rate (mg day−1), SA is the dermal surface exposure (cm2), AF is the dermal adherence factor (mg cm2 h−1), ABS is the dermal adsorption fraction, and PEF is particle emission factor (m3 kg−1)62. CSFIngestion, CSFDermal and CSFInhalation of BaP were addressed as 7.3, 25, and 3.85 (mg kg−1 day−1), respectively, determined by the cancer-causing ability of BaP63. All of the parameters included in this model were based on the United States Environmental Protection Agency's (US EPA) Risk Assessment Guidance and associated publications64, 65. Values of the parameters used in the above equations are presented in Table S1 (“Supplementary material S1”).
Non-carcinogenic risk
The non-cancer risk assessment of PAHs is essentially examining the association between PAHs dose and unfavourable health effects. It primarily consisted of estimating PAHs exposure dose using various environmental matrices (in this study, dust and air), exposure pathways and exposure frequency66. Although, other parameters such as age and body weight may influence the frequency and duration of exposure. The non-cancer risk of PAHs was evaluated in this study for five age groups based on ingestion and inhalation pathways using the Eqs. (5) and (6) to determine the health risk of daily oral and breathing intake of PAHs from dust and air, respectively10, 14, 67.
The equation to determine the health risk of breathing intake10:
Results and discussion
Distribution and levels of PAHs in air
The ∑16PAHs mean concentrations (pg m−3) in air during summer and winter seasons from the study area have shown in Table 1. Lowest to highest mean concentrations of ∑16PAHs ranged from 258.2 ± 860.7 to 303.3 ± 1010.9 (pg m−3) and 903.3 ± 271.0 to 1035.8 ± 310.7 (pg m−3) in summer and winter, respectively. The mean concentrations of lower molecular weights PAHs (∑LMPAHs) (and high molecular weight PAHs (∑HMPAHs) ranged from 366.3 ± 109.9 to 407.6 ± 122.3 and 494.4 ± 148.3 to 603.3 ± 181.0 (pg m−3) in summer and 376.1 ± 112.8 to 419.6 ± 125.9 and 527.2 ± 158.2 to 616.2 ± 184.9 (pg m−3) in winter seasons of the study period. The mean carcinogenic PAHs (∑C7PAHs) concentrations were ranged from 295.0 ± 88.5 to 358.9 ± 107.7 and 311.1 ± 93.3 to 365.9 ± 109.8 (pg m−3) in summer and winter, respectively. Among all the PAHs studied, Phe, Nap, and Pyr were found at significant levels in air. The concentration range of studied PAHs for summer and winter in air is mentioned in Tables S2 and S3, respectively.
The current study's findings showed a substantially lower range of ∑16 PAHs than earlier PAHs studies conducted in contaminated places, such as Khatmandu in Nepal (155,000 pg m−3)68, Tehran in Iran (57,000 pg m−3)58, Ningbo in China (46,000 pg m−3)69 and Xian in China (116,000 pg m−3)70. Due to the meteorological and geographical variations, the composition and concentration of PAHs vary in Lahore from the other urban environments of the world. The findings of the current study agreed with a study reported from twin cities of Pakistan (2132 pg m−3)10 and Paris in France (1000 pg m−3)71 (Table S6).
Levels and distribution of PAHs in road dust
Mean concentrations of ∑16PAHs in road dust ranged from 245.7 ± 818.9 to 283.6 ± 945.2 (ng g−1) and 256.8 ± 853.4 to 89.0 ± 963.4 (ng g−1) in summer and winter seasons, respectively (Table 2). The mean concentrations of ∑LMPAHs and ∑HMPAHs were ranged between 348.8 ± 104.6 and 387.3 ± 116.2 (ng g−1) and 470.1 ± 141.0 to 550.2 ± 165.1 (ng g−1) in summer while 359.8 ± 108.9 to 397.6 ± 119.3 (ng g−1) and 488.9 ± 146.7 to 565.8 ± 169.7 (ng g−1) during winter seasons. The mean ∑C7PAHs concentrations were ranged from 282.6 ± 84.8 to 331.3 ± 99.4 (ng g−1) and 285.2 ± 85.6 to 331.8 ± 99.5 (ng g−1) in summer and winter, respectively. Phe, Nap and Fla levels showed the highest concentrations in both seasons for dust samples. However, PAHs identified in dust samples showed higher winter concentrations than summer during the study period. The concentrations range of PAHs parameters for summer and winter dust are mentioned in Tables S4 and S5, respectively.
Findings of the present study have observed higher mean concentrations from the previous study conducted in Pakistan, i.e. Rawalpindi and Islamabad (89.8 ng g−1 d.w.)10 and showed similar findings with Chung Khurd, Lahore (882 ng g−1 d.w.)72. In comparison with other international cities, the current study has shown higher mean concentrations of Σ16PAHs of dust compared from Karaj, Iran (624 ng g−1 d.w.)73 and lower than Lanzhou city, China (3900 ng g−1 d.w.)65, Sydney, Australia (2910 ng g−1 d.w.)74, Tianjin, China (7993.3 ng g−1 d.w.)14, New Delhi, India (1100 ng g−1 d.w.), and Mashhad, Iran (2183.5 ng g−1 d.w.)75. Furthermore, the current study results depicted similar findings to the study conducted in PAHs of dust in Ulsan, Korea (960 ng g−1 d.w.)76 (Table S7).
Spatial distribution pattern of PAHs in Lahore
Lahore, the country's biggest traffic hub, had a tremendous influx of traffic every day, causing traffic congestion and eventually increasing vehicular emissions28. Furthermore, poor car engines maintenance and fuel quality had a substantial role in PAHs emissions77. The concentrations of PAHs in Lahore city air and dust varied with seasons. The greatest PAHs concentrations in the air were detected in Shah Jamal and UET, followed by Ravi road, where traffic pollution is to blame for the increasing PAHs levels in these areas. Among all study sites, the dust samples taken from UET road had the highest PAH concentrations. It is the oldest and largest high-traffic area globally, with heavily inhabited streets on both sides of a 2-km span78. Due to the high population density in the city, the sites are subjected to considerable traffic and domestic heating77. The study's findings revealed that the PAHs distribution in the dust around Lahore is not uniform but rather the result of a number of contributing elements like heavy traffic and distance from industry.
Profile of PAHs in air and dust samples
A significant variation was detected among PAHs concentrations in air, and dust samples. The PAHs profile distributions was in order of 4 rings (air: 31%, dust: 32%) > 3 rings (air: 26%, dust: 27%) > 5 rings (air: 19%, dust: 20%) > 2 rings (air: 14%, dust: 13%) and > 6 rings (air: 10%, dust: 8%) PAHs, respectively. The most dominant PAHs in this investigation were 3 rings (Acy, Flu, Phe, and Ant) and 4 rings (Pyr, BAA, and CHR) with a cumulative percentage contribution of 55% in air and 57% in dust (Fig. 2a,b). This compositional pattern is similar to previous studies showing 3 and 4 ring PAHs as the main contributors of PAHs2, 79, 80. The observed trend can be explained by considering the physicochemical characteristics of PAHs and their nearness to their origins79. In the present study, 4 rings PAHs were found in relatively higher concentrations, representing the pyrogenic origin and biomass fuel combustion, followed by 3 rings PAHs, suggesting the markers of petroleum-derived residues22, 41. The dominance of HMW (4, 5 and 6 rings) PAHs was similar to the results conducted by Najmeddin and Keshavarzi55 in Ahvaz city where HMW PAHs (dust: 68.8%) showed higher concentrations. The amount of PAHs in the atmosphere is also influenced by several variables such as emission sources and meteorological characteristics, including rainfall, temperature, wind speed and direction, resulting in seasonal variation in PAHs levels81. Higher PAHs emissions in the winter season are due to increased combustion of biomass and fossil fuels for household heating and the usual increase in primary pollutants in the colder months due to poorer dispersion conditions and lower atmospheric temperature compared to the summer (high-temperature) seasons82, 83. Thus, the overall results of the PAHs profile revealed not only biomass combustion but also vehicular emission as the source of PAHs deposition in Lahore because all of the selected sampling sites are known for traffic pollution due to the high rate of daily traffic flow on the roadways1, 4.
PAHs isomeric ratio in air and dust
In the present study, PAHs ratios such as Phen/Anth, Flan/Pyr, BaA/Chry, and BaP/BghiP, IP/(IP + BghiP), Flu/(Flu + Pyr) and Anth/(Anth + Phen) were determined to predict the origins of PAHs, which possesses a significant hazard to the population46, 54, 58. PAHs generated from various sources have considerable diverse compositional patterns84, 85. Ant/(Ant + Phe) ratios less than 0.1 suggest a petroleum source, whereas ratios greater than 0.1 implies that combustion is dominant65, 86. In the present study, Ant/(Ant + Phe) ratios ranged from (0.05–0.15; 0.05–0.13), indicating the dominance of petroleum sources. Furthermore, a Flu/(Flu + Pyr) ratio of 0.4 suggests a petroleum input source, 0.4–0.5 for liquid fossil fuel and crude oil combustion, > 0.5 for biomass and coal combustion and the ratio of InP/(InP + BP) < 0.20 indicates a petroleum source, > 0.50 for the biomass and coal combustion, and 0.20–0.50 for liquid fossil fuel combustion86.
In the current study, ratios of Flu/(Flu + Pyr) (0.51–0.58; 0.52–0.59) and InP/(InP + BP) (0.51–0.58; 0.52–0.59) showed the major contribution from biomass and coal combustion86. While the ratio of BaA/(BaA + Chr) < 0.2 stands for petroleum, 0.2–0.35 for liquid fossil fuel, vehicle and crude oil, and > 0.35 for combustion of coal, grass and wood86. As the ratio of BaA/(BaA + Chr) in this study ranged from (0.31–0.49; 0.49–0.53), representing vehicular emission and grass and wood combustion are highlighted as key sources of PAHs in air and dust of Lahore city (Fig. 3a,b). Furthermore, the LMW/HMW PAHs ratio was employed to estimate the extent of burning activities and pyrogenic and petrogenic sources of PAHs10. The current study's findings revealed that petroleum combustion was a prominent PAHs contributor since LMW/HMW PAHs ratio was less than 1 during both summer and winter seasons in air (0.68–0.74) and dust samples (0.69–0.75), respectively. It is further supported by the results of Spearmen Correlations, showing the negative relationship between LMW/HMW PAHs ratio for air (summer: R2 = 0.9691; winter: R2 = 0.9968) (Fig. 4a,b) and dust (summer: R2 = 0.9649; winter: R2 = 0.872) (Fig. 4c,d). Current results are consistent with the findings reported by He et al.84 from Nanjing, China, where fossil fuel burning was recognized as the primary source of PAHs and Hamid et al.10, where the indoor and outdoor air PAHs relation with fuel combustion in Pakistan's twin cities was found (Rawalpindi and Islamabad).
Health risk assessment of PAHs in air and dust
Carcinogenic risk
The total BaPeq of ∑16PAHs in air and dust samples ranged from 92.5 to 122.4 pg m−3 and 91.9–117.5 ng g−1 during the study period, respectively (Tables 1 and 2). The current study's findings were comparable to the PAHs levels in street dust of Nanjing, China, from 25.9 to 90.8 (ng g−1)84. The cancer risk from various routes of exposure was found to be in the order: dermal contact > ingestion > inhalation. Total ILCR estimates utilizing maximum concentrations showed a possible cancer risk for persons residing in sampling areas. The total ILCR values in air samples were children (summer: 9.61E − 02, winter: 2.09E − 02) and adults (summer: 1.45E − 01, winter: 3.14E − 02) and in dust samples of selected areas, children summer: 9.16E − 03, winter: 8.80E − 03 and adults summer: 1.38E − 02, winter: 1.33E − 02 during the study period (Table 3). According to the present study, both ingestion and dermal contact increased cancer with the magnitude of 1E − 02 and 1E − 03 in air and dust samples, respectively, contributing significantly to cancer risk in children and adults (Table 3).
According to epidemiological research, long-lasting PAHs exposure has been linked to increased skin, lung and gastrointestinal malignancies5, 22, 54. For adults, skin contact was the most common exposure route because PAHs enter in the body very easily by the dermal contact with soil, contaminated water, soot, tar or by applying few oils on the body that contain high levels of PAHs, which resulted in a substantially increased risk, followed by the ingestion pathway51. An ILCR between E − 06 and E − 04 specified a potential carcinogenic threat, whereas an ILCR between E − 04 and E − 042 indicated a high-potential health hazard4. In the current research, ILCRs of total cancer risk for both children and adults were greater than the baseline tolerable risk, suggesting a high cancer risk. According to the findings, dust-borne PAHs pose a risk to local inhabitants in Lahore, comparable to research done by Jiang et al.85, where ILCR was higher from 5.34 E − 05 to 4.50 E − 04.
Non-carcinogenic risk
PAHs can induce health hazards that are not always linked to cancer but can show significant consequences for non-cancerous health risks such as asthma, heart problems, acute lung dysfunction, jaundice, kidney and liver failure51, 52. Microbial diversity and metabolic profiles may serve as response markers to PAHs exposure in children with asthma. Inhaling PAHs causes hypersensitivity of immunoglobulin E (IgE) substance associated with increasing the asthma emergency department visits in all age groups87, 88. PAHs, such as Naphthalene, are extremely carcinogenic, can induce kidney and liver damage, cause redness and irritation of skin through dermal contact and cause red blood cell destruction when breathed. Primarily the industrial workers exposed to PAHs and other chemicals were shown to have an elevated risk of skin, lung, bladder, and gastrointestinal malignancies in many studies89. PAHs metabolites are related to increased risk of atherosclerotic cardiovascular disease (ASCVD) in the general population, changing the heart rate variability (HRV), an early marker of cardiac autonomic imbalance90. Chronic exposure to PAHs induced oxidative stress, involved in the development of diabetes91, 92. Additionally, additive effect of reduced lung function and urinary OH-PAHs on diabetes was also found93.
Total estimated daily intake (EDI) PAHs of oral intake in air samples ranged from 2.7 to 17.9 ng kg−1 day−1 for two age cohorts in the air samples, i.e. children: 17.9 ng kg−1 day−1 and adults: 2.7 ng kg−1 day−1 in summer seasons and a similar trend was observed during winter seasons, exhibiting relative higher winter EDI in children than adults. The oral PAHs intake in the dust samples varied as children summer: 16.2 ng kg−1 day−1, winter: 16.3 ng kg−1 day−1, adults summer: 0.01 ng kg−1 day−1, winter: 2.5 ng kg−1 day−1 (Fig. 5a). Total EDI range of breathing intake of air and dust PAHs for two age groups ranged from 695.1 to 1362.7 ng kg−1 day−1 during the study period. EDI for breathing intake of PAHs in air samples varied as children summer: 1317 ng kg−1 day−1, winter: 1362.7 ng kg−1 day−1 and adults summer: 695.1 ng kg−1 day−1, winter: 719.2 ng kg−1 day−1. Whereas, total EDI in dust samples were found to be children: 1234.5 ng kg−1 day−1, 1235.8 ng kg−1 day−1 and adults: 260 ng kg−1 day−1, 652.2 ng kg−1 day−1 during summer and winter seasons, respectively (Fig. 5b). The pattern for PAHs intake values through both oral and breathing exposure was higher in children than adults because PAHs can enter the body by breathing in the air contaminated with dust, cigarette smoke, wood, coal, or any other anthropogenic activity such as mining, oil refining, metallurgy, chemical manufacture, transportation, and the electrical sector. PAHs are inhaled through the lungs and are largely deposited in the kidneys, liver and fat. The present study's findings were consistent with previous research conducted in China and Pakistan10, 94.
Practical implications of this study
As a developing country, Pakistan is facing serious energy crises, and to generate required energy has adopted alternate inefficient fuels, which results in environmental deterioration, which substantially increases the PAHs emissions. Furthermore, the paradigm shift of natural gas vehicles to gasoline/diesel fuel engines has also substantially contributed to the outdoor concentration of PAHs10. Additionally, due to a shortage of natural gas (comparatively cleaner fuel), biomass combustion became the main source of household required energy, resulting in an upsurge of pyrogenic PAHs41, 72. As few past studies highlighted the significance of long-term strategies that intend to transition from allocating subsidies to unsustainable, environmentally-degrading fossil fuels to sustainably-produced renewable energy carriers10, 42, 95, 96. Therefore, The green technologies and dissemination of alternative fuels mainly biodiesel and solar power could be one of the environment-friendly alternatives and planning infrastructure, fuel quality, fuel subsidies, renewable energy industry, energy price change and abatement of industrial emissions will be highly essential to reduce the PAHs pollution and maintain the air quality to boost up the economy and achieve energy security of the city.
Conclusions and recommendations
The present study assessed sixteen US EPA priority listed PAHs in outdoor air and dust environments from ten selected areas of Lahore city, Pakistan. Results derived from the comparative analysis identified relative higher PAHs concentrations during the winter season in both air and dust matrices, with air being a more contaminated environmental compartment, which can be attributed to diesel combustion and heavy traffic. Naphthalene, Phenanthrene and Pyrene were the primary PAHs contributors to the air and dust PAHs in Lahore City. According to the particular isomer ratios, PAHs in the investigated region were largely produced by fuel combustion as well as petroleum emissions. Ingestion and dermal contact were the primary exposure routes for PAHs long term exposure. In comparison, inhalation was the least significant contributor to air and dust matrices. The estimated total ILCR from Σ16PAHs exposure signifies a high health risk to the exposed population. This research identifies the need for immediate actions of legislation to limit the semi-volatile organic compounds, particularly PAHs, in urban cities of the developing world and enhance environmental management and health conditions.
Overpopulation, rapid industrialization and urbanization have challenged the energy resources and resulted in a dramatic shift of non-environment friendly fuel choice, which has elevated the PAHs levels in the city. Therefore, the investigation of the gaseous PAHs in the atmosphere and dust of the second largest city of Pakistan suggests that green technologies should be introduced in the market to reduce the gap between energy need and supply and ensure public health. Furthermore, the government should formulate policies to minimize pollution load and improve air quality and associated health risks. Moreover, comprehensive research, including a wide range of environmental matrices with varying socioeconomic factors, is needed to determine the extent of chemical contamination in the world's worst air quality affected city, i.e. Lahore.
Data availability
All data generated or analyzed during this study are included in this article (and its supplementary information file).
Abbreviations
- PAHs:
-
Polycyclic aromatic hydrocarbons
- GS/MS:
-
Gas chromatography/mass spectrometry
- ILCR:
-
Incremental lifetime cancer risk
- HMW:
-
High molecular weight
- LMW:
-
Low molecular weight
- US EPA:
-
United States Environmental Protection Agency
- WHO:
-
World Health Organization
- PAS:
-
Passive air samplers
- EPD:
-
Environment Protection Department
- PUF:
-
Polyurethane foam
- DCM:
-
Dichloromethane
- AASHTO:
-
American Association of State Highway and Transportation Officials
- SIM:
-
Split injection mode
- TEF:
-
Toxic equivalency factor
- BaP-TEQ:
-
Benzo(a)pyrene toxicity equivalent
- BW:
-
Body weight
- CSF:
-
Carcinogenic slope factor
- EF:
-
Exposure frequency
- ED:
-
Exposure duration
- EDI:
-
Estimated daily intake
References
Abdel-Shafy, H. I. & Mansour, M. S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 25(1), 107–123 (2016).
Ali, N. et al. Polycyclic aromatic hydrocarbons (PAHs) in the settled dust of automobile workshops, health and carcinogenic risk evaluation. Sci. Total Environ. 601, 478–484 (2017).
Iwegbue, C. M. et al. Concentrations and health risk assessment of polycyclic aromatic hydrocarbons in soils of an urban environment in the Niger Delta, Nigeria. Toxicol. Environ. Health Sci. 8(3), 221–233. https://doi.org/10.1007/s13530-016-0279-8 (2016).
Wang, M., Jia, S., Lee, S. H., Chow, A. & Fang, M. Polycyclic aromatic hydrocarbons (PAHs) in indoor environments are still imposing carcinogenic risk. J. Hazardous Mater. 409, 124531 (2021).
Kamal, A., Malik, R. N., Martellini, T. & Cincinelli, A. Source, profile, and carcinogenic risk assessment for cohorts occupationally exposed to dust-bound PAHs in Lahore and Rawalpindi cities (Punjab Province, Pakistan). Environ. Sci. Pollut. Res. 22(14), 10580–10591 (2015).
Kastury, F., Smith, E. & Juhasz, A. L. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci. Total Environ. 574, 1054–1074. https://doi.org/10.1016/j.scitotenv.2016.09.056 (2017).
Yu, Y. et al. Novel in vitro method for measuring the mass fraction of bioaccessible atmospheric polycyclic aromatic hydrocarbons using simulated human lung fluids. Environ. Pollut. 242, 1633–1641. https://doi.org/10.1016/j.envpol.2018.07.114 (2018).
Hussain, K., Rahman, M., Prakash, A. & Hoque, R. R. Street dust bound PAHs, carbon and heavy metals in Guwahati City—Seasonality, toxicity and sources. Sustain. Cities Soc. 19, 17–25 (2015).
Kamal, A. et al. Profile of atmospheric PAHs in Rawalpindi, Lahore and Gujranwala districts of Punjab province (Pakistan). Aerosol Air Qual. Res. 16(4), 1010–1021 (2016).
Hamid, N. et al. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: Implications for changing energy demand. Sci. Total Environ. 619, 165–175 (2018).
Shi, Y., Bilal, M., Ho, H. C. & Omar, A. Urbanization and regional air pollution across South Asian developing countries—A nationwide land use regression for ambient PM2.5 assessment in Pakistan. Environ. Pollut. 266, 115145 (2020).
Ma, Y. & Harrad, S. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: A review. Environ. Int. 84, 7–16 (2015).
Suman, S., Sinha, A. & Tarafdar, A. Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Sci. Total Environ. 545, 353–360 (2016).
Yu, B., Xie, X., Ma, L. Q., Kan, H. & Zhou, Q. Source, distribution, and health risk assessment of polycyclic aromatic hydrocarbons in urban street dust from Tianjin, China. Environ. Sci. Pollut. Res. 21(4), 2817–2825 (2014).
Wang, X. T. et al. Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: Occurrence, source apportionment and potential human health risk. Sci. Total Environ. 447, 80–89 (2013).
Elbayoumi, M., Ramli, N. A. & Yusof, N. F. F. M. Spatial and temporal variations in particulate matter concentrations in twelve schools environment in urban and overpopulated camps landscape. Build. Environ. 90, 157–167 (2015).
Elbayoumi, M., Ramli, N. A., Yusof, N. F. F. M. & Madhoun, W. A. Seasonal variation in schools’ indoor air environments and health symptoms among students in an Eastern Mediterranean climate. Hum. Ecol. Risk Assess. Int. J. 21(1), 184–204 (2015).
Sun, K. et al. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 773, 145403 (2021).
Hopf, N. B. et al. Polycyclic aromatic hydrocarbons (PAHs) skin permeation rates change with simultaneous exposures to solar ultraviolet radiation (UV-S). Toxicol. Lett. 287, 122–130 (2018).
Yu, Y. et al. Risk of human exposure to polycyclic aromatic hydrocarbons: A case study in Beijing, China. Environ. Pollut. 205, 70–77 (2015).
Sangiorgi, G. et al. Indoor airborne particle sources and semi-volatile partitioning effect of outdoor fine PM in offices. Atmos. Environ. 65, 205–214 (2013).
Kamal, A., Qamar, K., Gulfraz, M., Anwar, M. A. & Malik, R. N. PAH exposure and oxidative stress indicators of human cohorts exposed to traffic pollution in Lahore city (Pakistan). Chemosphere 120, 59–67 (2015).
Dat, N. D. & Chang, M. B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 609, 682–693 (2017).
Gregoris, E. et al. Gas-particle distributions, sources and health effects of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs) in Venice aerosols. Sci. Total Environ. 476, 393–405 (2014).
Lai, Y. C., Tsai, C. H., Chen, Y. L. & Chang-Chien, G. P. Distribution and sources of atmospheric polycyclic aromatic hydrocarbons at an industrial region in Kaohsiung, Taiwan. Aerosol Air Qual. Res. 17, 776–787 (2017).
Wu, S. P., Yang, B. Y., Wang, X. H., Yuan, C. S. & Hong, H. S. Polycyclic aromatic hydrocarbons in the atmosphere of two subtropical cities in southeast china: seasonal variation and gas/particle partitioning. Aerosol Air Qual. Res. 14, 1232–1246 (2014).
Cao, H. et al. Urbanization-related changes in soil PAHs and potential health risks of emission sources in a township in Southern Jiangsu, China. Sci. Total Environ. 575, 692–700 (2017).
Lin, Y. C., Li, Y. C., Amesho, K. T., Chou, F. C. & Cheng, P. C. Characterization and quantification of PM2.5 emissions and PAHs concentration in PM2.5 from the exhausts of diesel vehicles with various accumulated mileages. Sci. Total Environ. 660, 188–198 (2019).
Feng, B. et al. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Beijing: Seasonal variations, sources, and risk assessment. J. Environ. Sci. 77, 11–19 (2019).
Yang, Y., Woodward, L. A., Li, Q. X. & Wang, J. Concentrations, source and risk assessment of polycyclic aromatic hydrocarbons in soils from Midway Atoll, North Pacific Ocean. PLoS ONE 9(1), e86441 (2014).
Azam, M. et al. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ. Int. 134, 105291 (2020).
El-Mubarak, A. H. et al. Polycyclic aromatic hydrocarbons and trace metals in mosque’s carpet dust of Riyadh, Saudi Arabia, and their health risk implications. Environ. Sci. Pollut. Res. 23(21), 21273–21287 (2016).
Gope, M., Masto, R. E., George, J., Hoque, R. R. & Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Safety. 138, 231–241 (2017).
Shin, H. M. et al. Determining source strength of semivolatile organic compounds using measured concentrations in indoor dust. Indoor Air 24(3), 260–271 (2014).
Tue, N. M. et al. Methylated and unsubstituted polycyclic aromatic hydrocarbons in street dust from Vietnam and India: Occurrence, distribution and in vitro toxicity evaluation. Environ. Pollut. 194, 272–280 (2014).
Jadoon, W. A. & Sakugawa, H. Concentrations of polycyclic aromatic hydrocarbons: Their potential health risks and sources at three non-urban sites in Japan. J. Environ. Sci. Health 51(11), 884–899 (2016).
Tarafdar, A. & Sinha, A. Health risk assessment and source study of PAHs from roadside soil dust of a heavy mining area in India. Arch. Environ. Occup. Health 74(5), 252–262 (2019).
Irfan, M. et al. Estimation and characterization of gaseous pollutant emissions from agricultural crop residue combustion in industrial and household sectors of Pakistan. Atmos. Environ. 84, 189–197 (2014).
Awan, A. B. & Khan, Z. A. Recent progress in renewable energy—Remedy of energy crisis in Pakistan. Renew. Sustain. Energy Rev. 33, 236–253 (2014).
Smith, D. J. T. et al. Concentrations of particulate airborne polycyclic aromatic hydrocarbons and metals collected in Lahore, Pakistan. Atmos. Environ. 30(23), 4031–4040 (1996).
Kamal, A., Cincinelli, A., Martellini, T., Malik, R. N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. 22(6), 4076–4098 (2015).
Ishtiaq, J. et al. Atmospheric polycyclic aromatic hydrocarbons (PAHs) at urban settings in Pakistan: Spatial variations, sources and health risks. Chemosphere 274, 129811 (2021).
Akhtar, M. M. & Tang, Z. Identification of contamination sources and TDS concentration in groundwater of second biggest city of Pakistan. Int. J. Environ. Sci. Development. 4(3), 341 (2013).
Jalees, M. I. et al. Hydrochemistry modelling: evaluation of groundwater quality deterioration due to anthropogenic activities in Lahore, Pakistan. Environ. Dev. Sustain. 23(3), 3062–3076 (2021).
Shirwani, R., Gulzar, S., Asim, M., Umair, M. & Al-Rashid, M. A. Control of vehicular emission using innovative energy solutions comprising of hydrogen for transportation sector in Pakistan: A case study of Lahore City. Int. J. Hydrogen Energy 45(32), 16287–16297 (2020).
Naz, S., Arooj, S., Ali, Z. & Farooq, Z. Potential consequences of captivity and environmental pollution in endoparasitic prevalence in different antelopes kept at wildlife parks. Environ. Sci. Pollut. Res. 28(13), 16308–16313 (2021).
Sarfaraz, S. A. R. D. A. R., Arsalan, M. H. & Fatima, H. I. R. A. Regionalizing the climate of Pakistan using Köppen classification system. Pakistan Geogr. Rev. 69, 111–132 (2014).
Wu, D. et al. Polycyclic aromatic hydrocarbons (PAHs) in atmospheric PM2.5 and PM10 at a coal-based industrial city: Implication for PAH control at industrial agglomeration regions, China. Atmos. Res. 149, 217–229 (2014).
Shoeib, M. & Harner, T. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 36(19), 4142–4151 (2002).
Harner, T., Shoeib, M., Diamond, M., Stern, G. & Rosenberg, B. Using passive air samplers to assess urban−rural trends for persistent organic pollutants. 1. Polychlorinated biphenyls and organochlorine pesticides. Environ. Sci. Technol. 38(17), 4474–4483 (2004).
Ali, N. Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population. Sci. Total Environ. 696, 133995 (2019).
Xu, L. Y. & Shu, X. Aggregate human health risk assessment from dust of daily life in the urban environment of Beijing. Risk Anal. 34(4), 670–682 (2014).
Yang, W., Lang, Y. H., Bai, J. & Li, Z. Y. Quantitative evaluation of carcinogenic and non-carcinogenic potential for PAHs in coastal wetland soils of China. Ecol. Eng. 74, 117–124 (2015).
Gope, M., Masto, R. E., George, J. & Balachandran, S. Exposure and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the street dust of Asansol City, India. Sustain. Cities Soc. 38, 616–626 (2018).
Najmeddin, A. & Keshavarzi, B. Health risk assessment and source apportionment of polycyclic aromatic hydrocarbons associated with PM10 and road deposited dust in Ahvaz metropolis of Iran. Environ. Geochem. Health 41(3), 1267–1290 (2019).
Li, J. L. et al. The source apportionment of polycyclic aromatic hydrocarbons (PAHs) in the topsoil in Xiaodian sewage irrigation area, North of China. Ecotoxicology 23(10), 1943–1950 (2014).
Li, Y., Zhang, L., Hou, W., Li, Y. & Li, X. Research on content, distribution and health risk assessment of PAHs in surface dust in Shenyang City. Nat. Environ. Pollut. Technol. 14(3), 721 (2015).
Hoseini, M. et al. Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric Particulate of Tehran, Iran. Environ. Sci. Pollut. Res. 23(2), 1820–1832 (2016).
EPA U. Provisional Guidance for Quantitative Risk Assessment of PAH. EPA/600/R-93/089 (US Environmental Protection Agency, 1993).
Nisbet, I. C. & Lagoy, P. K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 16(3), 290–300 (1992).
Franco, C. F. J. et al. Polycyclic aromatic hydrocarbons (PAHs) in street dust of Rio de Janeiro and Niterói, Brazil: Particle size distribution, sources and cancer risk assessment. Sci. Total Environ. 599, 305–313 (2017).
National Research Council. Sustainability and the US EPA (National Academies Press, 2011).
Peng, Y. & Gitelson, A. A. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agric. For. Meteorol. 151(9), 1267–1276 (2011).
US Environmental Protection Agency (EPA). Guidelines for Developmental Toxicity Risk Assessment. (EPA/600/FR‐91/001). (1997).
Jiang, Y., Hu, X., Yves, U. J., Zhan, H. & Wu, Y. Status, source and health risk assessment of polycyclic aromatic hydrocarbons in street dust of an industrial city, NW China. Ecotoxicol. Environ. Safety. 106, 11–18 (2014).
Ollson, C. A., Knopper, L. D., Aslund, M. L. W. & Jayasinghe, R. Site specific risk assessment of an energy-from-waste thermal treatment facility in Durham Region, Ontario, Canada. Part A: Human health risk assessment. Sci. Total Environ. 466, 345–356 (2014).
Li, Y., Song, N., Yu, Y., Yang, Z. & Shen, Z. Characteristics of PAHs in street dust of Beijing and the annual wash-off load using an improved load calculation method. Sci. Total Environ. 581, 328–336 (2017).
Chen, P. et al. Characteristics and sources of polycyclic aromatic hydrocarbons in atmospheric aerosols in the Kathmandu Valley, Nepal. Sci. Total Environ. 538, 86–92 (2015).
Liu, D. et al. Concentrations, seasonal variations, and outflow of atmospheric polycyclic aromatic hydrocarbons (PAHs) at Ningbo site, Eastern China. Atmos. Pollut. Res. 5(2), 203–209 (2014).
He, K. et al. Characteristics of indoor and personal exposure to particulate organic compounds emitted from domestic solid fuel combustion in rural areas of northwest China. Atmos. Res. 248, 105181 (2021).
Teil, M. J. et al. Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors. Chemosphere 146, 94–104 (2016).
Kamal, A., Malik, R. N., Martellini, T. & Cincinelli, A. Cancer risk evaluation of brick kiln workers exposed to dust bound PAHs in Punjab province (Pakistan). Sci. Total Environ. 493, 562–570 (2014).
Qishlaqi, A. & Beiramali, F. Potential sources and health risk assessment of polycyclic aromatic hydrocarbons in street dusts of Karaj urban area, northern Iran. J. Environ. Health Sci. Eng. 17(2), 1029–1044 (2019).
Nguyen, T. C. et al. Polycyclic aromatic hydrocarbons in road-deposited sediments, water sediments, and soils in Sydney, Australia: Comparisons of concentration distribution, sources and potential toxicity. Ecotoxicol. Environ. Saf. 104, 339–348 (2014).
Najmeddin, A., Moore, F., Keshavarzi, B. & Sadegh, Z. Pollution, source apportionment and health risk of potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in urban street dust of Mashhad, the second largest city of Iran. J. Geochem. Explor. 190, 154–169 (2018).
Kwon, H. O. & Choi, S. D. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci. Total Environ. 470, 1494–1501 (2014).
Cetin, B. Investigation of PAHs, PCBs and PCNs in soils around a heavily industrialized area in Kocaeli, Turkey: Concentrations, distributions, sources and toxicological effects. Sci. Total Environ. 560, 160–169 (2016).
Ashfaq, M. et al. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 688, 653–663 (2019).
Qi, H. et al. Concentrations and sources of polycyclic aromatic hydrocarbons in indoor dust in China. Sci. Total Environ. 491, 100–107 (2014).
Maragkidou, A. et al. Occupational health risk assessment and exposure to floor dust PAHs inside an educational building. Sci. Total Environ. 579, 1050–1056 (2017).
Dat, N. D., Lyu, J. M. & Chang, M. B. Variation of atmospheric PAHs in northern Taiwan during winter and summer seasons. Aerosol Air Qual. Res. 18(4), 1019–1031 (2018).
Bandowe, B. A. M. et al. PM2.5-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: Seasonal variation, sources and cancer risk assessment. Sci. Total Environ. 473, 77–87 (2014).
Alves, C. A. et al. Polycyclic aromatic hydrocarbons and their derivatives (nitro-PAHs, oxygenated PAHs, and azaarenes) in PM2.5 from Southern European cities. Sci. Total Environ. 595, 494–504 (2017).
He, J. et al. Polycyclic aromatic hydrocarbons (PAHs) associated with fine particulate matters in Nanjing, China: Distributions, sources and meteorological influences. Atmos. Environ. 89, 207–215 (2014).
Jiang, Y., Zhang, Z. & Zhang, X. Co-biodegradation of pyrene and other PAHs by the bacterium Acinetobacter johnsonii. Ecotoxicol. Environ. Saf. 163, 465–470 (2018).
Yunker, M. B. et al. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 33(4), 489–515 (2002).
Vandenplas, O. & Suojalehto, H. Manuscript cited in “Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement”. Eur. Respir. J. 44(4), 1100–1100 (2014).
Hu, J., Bao, Y., Huang, H., Zhang, Z., Chen, F., Li, L. & Wu, Q. The preliminary investigation of potential response biomarkers to PAHs exposure on childhood asthma. J. Exposure Sci. Environ. Epidemiol. 1–12 (2021).
Singh, A. et al. Association between PAHs biomarkers and kidney injury biomarkers among kitchen workers with microalbuminuria: A cross-sectional pilot study. Clin. Chim. Acta 487, 349–356 (2018).
Yang, L. et al. Heart rate variability mediates the association between polycyclic aromatic hydrocarbons exposure and atherosclerotic cardiovascular disease risk in coke oven workers. Chemosphere 228, 166–173 (2019).
Alshaarawy, O., Zhu, M., Ducatman, A. M., Conway, B. & Andrew, M. E. Urinary polycyclic aromatic hydrocarbon biomarkers and diabetes mellitus. Occup. Environ. Med. 71(6), 437–441 (2014).
Kuang, D. et al. Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ. Sci. Technol. 47(13), 7446–7456 (2013).
Hou, J. et al. Combined effect of urinary monohydroxylated polycyclic aromatic hydrocarbons and impaired lung function on diabetes. Environ. Res. 148, 467–474 (2016).
Soltani, N. et al. Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Sci. Total Environ. 505, 712–723 (2015).
Fazal, R., Rehman, S. A. U., Rehman, A. U., Bhatti, M. I. & Hussain, A. Energy–environment–economy causal nexus in Pakistan: A graph theoretic approach. Energy 214, 118934 (2021).
Khatibi, S. R., Karimi, S. M., Moradi-Lakeh, M., Kermani, M. & Motevalian, S. A. Fossil energy price and outdoor air pollution: Predictions from a QUAIDS model. Biofuel Res. J. 7(3), 1205–1216 (2020).
Author information
Authors and Affiliations
Contributions
R.A. has conducted the field surveys for data collection as well as have written the document. F.S. has provided the main idea of study and supervise whole practical work. M.B. has conducted the field surveys for data collection along with R.A. L.S. has proof read the manuscript and overall improve the grammar and comprehension.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aslam, R., Sharif, F., Baqar, M. et al. Source identification and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in air and dust samples of Lahore City. Sci Rep 12, 2459 (2022). https://doi.org/10.1038/s41598-022-06437-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06437-8
This article is cited by
-
Characterization, source identification and risk assessment of POPs (PAHs, PCBs, and OCPs) in indoor dusts from two metropolitan cities (Istanbul and Kocaeli) of Türkiye with intensive industrial and human activities
Stochastic Environmental Research and Risk Assessment (2024)
-
Characteristics, source analysis, and health risk of PM2.5 in the urban tunnel environment associated with E10 petrol usage
Environmental Science and Pollution Research (2024)
-
Polycyclic Aromatic Hydrocarbons in Air and Dust Samples from Vietnamese End-of-life Vehicle Processing Workshops: Contamination Status, Sources, and Exposure Risks
Bulletin of Environmental Contamination and Toxicology (2023)
-
Compositional and seasonal differences of gas and particle phase polycyclic aromatic hydrocarbons (PAHs) over the southern Baltic Sea coast
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.