Abstract
In 2015, we measured polycyclic aromatic hydrocarbons (PAHs) in atmospheric fine particulate matter (PM2.5) collected from 5 cities in Zhejiang Province. The mean toxic equivalent quotient (TEQ) values of benzo(a)pyrene (BaP) ranged between 1.2–3.1 ng/m3. The BaP-TEQ displayed seasonal trends, such that winter > spring and autumn > summer. During the winter, the most abundant individual PAHs were 4–6ring PAHs (84.04–91.65%). The median daily intake of atmospheric PAHs ranged between 2.0–7.4 ng/day for all populations, with seasonal trends identical to that of BaP-TEQ. The 95th incremental lifetime cancer risk (ILCR) values induced by PM2.5-bound PAHs were far lower than 10−6 for all populations. The data suggested that the pollution levels in the 5 Zhejiang Province cities were higher than the Chinese National Ambient Air Quality Standard (NAAQS). In the future, relevant measures should be taken to control atmospheric PAHs, especially 4–6 ring PAHs. The data also revealed no obvious cancer risk for populations residing in these 5 cities of Zhejiang Province.
Similar content being viewed by others
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large group of ubiquitous environmental poisons mainly sourced from the pyrolysis of organic matter or incomplete combustion1. Motor vehicles, home heating, fossil fuel combustion, and industrial processes are major sources of atmospheric PAHs2. After various combustion processes, gas phases or particulate-bound PAHs are emitted into the atmosphere3. The atmosphere is the most important means of PAHs dispersal4. Due to their widespread source and persistent characteristics, PAHs are dispersed through atmospheric transport and result in being ubiquitous in the environment5. Human beings are exposed to gas phases or particulate-bound PAHs in ambient air. Atmospheric PAHs adsorbed on fine particulate matter (PM2.5) enter into the human body through the respiratory system6. Several studies reported that 59–97% of atmospheric PAHs were adsorbed on PM2.57,8,9,10. Therefore, recent studies have used atmospheric PAHs in PM2.5 to determine respiratory exposure concentrations11,12,13,14.
The U.S. Environmental Protection Agency has listed 16 PAHs as priority pollutants to regard to potential exposure and adverse health effects on humans15. Some PAHs are classified as carcinogenic materials to humans by the International Agency for Research on Cancer16. According to these, Benzo[a]pyrene was classified as carcinogenic material (Group 1), naphthalene, chrysene, benz[a]anthracene, benzo[k]fluoranthene and benzo[b]fluoranthene were classified as probably carcinogenic materials (Group 2B)16. Therefore, PAHs pollution poses a substantial concern.
China generates the highest PAHs emission17, at a total of 120,611 tons as of 20122. There is a close correlation between human lung cancer and inhaled PAHs18. In recent years, lung cancer has become the 4th leading cause of death in the Chinese population due to severe air pollution19. Three methods were applied for the carcinogenic risk assessment of PAHs: toxic equivalency factors (TEFs), the comparative potency of mixtures and the use of Benzo[a]pyrene as a surrogate20,21. A UK study found that the TEFs approach is more preferable than other two approaches for the estimate the carcinogenic risk associated with exposure to atmospheric PAHs21. Thus, we used TEFs method to assess the inhalation risk of PAHs to human health in our study.
Zhejiang Province is located in the Yangtze River Delta (YRD) region, which is considered one of the most rapidly developing regions of China. Severe atmospheric PAHs pollution has been detected in some regions of Zhejiang Province22,23, as well as in many Chinese cities experiencing urbanization and industrialization. To date, several cities in China have conducted health risk assessments of atmospheric PAHs11,13,14,18,24,25,26. However, most researchers focused on the risk of atmospheric PAHs by using a 24-hour exposure time, which could result in overestimations due to significant differences in outdoor and indoor PAHs concentrations22. Hence, we carried out an investigation to obtain an accurate exposure time. Research on the human health risk of PAHs via inhalation exposure is limited, regarding the Zhejiang Province.
The objectives of this study are to measure the concentration and distribution of atmospheric PAHs in PM2.5, to assess the daily intake of atmospheric PAHs in PM2.5 for different population groups, and to evaluate the lifetime carcinogenic risk of atmospheric PAHs in PM2.5 for different populations in Zhejiang Province.
Results and Discussion
Atmospheric PAHs in PM2.5
The concentrations of PM2.5
The concentrations of atmospheric PM2.5 in Hangzhou(HZ), Ningbo(NB), Jinhua (JH), Lishui (LS), and Zhoushan (ZS) were 79.4 ± 40.1, 63.0 ± 35.9, 61.7 ± 32.4, 49.0 ± 24.4, and 41.9 ± 31.6 µg/m3, respectively (Table S1). In all 5 cities, the concentration of PM2.5 exceeded the Chinese National Ambient Air Quality Standard (NAAQS) annual average PM2.5 value of 35 μg/m3 (GB 3095–2012)27.
The concentrations of Σ16 PAHs
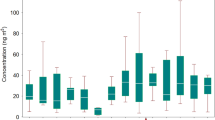
The concentrations of the sum of 16 atmospheric PAHs (Σ16 PAHs) in PM2.5 for the 5 selected cities in 2015 are shown in Table S1, Table S2, and Fig. 1. The concentrations of Σ16 PAHs in JH, HZ, LS, NB and ZS were 18.3 ± 16.0, 17.8 ± 15.8,16.9 ± 15.2, 13.5 ± 10.0, and 7.5 ± 4.0 ng/m3, respectively. For each city selected, the changing trends of Σ16 PAHs concentrations were similarly distributed by season, with a 1.7–5.0 times higher value in winter than summer. During the winter, the most abundant individual PAHs were 4–6 ring PAHs (84.0–91.7%). This result is in agreement with studies conducted in Taiyuan13 and other urban regions14,28. The potential reasons may be related with the more amount of incomplete combustion emissions from fossil fuel and other organic mass due to the residential heating, and lower levels of photochemical degradation in winter, as well as poor atmospheric diffusion in a certain meteorological condition with calm winds and temperature inversion during cold season29,30. Moreover, there is a smaller proportion of low molecular weight PAHs (2–3 ring) compared with 4–6 ring PAHs because there is a higher level of volatilization into the gaseous phase.
Concentration and TEQ of atmospheric PAHs in PM2.5 for different seasons by city: (a) Total of 16 PAH concentrations, (b) Total of 16 PAHs TEQ. The data are presented as the mean ± standard deviation. The figure was generated by R 64 3.3.1 with function plot: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org).
In winter, the concentrations of Σ16 PAHs in HZ, JH, and LS were 1.3–1.4 times higher than in NB, and 3.3–3.4 times higher than in ZS. Compared with the 1-year Σ16PAH concentrations in other areas of China, HZ, JH, and LS were similar to those in the Shenzhen suburbs (19.0 ng/m3 in PM2.5) of South China14, where our study area was also located. The Σ16 PAH concentrations in the 5 selected cities were 0.1–0.3 times that found in rural and urban Taiyuan (57.5–73.6 ng/m3 in PM2.5)13, and 0.01–0.8 times that observed in urban Tianjin in North China (23.4–513 ng/m3 in PM10)29. The latter location is a highly contaminated region due to a combination of high coal consumption and dense heavy traffic13,29. The decreasing trend of Σ16 PAH concentrations from North to South in China indicated spatial variation of the pollutants. This may be due to the different intensities of pollutant emission, geographical location, and adverse weather conditions31.
Potential Sources of PAHs
In our study, the ratio Fle/Pyr, BaP/BghiP, and BaP/(BaP + Chr) ranged from 0.75(LS) to 1.12(JH), 0.61(ZS) to 0.95(LS), and 0.47(NB) to 0.54(LS), respectively (Table S3). The ratios indicated that gasoline emissions may be the main source of PAHs in HZ, NB, and ZS, while the ratios in JH and LS suggested that gasoline emission and coal combustion both contributed.
Toxic equivalency quotient
The toxic equivalent quotient (TEQ) of the 5 selected cities is shown in Table S1 and Fig. 1. The TEQ in HZ, JH, LS, NB, and ZS were 2.8 ± 2.8, 3.1 ± 2.6, 2.8 ± 2.2, 2.1 ± 1.5, and 1.2 ± 0.7 ng/m3, respectively. Compared to the Chinese NAAQS (GB 3095-2012) annual average TEQ value for PAHs (1.0 ng/m3), the TEQs observed in HZ, JH, LS, NB, and ZS exceeded the standard. This finding was in accordance with the results of the PM2.5 concentrations. Thus, Σ16 PAHs may have negative effects in these cities.
During the study period, the TEQ displayed similar seasonal variation trends in each city. According to the seasonal distribution, the TEQ was listed, in descending order, as winter, spring and autumn, and summer. Coincidently, during the same study period, the seasonal trends of Σ16 PAH concentrations in each city were similar to those of the TEQ, due to a large proportion of 5–6 ring PAHs with high toxic equivalency factors (TEFs).
Compared to the 1-year TEQ in other areas of China, the TEQ in HZ, JH,LS, and NB were similar to those reported in China overall (2.4 ng/m3 based on high-resolution emission inventory)24 and in suburban Shenzhen (2.4 ng/m3 in PM2.5) in South China14, where our study area was also located. In the 5 studied cities, the TEQ is significantly lower than that reported in rural and urban Taiyuan (23.2 ± 30.8 and 27.4 ± 28.1 ng/m3 in PM2.5)13 and urban Tianjin (25.8 ng/m3 in PM10)29 in North China. The latter location is a highly contaminated region due to the spatial variation of pollutants, which is analysed as the Σ16 PAH concentrations. On the other hand, higher molecular weight PAHs with larger TEFs may be associated with vehicle emissions29, which suggests that vehicle combustion in North China may result in higher TEQs than observed in South China.
Overall, the 5 studied cities were only slightly polluted by PAHs when compared with other areas in China. However, the TEQs were all higher than the Chinese NAAQS. In the future, appropriate measures should be taken to control atmospheric PAHs, especially 4–6 ring PAHs, in Zhejiang.
Daily intake exposure to atmospheric PAHs
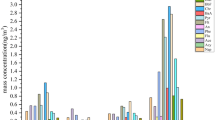
The cumulative probability distributions of the daily intake (DI) of PM2.5-bound Σ16PAHs for different population groups, over 4 seasons, in the 5 studied cities are shown in Table 1 and Figs 2, S1–S3. The median DI for adults in JH, LS, HZ, ZS, and NB were 7.4, 5.6, 4.4, 4.2, and 2.8 ng/day, which was basically in line with the ranking of mean of TEQ (Table S1), for children in JH, NB, LS, HZ, ZS as the decreasing order were 6.6, 3.5, 2.5, 2.3 and 2.0 ng/day, which was completely in line with the ranking of mean of exposure time (Table S4).
Probability distribution of daily intake exposure to atmospheric PAHs by inhalation from PM2.5 for different population groups in 5 selected cities of Zhejiang Province: (a) Adults, (b) Children. The figure was generated by R 64 3.3.1 with function plot: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org).
In the spring, the median DI was estimated to be 2.5–7.9 ng/day for adults and 1.7–7.0 ng/day for children. During summer, the median DI was approximately 1.8–3.8 ng/day for adults and 1.1–3.2 ng/day for children. In autumn, the median DI was 2.3–5.7 ng/day for adults and 1.7–5.2 ng/day for children. During winter, the median DI was 5.2–15.5 ng/day for adults and 3.2–13.9 ng/day for children (Table 1). According to the seasonal distribution, the DI was listed, in descending order, as winter, spring and autumn, and summer for both adults and children (Table 1 and Figs S1, S2). This result was in accordance with the TEQ ranking due to the large contribution of TEQ to DI when the constant effects of inhalation rate and exposure time are considered for different seasons.
According to the population, adults have higher DI levels than children (Table 1 and Fig. S3) owing to the fact that inhalation rate ratios were higher than exposure time ratios between children and adults (Table S4). However, NB is the exception to this observation, with an inhalation rate ratio (1.8) that was less than the exposure time ratio (2.5) between children and adults (Tables 1, S4 and Fig. S3). The result of the former was in line with that reported in Taiyuan13, while the latter was agreed with that reported in Shenzhen14. Children in HZ had an inhalation rate ratio (1.02) that was slightly less than the exposure time ratio (1.03). Males showed a slightly higher exposure dose than females in all cities for both adults and children because the male inhalation rates and exposure times were higher than females in all adults and in children from JH, LS, and ZS (Tables 1, S4 and Fig. S3). Although the exposure time of male children in NB was less than that of female children, the inhalation exposure of the male children was higher (Table S4), resulting in a higher DI in male children (Table 1 and Fig. S3). The result of the former was in line with that reported in Shenzhen14, while the latter was in line with that reported in Taiyuan13.
However, our results were far lower than that in previous reports conducted in Taiyuan (outdoors for adolescents, adults, and seniors: 104–307 ng/day)13, Tianjin (outdoors for children:321.6 ng/day; adults: 519 ng/day)11, Taiwan (rural, industrial, and high traffic for infants: 252 ng/day; children: 1590 ng/day; adults:1628 ng/day)18. The main reason for this difference could be that those reports assume that the daily exposure time for the population was 1 day, whereas most people may not remain outdoors for an entire day. The exposure time in the present study was 1.9–7.9 hours (approximately 0.1–0.3 of 1 day), which was calculated based on population surveys. The difference between the DI of PAHs given the real exposure time and 1-day exposure time was shown in Table S5. The TEQ values in the present study were also lower than those previously reported (except Shenzhen)14.
Incremental lifetime cancer risk
Under most regulatory programs, an incremental lifetime cancer risk (ILCR) less than 10−6 indicates acceptable risk, between 10−6–10−4 designates a potential risk, and greater than 10−4 suggests a public health concern13,32. The cumulative probability distributions of the ILCR induced by atmospheric PAHs are shown in Table 2 and Figs 3 and S4.
Cumulative probability distribution of incremental lifetime cancer risk induced by atmospheric PAHs via inhalation from PM2.5 for different population groups in 5 selected cities of Zhejiang Province: (a) Adults, (b) Children. The figure was generated by R 64 3.3.1 with function plot: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org).
For adults, the 95th ILCR induced by PM2.5-bound Σ16 PAHs in JH, LS, HZ, ZS, and NB were 4.8 × 10−7, 3.5 × 10−7, 3.4 × 10−7, 1.9 × 10−7, and 1.8 × 10−7 (Table 2 and Fig. 3), which was in line with the ranking of DI, for children in JH, NB, HZ, LS, ZS as the decreasing order were 1.5 × 10−7, 5.9 × 10−8, 4.5 × 10−8, 4.3 × 10−8 and 2.6 × 10−8 (Table 2 and Fig. 3), which was also basically in line with the ranking of DI.
According to the population, the 95th ILCR were estimated to be 2.1 × 10−7–5.4 × 10−7 for adult males, 1.4 × 10−7–4.3 × 10−7 for adult females, 2.7 × 10−8–1.5 × 10−7 for male children, and 2.4 × 10−8–1.3 × 10−7 for female children. The ILCR, in descending order, was listed as adult males, adult females, male children, and female children (Table 2 and Fig. 4). These results may be attributed to the higher level of DI exposure to atmospheric PAHs in the adult and male populations, as well as the lower exposure duration in children despite their lower body weights (Table S4). This result was in accordance with a study conducted in Shenzhen14.
Sensitivity analysis results for the incremental lifetime cancer risk assessment in 5 cities of Zhejiang Province: (a) Adults, (b) Children. The figure was generated by R 64 3.3.1 with function plot: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org).
Similar to the DI results, the ILCR values were far lower than the mean determined by previous reports in Taiyuan (children: 1.7 × 10−6, adults: 8.0 × 10−6)13, Taiwan (children: 6.5 × 10−6, adults: 1.0 × 10−4)18, and Tianjin (2.7 × 10−4)11. Though the TEQ in our study was similar to that in Shenzhen, the ILCR values were 0.01–0.3 times the level in Shenzhen (2.0 × 10−7–1.3 × 10−6)14. This is mainly due to overexposure in the daily outdoor exposure time, which is analysed in the DI exposure to atmospheric PAHs section. Those values were far higher than that in Guangzhou (children: 2.2 × 10−11, adults: 6.7 × 10−11)33.
Overall, the ILCR values were all less than 10−6 for adult males, adult females, male children, and female children. These results suggested that no obvious cancer risk existed for populations living in the 5 selected cities of Zhejiang Province.
Sensitivity and uncertain analysis
The results of the sensitivity analysis on the ILCR for different population groups in the 5 selected cities are shown in Table S6 and Fig. 4. For the adult population, the partial correlation coefficient with ILCR was listed, in descending order, as TEQ (0.8–0.9), exposure time (ET) (0.7–0.9), and cancer slope factor (CSF) (0.5–0.8). For the child population, the partial correlation coefficient with ILCR was listed, in descending order, as TEQ (0.8–0.9), ET (0.5–0.8), CSF (0.5–0.8), and body weight (BW) (−0.7 – −0.4). Based on the sensitivity results, TEQ and ET are the 2 most influential variables in the health risk assessments of both children and adults. Therefore, improving the accuracy of ET and TEQ could enhance the accuracy of health risk assessments.
Based on the modelling of the distribution parameter uncertainty results, Table S7 and Fig. 5 show the results of the 95% confidence interval with an ILCR of 100 values. We can see that the uncertainty in the “max” parameter has a small effect on the risk distribution for this scenario due to the narrower range of the 95% confidence interval for the 95th ILCR (Table S7 and Fig. 5). These data indicated that we could neglect the effect from the missing max values of CSF.
Uncertainty analysis results for the incremental lifetime cancer risk assessment of the cancer slope factor in 5 cities of Zhejiang Province: (a) Adults, (b) Children. The figure was generated by R 64 3.3.1 with function plot: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org).
Uncertainty and limitation of health risk assessments
In this study, only 1 sampling site was set up in each city’s traditional centre district. The results of the health risk assessment would be more representative, if more sampling sites were built in different regions, such as industrial or high traffic regions.
Although the atmospheric PAH concentrations in PM2.5 were detected for 7 days/month in this study, the day-to-day variations in the concentration of atmospheric PAHs in PM2.5 could lead to some uncertainties due to meteorological conditions such as temperature, wind speed, and humidity34.
Moreover, the mean time spent outdoors was investigated as 2.1 ± 1.7 to 5.9 ± 2.6 hours/day and 2.9 ± 0.7 to 7.0 ± 4.1 hours/day for adults and children, respectively. Hence, people likely spend most of their time indoors. In general, the concentration of atmospheric PAHs in PM2.5 were very different between outdoors and in doors22. In our study, only outdoor contaminants were used to calculate the exposure dose and ILCR; indoor contaminants were not taken into consideration. Furthermore, atmospheric PAHs in the gaseous phase were not considered in our study. These limitations would lead to a possible bias that partially underestimates the exposure dose and ILCR. Although there are some uncertainties and limitations, this study still provides a valuable evaluation of health risks associated with exposure to atmospheric PAHs in PM2.5.
Conclusions
Our data suggested that the pollution levels, in the 5 selected cities of Zhejiang Province, were higher than the Chinese NAAQS. In the future, appropriate measures should be taken to control atmospheric PAHs, especially 4–6 ring PAHs. The pollution level and inhalation exposure of atmospheric PAHs in PM2.5 were listed, in descending order, as winter, spring and autumn, and summer. The ILCR values were far lower than 10−6, which suggested no obvious cancer risk for populations residing in the 5 selected cities of Zhejiang Province. Due to the aforementioned limitations, future studies should consider indoor pollutant data and sample more sites in different regions.
Materials and Methods
Site description
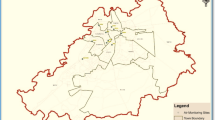
This study was conducted in Zhejiang Province (between 118°E–123°E and 27.12°N –31.31°N), an eastern coastal region of China, which covers 11cities. The province features a subtropical climate with 4 distinct seasons. In this study, cluster sampling was applied to select 5 traditional urban centres with populations greater than 10,000 in Zhejiang Province (Hangzhou (HZ), Jinhua (JH), Lishui (LS), Ningbo (NB), and Zhoushan (ZS)). One PM2.5 sampling site was set up in each selected community centre (Fig. 6). Detailed information on sampling sites is in Table S8.
Location of the survey areas in Zhejiang Province, China: (a) The Zhejiang province in China; (b) The sampling sites in each city: the red point is sampling site of each city; the blue point is major industrial area of each city; the arrow is dominant winds trajectories of each season. The figure was generated by R 64 3.3.1: A Language and Environment for Statistical Computing, author: R Core Team, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org), with Package ggplot2 version 2.2.1 (https://cran.r-project.org/web/packages/ggplot2/index.html).
HZ is the capital of Zhejiang Province and is a famous tourist city with more than 2.6 million vehicles. NB is the world’s 4th largest port city and is an important chemical industry base in Zhejiang Province. ZS is an island city, which is the largest seafood production, processing, and marketing base in China. JH is located in the middle of Zhejiang Province and is known for the manufacture of metal products and medicine. LS is mainly in mountainous and hilly terrain. In winter, electricity and natural gas was the main heating situations in these 5 selected cities.
Sampling
A total of 485 samples were collected for 23 h (starting at 9:00 a.m. local time each day and ending at 8:00 a.m. the next day) from the 10th to 16thbetween January and December 2015. The sampling sites were set up on rooftops in each traditional centre district; the sites were established 12–15 m above ground to avoid airflow obstacles. Atmospheric PM2.5 samples were collected on 47-mm glass fibre filters (QZ47DMCAN, MTL, America) using a mid-volume sampler (MVS6, LECKEL, Germany) at a constant flow rate of 38.3 L/min. The samples were weighed by an automated filter weighing system (WZZ-02, Weizhizhao, Hangzhou, China) before and after sampling to determine the mass of PM2.5. All the filters were transported immediately to the laboratory in a filter holder and stored at −20 °C until analysis. Meteorological parameters, such as temperature and wind speed, were also recorded at the time of sample collection. Detailed information on the temperature and wind speed is in Table S8.
Extraction and analysis of PAHs
The filter membrane sample was cut into pieces and placed into a 15-mL centrifuge tube. Meanwhile, 0.2 μg/mL decafluorobiphenyl was added as internal standard. Ultrasonic extraction was applied for 10 min, and the sample was centrifuged at a speed of 8000 r/min for 5 min. Finally, the extracts were filtered prior to separation with a 0.45 μm membrane (Agilent) and stored in a refrigerator until analysis3.
PAHs, including naphthalene (Nap), acenaphthylene (Acy), fluorene (Flu), acenaphthene (Ace), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fle), pyrene (Pyr), chrysene (Chr), benz(a)anthracene (BaA), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DBA), benzo(g,h,i)perylene (BghiP), and indeno(1,2,3-cd)pyrene (InP), were determined using high performance liquid chromatography (HPLC) with fluorescence and UV detectors (Agilent 1260). A 4.60 mm × 250 mm, 3.5 μm C18 reversed-phase column (Agilent) was used; the column temperature was 35 °C and the mobile phase flow rate was 0.8 mL/min. The gradient elution program was the following: 72% acetonitrile and 28% water were added and held for 27 min; 100% acetonitrile was added at a rate of 2.5% acetonitrile/min until the peak was achieved. The HPLC system was calibrated using an external standard. A standard reference material (O2Si, standard PAH mixture) soluble in acetonitrile was used at different dilutions to obtain calibration curves for each run. The concentration range of the calibration standards were 0, 0.05, 0.1, 0.5, 1.0, and 5.0 μg/mL. A good agreement existed between standard and sample chromatograms obtained on a given day. Since the fluorescence detector was unable to detect all 16 PAHs, we used fluorescence and UV detectors to determine the PAHs simultaneously3. The detection wavelengths of fluorescence and UV detectors were set at the values given in Table S9. QA/QC included reagent blanks, analytical duplicates, and analysis of the standard reference material (O2Si, standard PAH mixture). The detection limits of PAHs corresponding to the fluorescence and UV detectors are shown in Table S10. The relative standard deviation was less than 10%. The PAHs recovery percentage of 16 PAHs from the standard material was between 65% and 96% (Table S10). The method we used to determine the PAHs was based on the Chinese National Ambient Air Quality Standard (NAAQS)35.
Population survey
Exposure time in outdoor environments is an important parameter for health risk assessment. Approximately 1000 adults (>18 years old) and 600 school children (9–11 years old) were randomly selected from each community to conduct an exposure time investigation by questionnaire. Exposure time showed significant regional and individual differences.
In the study, systematic sampling was applied to select households from each community based on the household size. All family members greater than 18 years old were recruited. One primary school from each community was selected by the cluster sampling method. In each school, children aged 9 to 11 years were randomly selected as subjects. Subjects were introduced to the study protocol.
The first part of the questionnaire involved demographic characteristics, including age, gender, and occupation. The second part of the questionnaire was divided into 2 periods: rest and working days, including indoor and outdoor time at home, work (for adults), school (for children), travel, and other activities. Indoor and outdoor time at work was not included in the rest days of adults. The time in the questionnaires was accurate to the minute, and the investigation was carried out by well-trained public health doctors. Eventually, the average daily outdoor exposure time was obtained. Detailed information on the exposure time questionnaire is in the supplementary information. The weight measurement was performed using the World Health Organization’s (WHO) standard methods36, which require the child to take off their shoes, belt, and wear light clothing. Weight measurements were precise to 0.1 kg. Detailed results for exposure time and weight are in Table S4.
Data analysis
Toxic equivalency quotient
The carcinogenic effect of mixtures of atmospheric PAHs was expressed as the TEQ of BaP. The TEQ of atmospheric PAHs in PM2.5 were calculated according to formula (1):
where Ci = concentration of PAHs congener i (pg/m3); TEFi = Toxic equivalency Factor of PAHs congener i37 (Table S1).
Daily intake estimates
The DI of atmospheric PAHs in PM2.5 for different populations during 4 seasons was calculated according to formula (2):
where TEQ = the toxic equivalent quotient of BaP (pg/m3); IR = Inhalation rate (m3/day); ET = Exposure times (hours/day); unit of 24 is hours/day (Table S4). The cumulative probability of DI is calculated by the Monte Carlo simulation method running 10,000 iterations in which TEQ obeys lognormal distribution and ET obeys normal distribution (R 3.3.1 software with EnvStats packages version 2.1.0).
Incremental lifetime cancer risk
ILCR was calculated according to formula (3):
where TEQ = the toxic equivalent quotient of BaP (pg/m3); IR = Inhalation rate(m3/d); CF = Conversion factor (mg/pg); ED = Exposure duration (years); ET = Exposure times (hours/day); EF = Exposure frequency (days/year); CSF = Cancer slope factor (mg/kg-day)−1, which was regarded as 3.14(1.80) (Geometric Mean (Geometric Standard Deviation))18; BW = Body weight (kg); AT = Averaging time (hours). Detailed information is presented in Table S4. The cumulative probability of ILCR is calculated by the Monte Carlo simulation method running 10,000 iterations in which TEQ obeys lognormal distribution, ET obeys normal distribution, CSF obeys lognormal distribution, and BW (for children) obeys normal distribution (R 3.3.1 software with EnvStats packages vision 2.1.0).
Potential Sources of PAHs
Atmosphere PAHs are mainly derived from incomplete combustion of fuels such as gasoline, diesel and coal. Due to different fuel type and combustion conditions, the amount and range of PAHs produced from any pyrolytic process varies widely38. Therefore, the potential source of PAHs could be determined based on the characteristic ratio of PAHs. The ratios of Fle/Pyr, BaP/BghiP, and BaP/(BaP + Chr) are employed as diagnostic tools to identify the potential dominant sources of PAHs in ambient air due to the factor that the values are different to various sources such as gasoline, diesel and coal combustion, respectively39,40,41,42 (Table S3).
Sensitivity and uncertain analysis
The partial correlation coefficient between input variables (TEQ, ET, CSF for adults, TEQ, ET, BW, and CSF for children) and the output variable (ILCR) was used to determine the sensitivity of the input variable43,44. Since the max values of input variable, CSF, was not given in Chen et al.18, we used the value of log (Geometric Mean) + 3 × log (Geometric Standard Deviation) to replace the max value using uncertainty quantification based modelling of distribution parameter uncertainty43,44. In brief, we simulated the max value 100 times, assuming a uniform distribution ranging from 2 to 4 times the standard deviation in the uncertainty analysis. The 2-dimensional Monte Carlo analysis (2-D MCA) was performed by first generating 100 random “max” parameter values of the truncated lognormal distribution for CSF using Latin hypercube sampling (100 iterations of the outer loop representing uncertainty). Then, for each of these 100 values, 10,000 iterations of the inner loop (representing variability) were run to calculate the 95% confidence interval for the 95th ILCR (Table S11). The sensitivity and uncertainty analyses were conducted based on the EnvStats packages43,44.
Definition of variables
Seasons were categorized into 4 groups: March, April, and May were defined as spring; June, July, and August were defined as summer; September, October, and November were defined as autumn; and December, January, and February were defined as winter. Since lifetime cancer risk was regarded as the lifetime exposure risk, seasonal differences in cancer risk were not analysed in this study. Based on age and gender, populations were categorized into 4 groups: male children, female children, male adults, and female adults. All calculations, plotting, and statistical analysis were performed using R 3.3.1 software.
Ethical approval and informed consent
All research protocols involved in this study were approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention. All methods were carried out in accordance with the relative guidelines and regulations. Written informed consents were obtained from all participants or, if subjects are under 18, from a parent and/or legal guardian prior to enrolment.
References
Zheng, Y. et al. Assessing the polycyclic aromatic hydrocarbon (PAH) pollution of urban stormwater runoff: a dynamic modeling approach. Sci Total Environ 481, 554–563 (2014).
Mu, X., Zhu, X. & Wang, X. An Emission Inventory of Polycyclic Aromatic Hydrocarbons in China in EGU General Assembly Conference Abstracts, Print at http://adsabs.harvard.edu/abs/2015EGUGA.1710012M (2015).
Halek, F., Nabi, G. & Kavousi, A. Polycyclic aromatic hydrocarbons study and toxic equivalency factor (TEFs) in Tehran, IRAN. Environ Monit Assess 143, 303–311 (2008).
Abdel-Shafy, H. I. & Mansour, M. S. M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum 25, 107–123 (2016).
Vu, V. T., Lee, B. K., Kim, J. T., Lee, C. H. & Kim, I. H. Assessment of carcinogenic risk due to inhalation of polycyclic aromatic hydrocarbons in PM10 from an industrial city: a Korean case-study. J Hazard Mater 189, 349–356 (2011).
Offenberg, J. H. & Baker, J. E. Aerosol size distributions of elemental and organic carbon in urban and over-water atmospheres. Atmospheric Environment 34, 1509–1517 (2000).
Kaupp, H. & McLachlan, M. S. Distribution of polychlorinated dibenzo-P-dioxins and dibenzofurans (PCDD/Fs) and polycyclic aromatic hydrocarbons (PAHs) within the full size range of atmospheric particles. Atmospheric Environment 34, 73–83 (2000).
Bi, X., Sheng, G., Peng, Pa, Chen, Y. & Fu, J. Size distribution of n-alkanes and polycyclic aromatic hydrocarbons (PAHs) in urban and rural atmospheres of Guangzhou, China. Atmospheric Environment 39, 477–487 (2005).
Wu, S. P., Tao, S. & Liu, W. X. Particle size distributions of polycyclic aromatic hydrocarbons in rural and urban atmosphere of Tianjin, China. Chemosphere 62, 357–367 (2006).
Zhu, L., Lu, H., Chen, S. & Amagai, T. Pollution level, phase distribution and source analysis of polycyclic aromatic hydrocarbons in residential air in Hangzhou, China. Journal of Hazardous Materials 162, 1165–1170 (2009).
Bai, Z., Hu, Y., Yu, H., Wu, N. & You, Y. Quantitative health risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons on citizens in Tianjin, China. Bull Environ Contam Toxicol 83, 151–154 (2009).
Ma, J. et al. Airborne PM2.5/PM10-associated chlorinated polycyclic aromatic hydrocarbons and their parent compounds in a suburban area in Shanghai, China. Environ Sci Technol 47, 7615–7623 (2013).
Xia, Z. et al. Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut 173, 150–156 (2013).
Sun, J. L., Jing, X., Chang, W. J., Chen, Z. X. & Zeng, H. Cumulative health risk assessment of halogenated and parent polycyclic aromatic hydrocarbons associated with particulate matters in urban air. Ecotoxicol Environ Saf 113, 31–37 (2015).
Keith, L. H. & Telliard, W. A. Priority pollutants. I. A perspective view. Environ.sci.technol 13, 416–423 (1979).
IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. Iarc Monogr Eval Carcinog Risks Hum 92, 1–853 (2010).
Zhang, Y. & Tao, S. Global Atmospheric Emission Inventory Of Polycyclic Aromatic Hydrocarbons (pahs) For 2004. Atmospheric Environment 43, 812–819 (2009).
Chen, S. C. & Liao, C. M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ 366, 112–123 (2006).
Zhou, M. et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. The Lancet 387, 251–272 (2016).
World Health Organisation. Environmental Health Criteria 202: Selected Non-heterocyclic Polycyclic Aromatic Hydrocarbons. (International Programme on Chemical Safety, 1998).
Pufulete, M., Battershill, J., Boobis, A. & Fielder, R. Approaches to carcinogenic risk assessment for polycyclic aromatic hydrocarbons: a UK perspective. Regulatory Toxicology & Pharmacology. Rtp 40, 54–66 (2004).
Liu, Y., Zhu, L. & Shen, X. Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor and Outdoor Air of Hangzhou, China. Environmental Science & Technology 35, 840–844 (2001).
Zhong, Y. & Zhu, L. Distribution, input pathway and soil-air exchange of polycyclic aromatic hydrocarbons in Banshan Industry Park, China. Science of The Total Environment 444, 177–182 (2013).
Zhang, Y., Tao, S., Shen, H. & Ma, J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc Natl Acad Sci USA 106, 21063–21067 (2009).
Wang, J. et al. Inhalation Cancer Risk Associated with Exposure to Complex Polycyclic Aromatic Hydrocarbon Mixtures in an Electronic Waste and Urban Area in South China. Environmental Science & Technology 46, 9745–9752 (2012).
Zhou, B. & Zhao, B. Analysis of intervention strategies for inhalation exposure to polycyclic aromatic hydrocarbons and associated lung cancer risk based on a Monte Carlo population exposure assessment model. PLoS One 9, e85676 (2014).
Chinese Research Academy of Environmental Sciences. Ambient Air Quality Standard (China Environment Science Press, 2012).
Liu, N. et al. Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the Nantong Coast, China. Marine Pollution Bulletin 114, 571–576 (2017).
Shi, J. et al. Characterization and source identification of PM10-bound polycyclic aromatic hydrocarbons in urban air of Tianjin, China. Aerosol Air Qual. Res 10, 507–518 (2010).
Liu, S. et al. Seasonal and spatial occurrence and distribution of atmospheric polycyclic aromatic hydrocarbons (PAHs) in rural and urban areas of the North Chinese Plain. Environmental Pollution 156, 651–656 (2008).
Yanxu, Z., Shu, T., Huizhong, S. & Jianmin, M. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proceedings of the National Academy of Sciences of the United States of America 106, 21063–21067 (2009).
Boström, C.-E. et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environmental health perspectives 110, 451 (2002).
Wang, W. et al. Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: Status, sources and human health risk assessment. Science of The Total Environment 409, 4519–4527 (2011).
Chang, K.-F., Fang, G.-C., Chen, J.-C. & Wu, Y.-S. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: a review from 1999 to 2004. Environmental Pollution 142, 388–396 (2006).
Chinese Research Academy of Environmental Sciences. (China Environment Science Press, 2013).
Committee, W. E. Physical status: the use and interpretation of anthropometry. WHO technical report series 854, 55 (1995).
Nisbet, I. C. T. & LaGoy, P. K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regulatory Toxicology and Pharmacology 16, 290–300 (1992).
Simoneit, B. R. T. Organic matter of the troposphere—III. Characterization and sources of petroleum and pyrogenic residues in aerosols over the western united states ☆. Atmospheric Environment 18, 51–67 (1984).
Sawicki, E. Analysis for airborne particulate hydrocarbons: their relative proportions as affected by different types of pollution. National Cancer Institute Monograph 9, 201–220 (1962).
Lee, M. L., Vassilaros, D. L. & Later, D. W. Capillary column gas chromatography of environmental polycyclic aromatic compounds. International Journal of Environmental Analytical Chemistry 11, 251–262 (1982).
Khalili, N. R., Scheff, P. A. & Holsen, T. M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment 29, 533–542 (1995).
Yingjun, C. et al. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science & Technology 39, 1861–1867 (2005).
Millard, S. P., Neerchal, N. K. & Dixon, P. Environmental Statistics with R (CRC, 2012).
Millard, S. P. EnvStats, an R Package for Environmental Statistics (Wiley Online Library, 2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81502786); Zhejiang Province Science and Technology Fund (2014C03025); and Zhejiang Province Medical and Technology Fund (2017KY288, 2019KY053). The funds had no role in the design, analysis, or writing of this article. We thank the staff of the Centre for Disease Control and Prevention in HZ, JH, LS, NB, and ZS in Zhejiang Province, China, who collected the samples and epidemiological data. We particularly thank all the participants and their families for their contributions and support.
Author information
Authors and Affiliations
Contributions
Z.M., X.W., Z.C. and X.L. designed the research; Z.W., G.M., S.C., A.W., Y.Z., J.L. and X.Y. performed the research; Z.M., P.X., L.W. and X.P. analysed the data, and Z.M. wrote the main manuscript. All authors have reviewed the manuscript and have given approval to the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_43557_MOESM1_ESM.doc
Characterization and health risk assessment of PM2.5-bound polycyclic aromatic hydrocarbons in 5 urban cities of Zhejiang Province, China
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mo, Z., Wang, Z., Mao, G. et al. Characterization and health risk assessment of PM2.5-bound polycyclic aromatic hydrocarbons in 5 urban cities of Zhejiang Province, China. Sci Rep 9, 7296 (2019). https://doi.org/10.1038/s41598-019-43557-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43557-0
This article is cited by
-
Traffic influenced respiratory deposition of particulate polycyclic aromatic hydrocarbons over Dhaka, Bangladesh: regional transport, source apportionment, and risk assessment
Air Quality, Atmosphere & Health (2024)
-
PAH Pollution in Particulate Matter and Risk in Chinese Cities
Exposure and Health (2024)
-
PM2.5-bound polycyclic aromatic hydrocarbons (PAHs): quantification and source prediction studies in the ambient air of automobile workshop using the molecular diagnostic ratio
Asian Journal of Atmospheric Environment (2024)
-
Non-carcinogenic and cumulative risk assessment of exposure of kitchen workers in restaurants and local residents in the vicinity of polycyclic aromatic hydrocarbons
Scientific Reports (2023)
-
Emissions monitoring and carcinogenic risk assessment of PM10-bounded PAHs in the air from Candiota’s coal activity area, Brazil
Environmental Geochemistry and Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.