Abstract
We examined the epidemiological trends, including the distribution of sex, age, and disease etiology, in HCC incident cases, over 24 years. Data of 20,547 HCC patients (1996–2019) were analyzed in this prospective study. We divided the study period into four 6-yearly quarters. HCC etiology was categorized as hepatitis B virus (HBV) infection, HBV + hepatitis C virus (HCV) infection, HCV infection, and both negative (non-BC). The incident cases of HCC per quarter of the study period were 4311 (21.0%), 5505 (26.8%), 5776 (28.1%), and 4955 (24.1%), sequentially. Overall, 14,020 (68.2%) patients were male. The number of HCC cases in patients < 60 years, 60–69 years, 70–79 years, and ≥ 80 years were 3711 (18.1%), 6652 (32.4%), 7448 (36.2%), and 2736 (13.3%), respectively. The average age of newly-diagnosed patients increased in each quarter. HCC was associated with HBV, HBV + HCV, and HCV infections and non-BC in 2997 (14.6%), 187 (0.9%), and 12,019 (58.5%), and 5344 (26.0%) cases, respectively. The number of HCV-associated cases decreased in each quarter, while that of non-BC-associated cases increased. HCC incident cases tend to increase in the elderly and in non-BC patients; in contrast, HCC incident cases due to HCV tend to decrease.
Similar content being viewed by others
Introduction
In 2018, liver cancer was the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide, following lung, colorectal, and stomach cancers, with an estimated 841,000 new cases and 782,000 deaths annually1,2,3,4. Primary liver cancer includes hepatocellular carcinoma (HCC, comprising 75–85% of cases) and intrahepatic cholangiocarcinoma (comprising 10–15% of cases), as well as some rare disease types1,2. The main risk factors for HCC, are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), high alcohol intake, obesity, and type 2 diabetes1,2,5,6,7,8,9,10.
Globally, HBV infection is the leading cause of incident liver cancer and associated mortality, followed by alcohol consumption, HCV infection, and other causes, which account for 33%, 30%, 21%, and 16% of the total burden of this disease, respectively2. The major risk factors associated with HCC vary between regions. In areas considered “high-risk” for HCC, for example, China and East Africa, the critical disease determinant is chronic HBV infection; in contrast, in countries such as Egypt or Japan, HCV infection is likely the predominant cause1,2. Recent developments in HCV treatment suggest that a large proportion of liver cancer cases can be prevented1,11,12. An interferon (IFN)-free direct-acting antiviral agent (DAA, daclatasvir plus asunaprevir) was approved for use in Japanese patients with HCV infection in July 2014. Therefore, high rates of sustained virological response (SVR) have been achieved in patients with chronic HCV infection13,14,15,16. Recently, DAAs have been introduced as an easy and safe antiviral oral therapy for HCV infection16.
In Japan, viral hepatitis remains the leading cause of HCC; however, the decrease in the prevalence of HCV-related HCC has changed the distribution of the disease etiology7. Specifically, while the proportion of HCC cases with non-viral etiology continues to increase in Japan17, epidemiological trends in sex and age distribution of new HCC cases remain unclear. Therefore, this study aimed to examine the epidemiological trends in HCC incident cases in Japan over the past 24 years (1996–2019).
Results
A total of 20,547 patients were newly diagnosed with HCC during 1996–2019 (24 years, Table 1). The number of new HCC cases in the first, second, third, and fourth quarters was 4311 (21.0%), 5505 (26.8%), 5776 (28.1%), and 4955 (24.1%), respectively (Tables 2, 3, 4).
Sex
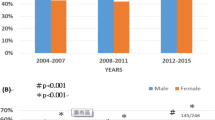
Overall, 14,020 (68.2%) and 6527 (31.8%) patients were males and females, respectively (Table 1). The number of cases in men and women per quarter were 3048 (70.7%) and 1263 (29.3%); 3732 (67.8%) and 1773 (32.2%); 3842 (66.5%) and 1934 (33.5%); 3398 (68.6%) and 1557 (31.4%), respectively (Table 2, Supplementary Fig. S1, S2). There were more female patients in the second quarter than in the first (P = 0.0020, Table 2); concurrently, there were more male patients in the fourth quarter than in the third (P = 0.0231, Table 2). Therefore, there was no noticeable change in the sex distribution of HCC incident cases throughout the study period (Fig. 1).
Age distribution of new hepatocellular carcinoma cases. 65.4 ± 9.2 years during 1996–2001 to 67.4 ± 9.8 years during 2002–2007, P < 0.0001; 67.4 ± 9.8 years during 2002–2007 to 69.5 ± 10.1 years during 2008–2013, P < 0.0001; and 69.5 ± 10.1 years during 2008–2013 to 71.5 ± 10.1 years during 2014–2019, P < 0.0001, respectively (mean ± standard deviation).
Age
Overall, the patients’ mean (± standard deviation, SD) age at diagnosis was 68.6 ± 10.1 years (Table 1). The numbers of HCC cases in patients < 60 years, 60–69 years, 70–79 years, and ≥ 80 years were 3711 (18.1%), 6652 (32.4%), 7448 (36.2%), and 2736 (13.3%), respectively (Table 3, Supplementary Fig. S3, S4). The mean ages (± SD) of the patients diagnosed with HCC in the first, second, third, and fourth quarters were 65.4 ± 9.2, 67.4 ± 9.8, 69.5 ± 10.1, and 71.5 ± 10.1 years, respectively, showing an increase over time (P < 0.0001, P < 0.0001, and P < 0.0001, respectively, Table 3, Fig. 1).
Etiology
The HCC diagnosis was associated with HBV, HBV + HCV, HCV, and non-BC exposure in 2997 (14.6%), 187 (0.9%), 12,019 (58.5%), and 5344 (26.0%) cases, respectively (Table 1). There were 579 (13.4%), 853 (15.5%), 889 (15.4%), and 676 (13.7%) new HCC cases associated with HBV in the first, second, third, and fourth quarters, respectively, showing no change in the overall incident cases over time (Table 4, Supplementary Fig. S5, S6). The corresponding values for HCV were 3147 (73.0%), 3636 (66.0%), 3233 (56.0%), and 2003 (40.4%) cases, respectively, showing a decrease over time (P < 0.0001, P < 0.0001, and P < 0.0001, respectively, Table 4). Finally, the corresponding values for non-BC were 518 (12.0%), 951 (17.3%), 1615 (27.9%), and 2260 (45.6%) cases, respectively, showing an increase over time (P < 0.0001, P < 0.0001, and P < 0.0001, respectively, Table 4).
Correlation between age and etiology
The mean ages (± SD) of the patients with HCC associated with HBV, HBV + HBC, HCV, and non-BC were 60.2 ± 10.9, 63.6 ± 10.0, 69.7 ± 8.8, and 71.0 ± 9.9 years, respectively (Fig. 2). There was a significant association between patient age and disease etiology (P < 0.0001, P < 0.0001, and P < 0.0001, respectively, Fig. 2).
Correlation between patient age and disease etiology. 60.2 ± 10.9 years in HBV to 63.6 ± 10.0 years in HBV + HCV, P < 0.0001; 63.6 ± 10.0 years in HBV + HCV to 69.7 ± 8.8 years in HCV, P < 0.0001; 69.7 ± 8.8 years in HCV to 71.0 ± 9.9 years in non-BC, P < 0.0001, respectively (mean ± standard deviation).
Discussion
In the present study, we examined epidemiological trends in HCC incident cases, including the distribution of sex, age, and disease etiology over 24 years. Although there was no noticeable change in the sex distribution of HCC incident cases throughout the study period, the average age of newly diagnosed HCC patients increased along the quarters. The number of HCV-associated cases decreased over time, while non-BC-associated cases increased over time. Moreover, there was a significant association between patient age and disease etiology.
In July 2014, DAAs were approved for Japanese patients with HCV infection. The development of DAAs has made it easier to treat HCV infections in the elderly and cirrhotic patients, and not only at specialized high-volume centers but also at general practice clinics16. As a result, the eradication of HCV infection has been reported to reduce HCC risk18. In the present study, the number of new HCC cases increased from the first to the third quarter (1996–2013), decreasing, after that, from the third to the fourth quarter (2014–2019). This relative reduction in HCC cases observed in the fourth quarter (2014–2019) may be associated with improved management of HCV infections with DAAs.
HCC incidence is two–threefold higher among men than women in most regions worldwide, while liver cancer ranks fifth and second in the global number of cases and associated deaths among men, respectively1. Meanwhile, liver cancer incidence is forecasted to decrease among men in Japan and China and women in Japan and Denmark2. In the present study, there was no association between sex and HCC incident cases over time. Therefore, while the incident case of HCC is expected to decrease among both men and women in Japan, a sex gap in the burden of this disease remains.
In the present study, patient age among newly diagnosed HCC cases increased over time. Meanwhile, the number of HCV-associated HCC cases decreased over time, in contrast to non-BC-associated cases, which increased over time. Additionally, we observed an association between patients’ age and disease etiology. Overall, HBV-associated HCC patients tended to be younger (mean age ± SD at onset 60.2 ± 10.9 years) than HCV-associated HCC patients (mean age ± SD at onset 69.7 ± 8.8 years). Therefore, it was predicted that the age of new-onset HCC patients would decrease by the decrease in new-onset HCV-associated HCC cases; however, the age of new-onset HCC patients has instead increased. This is because in the present study, the proportion of non-BC-associated HCC cases (mean age ± SD at onset 71.0 ± 9.9 years) was higher than that of HCV-associated HCC cases (mean age ± SD at onset 69.7 ± 8.8 years), and it continued to increase. In the future, as the incident case of non-BC-associated HCC increases, the age of the incident case of HCC will also increase.
The use of DAAs in HCV-infected patients has been shown to lower the risk of liver-related events, including HCC19. However, despite an SVR of > 95%, the HCC risk in DAA-treated HCV-infected patients—with advanced fibrosis or cirrhosis—was shown to remain between 0.3 and 1.8% per year20,21. The current European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) guidelines recommend lifetime surveillance of HCV-cured patients with cirrhosis22,23. Identifying clinical and molecular markers associated with HCC risk among these patients may improve the treatment effectiveness and resource allocation22,23. Studies on epidemiology and precision medicine may help inform, refine, and customize clinical guidelines for disease surveillance in this context24.
In the present study, the average age of patients newly diagnosed with HCC and those newly diagnosed with non-BC-associated HCC increased over time, while the patients newly diagnosed with HCV-associated HCC decreased over time. The increasing age of patients newly diagnosed with non-BC-associated HCC is likely to become a public health concern in the future. Recent studies have reported an association between metabolic syndrome (diabetes and obesity), excessive alcohol consumption (alcoholic fatty liver disease), and high-calorie intake (nonalcoholic fatty liver disease), and HCC risk in countries characterized by Westernized sedentary lifestyles25. A detailed understanding of the relevant risk factors is paramount for improving HCC screening, diagnosis, management, and prevention strategies25.
This study had some limitations. Firstly, while the hepatic reserve effect in liver carcinogenesis was known, in the present study, we focused mainly on three factors, sex, age, and disease etiology. Therefore, a multicenter study with additional clinical information is suggested in the future. Secondly, we did not investigate the size or number of HCC incident cases, namely, its stages. Future studies investigating the correlation between the HCC stage and sex, age, or disease etiology are required.
In conclusion, the present study suggests that HCC incident cases in the elderly and due to non-BC tended to increase, while the incident cases of HCV-associated HCC tended to decrease between quarters. In countries where HCV infection is likely the predominant cause of HCC, like Japan, similar trends in HCC incident case are anticipated in the future.
Methods
This prospective study was approved by the Ethics Committee of the National Hospital Organization Nagasaki Medical Center (no. 2020053) and was conducted according to the guidelines of the 1975 Declaration of Helsinki26.
We included only those newly diagnosed HCC patients in this study diagnosed at one of the 19 participating institutions of the Liver Cancer Study Group of Kyushu between 1996 and 2019 (24 years). The distribution of factors such as sex, age, and disease etiology was examined among the new HCC cases. The study period was divided into four quarters of six years each: 1996–2001(first quarter), 2002–2007 (second quarter), 2008–2013 (third quarter), and 2014–2019 (fourth quarter).
Diagnosis
We diagnosed HCC by measuring alpha-fetoprotein and des-gamma-carboxy prothrombin serum levels and via imaging techniques, including ultrasonography, contrast-enhanced computerized tomography, magnetic resonance, and/or tumor biopsies.
Etiology
The etiology of HCC was categorized as follows: HBsAg positive and HCV-antibody negative (HBV), both HBsAg and HCV-antibody positive (HBV + HCV), HBsAg negative and HCV-antibody positive (HCV), and both HBsAg and HCV-antibody negative (non-BC).
Statistical analysis
Inter-quarter differences in sex and disease etiology frequencies were calculated using the chi-square test, and age differences were examined using the one-way analysis of variance (ANOVA) and post-hoc analysis (Bonferroni) methods. All the statistical analyses were performed using JMP software version 15 (SAS Institute, Inc., Cary, NC, USA). P-values of < 0.05 were considered significantly different.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethics Committee of the National Hospital Organization Nagasaki Medical Center, no. 2020053) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
The data that support the findings of this study are available from the corresponding author, HY, on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424, doi:https://doi.org/10.3322/caac.21492 (2018).
Singal, A. G., Lampertico, P. & Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 72, 250–261. https://doi.org/10.1016/j.jhep.2019.08.025 (2020).
Akinyemiju, T. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 3, 1683–1691. https://doi.org/10.1001/jamaoncol.2017.3055 (2017).
Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (Lond. Engl.) 388, 1459–1544, doi:https://doi.org/10.1016/s0140-6736(16)31012-1 (2016).
Ioannou, G. N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. https://doi.org/10.1016/j.jhep.2020.10.016 (2020).
Campbell, C., Wang, T., McNaughton, A. L., Barnes, E. & Matthews, P. C. Risk factors for the development of hepatocellular carcinoma (HCC) in chronic hepatitis B virus (HBV) infection: a systematic review and meta-analysis. J. Viral Hepat. https://doi.org/10.1111/jvh.13452 (2020).
Enomoto, H. et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J. Gastroenterol. https://doi.org/10.1007/s00535-020-01748-x (2020).
Wen, B. et al. Targeted treatment of alcoholic liver disease based on inflammatory signalling pathways. Pharmacol. Ther. https://doi.org/10.1016/j.pharmthera.2020.107752 (2020).
Kucukoglu, O., Sowa, J. P., Mazzolini, G. D., Syn, W. K. & Canbay, A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2020.10.030 (2020).
Younossi, Z. M., Corey, K. E. & Lim, J. K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease (NAFLD): expert review. Gastroenterology https://doi.org/10.1053/j.gastro.2020.11.051 (2020).
Setiawan, V. W. & Rosen, H. R. Stratification of residual risk of hepatocellular carcinoma following HCV clearance with DAA in patients with advanced fibrosis and cirrhosis. Hepatology (Baltimore, Md.), doi:https://doi.org/10.1002/hep.31639 (2020).
Tanaka, Y. et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol. Int. https://doi.org/10.1007/s12072-020-10105-2 (2020).
Alqahtani, S. A. et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: analysis of phase III ION trials. Hepatology (Baltimore, Md.) 62, 25–30, doi:https://doi.org/10.1002/hep.27890 (2015).
Kwo, P. et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology (Baltimore, Md.) 64, 370–380, doi:https://doi.org/10.1002/hep.28467 (2016).
Pol, S. et al. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J. Hepatol. 66, 39–47. https://doi.org/10.1016/j.jhep.2016.08.021 (2017).
Nakano, M. et al. Predictors of hepatocellular carcinoma recurrence associated with the use of direct-acting antiviral agent therapy for hepatitis C virus after curative treatment: a prospective multicenter cohort study. Cancer Med. 8, 2646–2653. https://doi.org/10.1002/cam4.2061 (2019).
Tateishi, R. et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 54, 367–376. https://doi.org/10.1007/s00535-018-1532-5 (2019).
Roche, B., Coilly, A., Duclos-Vallee, J. C. & Samuel, D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 38(Suppl 1), 139–145. https://doi.org/10.1111/liv.13659 (2018).
Carrat, F. et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet (Lond. Engl.) 393, 1453–1464, doi:https://doi.org/10.1016/s0140-6736(18)32111-1 (2019).
Kanwal, F. et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996-1005.e1001. https://doi.org/10.1053/j.gastro.2017.06.012 (2017).
Ide, T. et al. Direct-acting antiviral agents do not increase the incidence of hepatocellular carcinoma development: a prospective, multicenter study. Hepatol. Int. 13, 293–301. https://doi.org/10.1007/s12072-019-09939-2 (2019).
EASL recommendations on treatment of hepatitis C 2018. J. Hepatol. 69, 461–511, doi:https://doi.org/10.1016/j.jhep.2018.03.026 (2018).
Ghany, M. G. & Morgan, T. R. Hepatitis C guidance 2019 update: american association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology (Baltimore, Md.) 71, 686–721, doi:https://doi.org/10.1002/hep.31060 (2020).
Fujiwara, N., Friedman, S. L., Goossens, N. & Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 68, 526–549. https://doi.org/10.1016/j.jhep.2017.09.016 (2018).
Suresh, D., Srinivas, A. N. & Kumar, D. P. Etiology of hepatocellular carcinoma: special focus on fatty liver disease. Front. Oncol. 10, 601710. https://doi.org/10.3389/fonc.2020.601710 (2020).
Taura, N. et al. The incidence of hepatocellular carcinoma associated with hepatitis C infection decreased in Kyushu area. Med. Sci. Monit. https://doi.org/10.12659/msm.881375 (2011).
Author information
Authors and Affiliations
Contributions
M.N.: concept and design, acquisition of data, writing of the manuscript; H.Y.: concept and design, acquisition of data, study supervision; S.B.: acquisition of data; Y.T.: acquisition of data; Y.T.: acquisition of data, study supervision; Y.Y.: acquisition of data; K.H.: acquisition of data; Y.K.: acquisition of data; M.H.: acquisition of data, study supervision; M.S.: acquisition of data; S.S.: acquisition of data, study supervision; S.S.: acquisition of data; K.N.: acquisition of data; T.Y.: acquisition of data, study supervision; S.I.: acquisition of data; T.S.: acquisition of data; S.O.: acquisition of data; K.N.: acquisition of data, study supervision; R.S.: acquisition of data; T.Y.: acquisition of data; A.I.: acquisition of data, study supervision; S.M.: acquisition of data; M.N.: acquisition of data, study supervision; Y.A.: acquisition of data; S.M.: acquisition of data; T.M.: acquisition of data; T.G.: acquisition of data; T.T.: acquisition of data, study supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakano, M., Yatsuhashi, H., Bekki, S. et al. Trends in hepatocellular carcinoma incident cases in Japan between 1996 and 2019. Sci Rep 12, 1517 (2022). https://doi.org/10.1038/s41598-022-05444-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05444-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.