Abstract
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide, but its current status is unclear. We aimed to investigate the evolution of etiology, presentation, management and prognostic tool in HCC over the past 12 years. A total of 3349 newly diagnosed HCC patients were enrolled and retrospectively analyzed. The comparison of survival was performed by the Kaplan-Meier method with the log-rank test. Hepatitis B and C virus infection in HCC were continuously declining over the three time periods (2004–2007, 2008–2011, 2012–2015; p < 0.001). At diagnosis, single tumor detection rate increased to 73% (p < 0.001), whereas vascular invasion gradually decreased to 20% in 2012–2015 (p < 0.001). Early stage HCC gradually increased from 2004–2007 to 2012–2015 (p < 0.001). The probability of patients receiving curative treatment and long-term survival increased from 2004–2007 to 2012–2015 (p < 0.001). The Cancer of Liver Italian Program (CLIP) and Taipei Integrated Scoring (TIS) system were two more accurate staging systems among all. In conclusion, the clinical presentations of HCC have significantly changed over the past 12 years. Hepatitis B and C virus-associated HCC became less common, and more patients were diagnosed at early cancer stage. Patient survival increased due to early cancer detection that results in increased probability to undergo curative therapies.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth leading cause of cancer-associated mortality in 2018 globally1. The incidence of HCC is high in East Asia with male prediomance2,3,4. Also, HCC is the second most common reason for cancer-related mortality in Taiwan5, an endemic area for hepatitis B virus (HBV).
HBV infection in Taiwan in mainly due to perinatal mother-to-infant transmission of the virus. The carrier rate of hepatitis B surface antigen (HBsAg) in general population was as high as 15–20%6. Prospective cohort and meta-analyses studies showed a 10- to 100-fold increase in the risk of HCC development among subjects chronically infected with HBV2,7. In Taiwan, the vaccination program against HBV was initiated in July 1984, and has reduced the incidence of HCC in children and adolescent successfully8,9. In addition to HBsAg, positive serum HBV e antigen and HBV DNA level were also associated with increased cancer risk10. Cumulative evidence showed that antiviral therapy might suppress HBV replication and lead to reduced risk of HCC formation11,12,13. HCV infection is another risk factor for HCC. The risk for HCC development in patients with serologically confirmed HCV infection was estimated to be 17-fold2. Notably, in recent years, nonalcoholic fatty liver disease (NAFLD), which is often associated with obesity and diabetes mellitus, emerged as potentially new risk factor for HCC3,14,15.
For patients with early stage HCC, surgical resection, liver transplantation or local ablation therapy are usually indicated, with 5-year survival rate up to 75%14,16. Transarterial chemoembolization is often considered for unresectable HCC without liver decompensation, and 2-year survival rate was estimated 20–25%3. Alternatively, in patients with far-advanced or terminal stage HCC, a dismal prognosis is anticipated and usually best supportive care could be provided3,4. Due to the fast changes in cancer prevention and treatment selection over the past decades, the current disease pattern and therapeutic strategy for HCC are largely unknown. We aimed to investigate the evolution of etiology, presentation, management, survival and prognostic tool in HCC patients from 2004 to 2015.
Patients and Methods
Patients
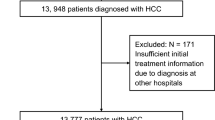
Over a 12-year period from 2004 to 2015, 3349 patients with newly diagnosed HCC in Taipei Veterans General Hospital, Taiwan, were prospectively identified and retrospectively analyzed. This study was categorized into three time periods by each 4-year interval, 2004–2007, 2008–2011 and 2012–2015, for comparison. Part of the enrolled patients have been described in our previous studies16,17. The baseline demographics, etiology of liver disease, serum biochemistry, tumor burden, performance status, cancer stage and treatment modality were comprehensively collected. All these patients were staged according to Barcelona Clínic of Liver Cancer (BCLC) staging at the time of diagnosis. The survival of patients was inspected and calculated every 3–4 months until death or dropout from the follow-up program and was cross-referenced from the database of Taiwan National Cancer Registry. This study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital, Taiwan, and complies with the standards of Declaration of Helsinki and current ethical guidelines. Waiver of consent was approved by the IRB of Taipei Veterans General Hospital, and patient’s personal information was anonymized and de-identified prior to analysis.
Diagnosis and definitions
The diagnosis of HCC was based on the findings of typical radiological features contrast-enhanced dynamic computed tomography (CT) and magnetic resonance imaging (MRI) or histology confirmed if atypical radiological features3,14. The performance status was evaluated by using Eastern Cooperative Oncology Group (ECOG) scale: 0 (asymptomatic) to 4 (confined to bed)18. All clinical data analyzed in this study were recorded at the time of diagnosis.
Surveillance
Current practice guidelines from the American Association for the Study of Liver Diseases (AASLD), European Association of the Study of Liver Diseases (EASL) and Asia-Pacific Association of the Study of Liver diseases (APASL) recommend surveillance for patients at high risk for hepatocellular carcinoma2,3,5. The combined use of serum ɑ-fetoprotein (AFP) level and abdominal sonography was regularly performed every 4–6 months for screening high-risk subjects including chronic hepatitis B and C, and subclinical or overt cirrhosis2,19.
Treatment
The newly diagnosed HCC patients at Taipei Veterans General Hospital were discussed in the multidisciplinary board for diagnosis confirmation and treatment recommendation. The criteria of surgical resection for HCC were (1) patients with tumor involving no more than three Healey’s segments, (2) Child-Turcotte-Pugh (CTP) class A with less than 25% of retention of indocyanine green 15 min after injection, and (3) no main portal trunk invasion or distant metastasis20. Radiofrequency ablation (RFA) was indicated in patients who had preserved liver function but were not eligible for surgical resection17,21. Transarterial chemoembolization (TACE) was performed in patients who had unresectable lesions or unwilling to receive curative treatment in the absence of distant metastasis and hepatic decompensation14. Systemic therapy or targeted therapy was given to selected patients with adequate liver functional reserve3,14. For patients with poor liver functional reserve or decreased performance status, best supportive care was given. Shared-decisions were made between physicians and patients prior to initiation of any definite treatment. In this study, resection, ablation and liver transplantation were classified as curative treatments. Other managements were collectively labeled as non-curative treatments.
Cancer staging
According to the AASLD and EASL HCC management guidelines, the Barcelona Clínic of Liver Cancer (BCLC) is endorsed as the standard staging system for HCC3,14. In addition, the prognostic power for HCC among the Cancer of the Liver Italian Program (CLIP), tumor-node-metastasis (TNM) system, Tokyo score, Japan Integrated Scoring (JIS) system, Taipei Integrated Scoring (TIS) system, and Hong Kong Liver Cancer (HKLC) staging system was also compared in this study22,23,24,25.
Statistics
The Chi-squared test and two-tailed Fisher exact test were used to compare categorical data. The Mann-Whitney ranked sum test or Kruskal-Wallis test was used to compare continuous variables between different groups. Data were expressed as the mean ± standard deviation (SD) and median with interquartile range (IQR). The comparison of survival distribution was performed by the Kaplan-Meier method with the log-rank test. Corrected Akaike information criteria (AICc) was obtained to reveal how the staging system correlated with patient’s survival. The AICc was chosen over Akaike information criteria to compensate for the different number of parameters in each staging system. Homogeneity was measured by χ2 test to evaluate the differences in survival among patients in the same stage within each system26. The lower AIC, the more explanatory and informative the staging system is27. A p-value < 0.05 was considered statistically significant by 2-tailed tests. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics and staging
A total of 3349 newly diagnosed HCC patients were consecutively identified during the study period. Their baseline characteristics and clinical information are shown in Table 1. There were 1308, 1136, and 905 HCC patients in the time periods of 2004–2007, 2008–2011 and 2012–2015, respectively. The mean age was 65 years, and the majority of patients were male in these three cohorts. Regarding the etiology of liver disease, the prevalence of HBV infection continuously declined from 43% in 2004–2007 to 39% in 2008–2011 and 36% in 2012–2015 (p < 0.001). The prevalence of HCV-related HCC was 24% in 2004–2007, and gradually decreased to 22% in 2008–2011 and 20% in 2012–2015 (p < 0.001). The prevalence of cryptogenic HCC increased from 14% in 2004–2011 to 20% in 2012 to 2015 (p < 0.001). The prevalence of diabetes mellitus in HCC was 24%, 24% and 27% in 2004–2007, 2008–2011 and 2012–2015 cohort, respectively (p = 0.001).
Single tumor detection rate was 59% in 2004–2007, and the rate increased to 61% in 2008–2011 and 73% in 2012–2015 (p < 0.001). Patients with a poor performance status (status 2–4) at the time of diagnosis decreased from 22% in 2004–2007 to 8% in 2012–2015 (p < 0.001). Patients of the 2012–2015 cohort had significantly better performance status, less multiple tumor nodules, less vascular invasion, better liver functional reserve and increased overall survival when specifically compared with 2004–2007 cohort (all p < 0.001).
The proportion of patients diagnosed at early cancer stage gradually increased, whereas the proportion of terminal stage decreased in three time periods according to the BCLC, HKLC, CLIP, TIS, JIS and TNM staging systems (all p < 0.01), except Tokyo score (p = 0.154).
Treatment
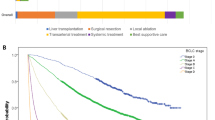
The probability for patients receiving curative treatment was 44% in 2004–2007 and the rate increased to 45% in 2008–2011 and 55% in 2012–2015 (p < 0.001) independent of age and sex (Fig. 1), and the rate was the highest in the 2012–2015 when compared with 2004–2007 cohort (p < 0.001). By contrast, the percentages of TACE and best supportive care consistently decreased over the three time periods (Table 1, p < 0.001).
Prognostic performance of the seven staging systems
The prognostic accuracy of 7 HCC staging systems was evaluated in three time periods (Table 2). In both 2004–2007 and 2008–2011, the CLIP staging system had the highest homogeneity and lowest AICc, suggesting superior prognostic capability than other staging systems. By contrast, in 2012–2015, the TIS system had the highest homogeneity and lowest AICc, indicating a better prognostic performance in this time period.
Long-term survival
The median survival of HCC patients was 30 months (95% confidence interval [CI]): 25–32 months) in 2004–2007, 23 months (95% CI: 19–27 months) in 2008–2011, and 72 months (95% CI: not reached) in 2012–2015 cohort (Fig. 2). A total of 1051 (80%), 839 (74%) and 375 (41%) patients died in 2004–2007, 2008–2011 and 2012–2015 period, respectively. Survival analysis disclosed that patient survival tended to be better in 2012–2015 (p < 0.001). The estimated survival probability at 1, 3 and 5 years were 69%, 45%, 31% for 2004–2007 cohort, 62%, 42%, 29% for 2008–2011 cohort, and 72%, 56%, 52% for 2012–2015 cohort, respectively.
In subgroup analysis, the survival probability at 1, 3 and 5 years were 90%, 67%, 49% for 2004–2007 cohort, 87%, 70%, 52% for 2008–2011 cohort, and 91%, 78%, 74% for 2012–2015 cohort, respectively, in patients undergoing curative treatment. The survival probability at 1, 3 and 5 years were 53%, 26%, 15% for 2004–2007 cohort, 42%, 19%, 11% for 2008–2011 cohort, and 50%, 27%, 24% for 2012–2015 cohort, respectively, in patients undergoing non-curative treatment. There were significant survival differences among 2004–2007, 2008–2011 and 2012–2015 cohort in both treatment groups (Fig. 3A,B; both p < 0.001).
The survival probability at 1, 3 and 5 years were 91%, 67%, 47% for 2004–2007 cohort, 88%, 67%, 49% for 2008–2011 cohort, and 90%, 75%, 72% for 2012–2015 cohort, respectively, in patients within the Milan criteria. The survival probability at 1, 3 and 5 years were 53%, 29%, 18% for 2004–2007 cohort, 45%, 25%, 16% for 2008–2011 cohort, and 56%, 37%, 33% for 2012–2015 cohort, respectively, in patients beyond the Milan criteria. Significant survival distributions were found in three different periods of HCC patients for both groups (Fig. 4A,B; p < 0.001).
The survival probability at 1, 3 and 5 years were 80%, 55%, 37% for 2004–2007 cohort, 76%, 54%, 38% for 2008–2011 cohort, and 81%, 65%, 62% for 2012–2015 cohort, respectively in patients with CTP class A. The survival probability at 1, 3 and 5 years were 38%, 17%, 10% for 2004–2007 cohort, 31%, 16%, 10% for 2008–2011 cohort, and 44%, 23%, 18% for 2012–2015 cohort, respectively, in patients with CTP class B or C. The 2012–2015 patient cohort had the best survival probability as compared with the 2004–2007 and 2008–2011 cohort for both CTP class A (p < 0.001; Fig. 5A) and class B-C patients (p = 0.008; Fig. 5B).
Discussion
Our study shows that there was 13.1% reduction and 20.3% further reduction in the incident cases of HCC from 2004–2007 (n = 1308) to 2008–2011 (n = 1136), and from 2008–2011 to 2012–2015 (n = 905), respectively. HBV is one of the etiologies of liver cirrhosis and HCC. The incidence of HBV-related HCC declined in this 12-year study period. Consistent with previous cohort studies6,8,9, HBV infection significantly decreased after the implementation of the vaccination program8,9. Notably, the impact of national HBV vaccination program not only reduced the carrier rate of HBsAg, but also decreased the incidence of HBV-related HCC.
High levels of serum HBsAg and DNA are tightly associated with the occurrence of HCC28,29,30. Antiviral therapy using nucleoside or nucleotide analogues may inhibit HBV replication, and leads to improvement in liver histology and reduced risk of HCC12,31. In addition to HBV, HCV is also another risk factor for HCC globally32. Antiviral therapy for hepatitis C with interferon and ribavirin results in improved clinical outcomes by decreasing the risk of hepatic decompensation and HCC33. Alternatively, the development of cirrhosis is associated with non-alcoholic steatohepatitis (NASH). In addition, metabolic syndrome such as diabetes and obesity, could increase the risk of HCC in NASH patients2,3. In our study, the percentage of cryptogenic cause of HCC increased from 14% in 2004–2007 to 20% in 2012–2015; suggesting NASH may play an important role in inducing HCC.
The changing incidence of HCC in the international setting revealed that the incidence of HBV-related HCC has declined in most Asian countries such as China, Philippines and South Korea after the implementation of HBV vaccination. The decreasing rate of HCV-related HCC was reported in Japan and Italy due to specific antiviral therapy, including direct acting antiviral agents. The increasing rates of cryptogenic HCC were observed in US, Western countries and some Asian countries possibly due to the emergence of metabolic syndrome and non-alocholic steatohepatitis (NASH)34. These results are mostly consistent with our single center study in Taiwan.
The diagnosis of early stage HCC increased from 33% in 2004–2007 to 37% in 2012–2015 in this survey. The percentage of patients receiving curative treatment also increased from 44% in 2004–2007 to 55% in 2012–2015. Previous studies suggested that serum AFP and abdominal sonography were useful screening tools in high risk patients2,35. Our data were consistent with previous study36, indicating that screening patients with known risk factors may result in early cancer detection. Consistently37,38, our results also show that there is increased long-term survival in HCC patients because of a higher rate of curative treatments, including surgical resection and local ablation therapy.
The key prognostic predictors for HCC are liver functional reserve, tumor burden and therapeutic strategy. We further performed a subgroup analysis to delineate the pattern and cause of a better long-term survival in the 2012–2015 cohort. Importantly, we found that such survival impact is independent of CTP class, Milan criteria and treatment modality. An overall improvement in antiviral therapy for chronic viral hepatitis, cancer detection and active anti-cancer therapy over the study period may greatly contribute to the survival advantage36,37.
To date, several HCC staging systems have been implemented for prognostic prediction. However, the optimal staging system has been in intense debate for a decade. Our results suggest that the CLIP score was the best staging system for prognostic prediction in the cohort of 2004–2007 and 2008–2011. However, TIS system is a better system in discriminating clinical outcomes than the other 6 models for the 2012–2015 cohort. This discrepancy could be due to the change in patient demographics and pattern of treatment strategy, and also well explains why published studies addressing this issue revealed discordant results.
Our study has some limitations. First, this is a single center study in an area where HBV is commonly seen, which is different from Western countries. Second, selection bias could not be completely avoided because of the retrospective nature of this study. Third, a direct causal relationship between the changes of disease presentation and patient outcome cannot be confidently confirmed. Further studies are needed to validate our result.
In conclusion, the characteristics of patients with HCC have significantly changed over the last 12 years. HBV- and HCV-associated HCC became less common and cryptogenic HCC, probably related to NASH, was increasing. Early cancer detection and implementation of active anti-cancer treatment become possible and can be expected to prolong the long-term survival of HCC patients further.
Change history
23 October 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424 (2018).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11, 317–370 (2017).
EASL. Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69, 182–236 (2018).
El-Serag, H. B. Hepatocellular carcinoma. N Engl J Med 365, 1118–1127 (2011).
Hsu, H. Y. et al. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for hepatocellular carcinoma. BMC Cancer 17, 742 (2017).
Chien, Y. C., Jan, C. F., Kuo, H. S. & Chen, C. J. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev 28, 126–135 (2006).
El-Serag, H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273.e1261 (2012).
Chang, M. H. et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 336, 1855–1859 (1997).
Chiang, C. J., Yang, Y. W., You, S. L., Lai, M. S. & Chen, C. J. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 310, 974–976 (2013).
Chen, C. J., Yang, H. I. & Iloeje, U. H. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 49, S72–84 (2009).
Singal, A. K., Salameh, H., Kuo, Y. F. & Fontana, R. J. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther 38, 98–106 (2013).
Wong, G. L. et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 58, 1537–1547 (2013).
Chiang, C. J. et al. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology 61, 1154–1162 (2015).
Villanueva, A. Hepatocellular Carcinoma. N Engl J Med 380, 1450–1462 (2019).
Wong, R. J., Cheung, R. & Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 59, 2188–2195 (2014).
Ho, S. Y. et al. Comparison of twelve liver functional reserve models for outcome prediction in patients with hepatocellular carcinoma undergoing surgical resection. Scientific Reports 8, 4773 (2018).
Ho, S. Y. et al. Prognostic Performance of Ten Liver Function Models in Patients with Hepatocellular Carcinoma Undergoing Radiofrequency Ablation. Sci Rep 8, 843 (2018).
Hsu, C. Y. et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 57, 112–119 (2013).
Kim, D. Y. & Han, K. H. Epidemiology and surveillance of hepatocellular carcinoma. Liver cancer 1, 2–14 (2012).
Hsu, C. Y. et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol 19, 842–849 (2012).
Fontana, R. J. et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl 8, 1165–1174 (2002).
Tateishi, R. et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut 54, 419–425 (2005).
Hsu, C. Y. et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol 53, 108–117 (2010).
Yau, T. et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 146, 1691–1700.e1693 (2014).
Liu, P. H. et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol 64, 601–608 (2016).
Pourhoseingholi, M. A. et al. Comparing Cox regression and parametric models for survival of patients with gastric carcinoma. Asian Pac J Cancer Prev 8, 412–416 (2007).
Forster, M. R. Key Concepts in Model Selection: Performance and Generalizability. J Math Psychol 44, 205–231 (2000).
Tseng, T. C. et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 57, 441–450 (2013).
Iloeje, U. H. et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130, 678–686 (2006).
Chan, H. L. et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol 26, 177–182 (2008).
Colombo, M. & Lleo, A. The impact of antiviral therapy on hepatocellular carcinoma epidemiology. Hepat Oncol 5, Hep03 (2018).
Huang, Y. T. et al. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol 29, 3643–3650 (2011).
Yu, M. L. & Chuang, W. L. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol 24, 336–345 (2009).
Petrick J. L et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer https://doi.org/10.1002/ijc.32723.(2019).
Poh, Z., Goh, B. B., Chang, P. E. & Tan, C. K. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: a 10-year follow-up of 673 patients. Eur J Gastroenterol Hepatol 27, 638–643 (2015).
Rodriguez, D. N., Torruellas, C. & Cress, R. D. Trends in early-stage hepatocellular carcinoma, California 1988-2010. Cancer causes & control: CCC 27, 325–331 (2016).
Altekruse, S. F., Henley, S. J., Cucinelli, J. E. & McGlynn, K. A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 109, 542–553 (2014).
Kuo, Y. H. et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer 46, 744–751 (2010).
Acknowledgements
This study was supported by the grants from Taipei Veterans General Hospital (V107C-003, V107A-008, V108A-002, VN109-06), Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: Teh-Ia Huo. Specific author contributions: S.-Y. Ho and T.-I. Huo performed the research and wrote the paper. C.-Y. Hsu, P.-H. Liu, C.-Y. Hsia, and C.-W. Su collected and analyzed the data. R.-C. Lee, H.-J. Lei, Y.-H. Huang and M.-C. Hou contributed to study design and data collection. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ho, SY., Liu, PH., Hsu, CY. et al. Evolution of etiology, presentation, management and prognostic tool in hepatocellular carcinoma. Sci Rep 10, 3925 (2020). https://doi.org/10.1038/s41598-020-61028-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61028-9
This article is cited by
-
Surgical resection could provide better outcomes for patients with hepatocellular carcinoma and tumor rupture
Scientific Reports (2022)
-
Reply to the letter by Huo et al. regarding our manuscript “The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan”
Journal of Gastroenterology (2021)
-
Changing patterns of etiology and management of hepatocellular carcinoma: need for global reappraisal
Journal of Gastroenterology (2021)
-
Methionine metabolism in chronic liver diseases: an update on molecular mechanism and therapeutic implication
Signal Transduction and Targeted Therapy (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.