Abstract
The insulin promoter is regulated by ubiquitous as well as pancreatic β-cell-specific transcription factors. In the insulin promoter, GG2–GG1/A2–C1 (bases − 149 to − 116 in the human insulin promoter) play important roles in regulating β-cell-specific expression of the insulin gene. However, these events were identified through in vitro studies, and we are unaware of comparable in vivo studies. In this study, we evaluated the activity of GG2–GG1/A2 elements in the insulin promoter region in vivo. We generated homozygous mice with mutations in the GG2–GG1/A2 elements in each of the Ins1 and Ins2 promoters by CRISPR–Cas9 technology. The mice with homozygous mutations in the GG2–GG1/A2 elements in both Ins1 and Ins2 were diabetic. These data suggest that the GG2–GG1/A2 element in mice is important for Ins transcription in vivo.
Similar content being viewed by others
Introduction
The promoter of the insulin gene (Ins) consists of DNA sequences immediately upstream of the site of transcription initiation1,2,3,4,5. In the insulin promoter, three highly conserved enhancer elements, A3 (bases − 225 to − 220 in the human insulin promoter)6,7,8,9, GG2–GG1/A2–C1 (bases − 149 to − 116)10 and E1 (bases − 111 to − 102)11,12, play important roles in regulating β-cell-specific expression of the insulin gene. In the human insulin gene, GG elements with the consensus core sequence of GGAAAT, such as GG2 at bases − 145 to − 140 and GG1 at bases − 134 to − 129, are located immediately upstream of C113. GG1 is also identified as A2 according to simplified nomenclature14. The GG1/A2 element overlaps with the C1 element14, and this region in the rat insulin II gene (Ins2) has been studied in detail10,12,15. The GG1/A2–C1 region, which is also called rat insulin promoter element 3b (RIPE3b), works together with the nearby E1 element, and this synergy is dependent on the C1 element15. The V-maf musculoaponeurotic fibrosarcoma oncogene family protein A (MafA) binds to the C1 element16. The A2.2 factor, which is a β-cell-specific activator that has not yet been identified, binds to the A2 element17. The MafA and A2.2 factors cooperatively activate insulin gene expression18.
The GG2 element contributes to the β-cell-specific transcription of the human insulin gene13,19, and it is a site for pancreatic and duodenal homeobox1 (PDX1) activation in the human insulin gene20. In contrast, the rodent GG2 element is negatively regulated by the Nkx2.2 transcription factor, although the sequences surrounding human GG2 (GGAAAT) and rat GG2 are only different at nucleotides − 144 and − 141 (Insulin I (Ins1); GCAAGT) or nucleotide − 141 (Ins2; GGAAGT) in humans denoted20,21. In the human GG element, the nucleotide − 141 mutant (GGAAAT → GGAAGT) exhibited decreased activity from the insulin promoter, while the nucleotide − 144 mutant (GGAAAT → GCAAAT) resulted in 3–4-fold activation over the wild type20,21. However, these events were identified through in vitro studies, and we are unaware of comparable in vivo studies.
MafA-deficient mice display intolerance to glucose and develop diabetes mellitus. Ins1 and Ins2 transcripts are diminished in MafA-deficient mice22. Mice lacking Pdx1 fail to form a pancreas23, and β-cell-specific inactivation of the mouse Pdx1 gene results in loss of the β-cell phenotype and maturity-onset diabetes24. Nkx2.2-deficient mice develop severe hyperglycemia and die shortly after birth25. These data show that MafA, Pdx1, and Nkx2.2 are important for pancreas development and that MafA affects the Ins1 and Ins2 transcripts in vivo. However, it is unknown whether the GG2–GG1/A2 sequence in insulin promoters is important for Ins1 and Ins2 transcripts in vivo because there are several binding sites of MafA and Pdx1 in insulin promoters and the three transcription factors affect pancreas development.
In this study, we evaluated the activity of GG2–GG1/A2 elements of the insulin promoter region in vivo. Clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 technology was used to generate mice with partial deletions of the Ins1 and Ins2 promoters, and the results showed that only homozygous mice with mutations in the highly conserved GG2–GG1/A2 elements of the Ins1 and Ins2 promoters developed diabetes.
Results
Generation of mice with insulin promoter mutations
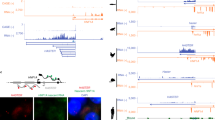
We previously reported the generation of mice with mutations in C1 elements of the Ins1 and Ins2 genes using CRISPR–Cas9 technology26. We comicroinjected the same pX330 vectors to express two guide RNAs (gRNAs) (Fig. 1A) and Cas9 (pX330-1st/2nd gRNA vectors) in mouse zygotes27,28,29,30. Among the neonates, we detected mutations in the GG2–GG1/A2 elements of the Ins1 and Ins2 promoters (Fig. 1B,C). We also analyzed mutations in 3 and 4 other sequences bordering the C1 elements of the Ins1 and Ins2 promoters, respectively (Fig. 1B,C). To evaluate the importance of the GG2–GG1/A2 elements in vivo, mice with 4 and 5 of these mutations in the Ins1 and Ins2 promoters, respectively, were crossed with wild-type C57BL/6J mice. Nine heterozygous mice that had deletions in the Ins1 or Ins2 promoter were generated, and they were not diabetic (Fig. 2A, Supplementary Table 1). The F1 mice were intercrossed to obtain homozygous F2 mice that had deletions in only the Ins1 promoter or the Ins2 promoter. These mice were also not diabetic (Fig. 2B, Supplementary Table 2).
Generation of mice with mutations of the insulin promoter. (A) Structures and sequences of the human and mouse insulin promoters. Bases − 170 to − 147 and − 145 to − 104 of the mouse Ins1 and Ins2 promoters are identical, and bases − 149 to − 147 (AGG) and − 114 to − 112 (TGG) were used as the PAM sequences of the guide RNAs (gRNAs) for the CRISPR–Cas9 system. The 20-bp double-stranded DNAs (dsDNAs) derived from positions − 169 to − 150 and − 134 to − 115 of the Ins1/2 promoters were inserted into pX330. (B, C) Generation of mice with mutated insulin promoters. The mice with 4 types of deletions in the Ins1 promoter (B) and 5 types of deletions in the Ins2 promoter (C) were generated. Sequences shaded in gray were deleted.
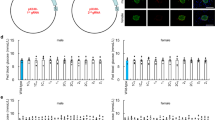
Blood glucose levels of mice with mutations of the insulin promoter and luciferase assay results. (A) Blood glucose levels of fed wild-type (n = 6) and heterozygous mice that had deletions in the Ins1/2 promoters (n = 6 each) at 12 weeks of age. (B) Blood glucose levels of fed wild-type (n = 6) and homozygous mice that had deletions in the Ins1/2 promoters (n = 6 each) at 12 weeks of age. (C) Mouse Ins1 promoter-luciferase plasmid containing approximately 1000 bp of the 5′-flanking sequences of the wild-type or mutated Ins1 promoter region was transfected into βTC6 cells (β-cell line). Forty-eight hours after transfection, cells were harvested and assayed (n = 3). **p < 0.01. (D) Mouse Ins2 promoter-containing luciferase plasmid containing approximately 1000 bp of the 5′-flanking sequences of the wild-type or Ins2 deletion promoter region was transfected into βTC6 cells (β-cell line). Forty-eight hours after transfection, cells were harvested and assayed (n = 3). **p < 0.01. The error bars represent the standard error.
The homozygous F2 mice that had deletions in only the Ins1 promoter or the Ins2 promoter were intercrossed to obtain heterozygous F3 mice that had deletions in both the Ins1 promoter and the Ins2 promoter. These mice were not diabetic (Fig. 2A, Supplementary Table 3). Twenty homozygous mice that had deletions in both the Ins1 and Ins2 promoters were obtained by crossing the F3 and F4 mice (Supplementary Table 4). Only homozygous mice with mutations in the GG2–GG1/A2 elements of both the Ins1 and Ins2 promoters (1GG2GG mice) were diabetic (Fig. 2B). Insulin promoter activity was significantly decreased for the 2 types of mutations in the Ins1 and Ins2 promoters (Fig. 2C,D).
Immunohistochemistry of the pancreatic tissue of 1GG2GG mice
Immunohistochemical analysis of the pancreatic tissue of both 1GG2GG male and female mice showed reduced insulin expression in islets compared with what was observed in the islets of wild-type C57BL/6 mice (Fig. 3).
Blood glucose levels of 1GG2GG mice
Fasted blood glucose levels of both male and female 1GG2GG mice at 6 weeks of age were elevated compared with those of wild-type C57BL/6 mice (Fig. 4A, B). Fed blood glucose levels of both male and female 1GG2GG mice at 6 weeks of age were also elevated compared with those of wild-type C57BL/6 mice (Fig. 4C, D). Blood glucose levels of fasted and fed 1GG2GG mice at 12 weeks of age were elevated compared with those of wild-type C57BL/6 mice (Fig. 4A–D).
Blood glucose levels of 1GG2GG mice. (A, B) Fasting blood glucose concentrations of wild-type (n = 6) and 1GG2GG mice (n = 6) at 6 and 12 weeks of age [(A): male, (B): female]. (C, D) Blood glucose levels of fed wild-type (n = 6) and 1GG2GG mice (n = 6) at 6 and 12 weeks of age [(C): male, (D): female]. *p < 0.05. **p < 0.01.
mRNA levels in pancreatic islets of 1GG2GG mice
Quantitative RT-PCR analysis of mRNA extracted from the pancreatic islets of male and female 1GG2GG mice (fasted) with diabetes showed that Ins1 and Ins2 mRNA levels were decreased compared with those of wild-type C57BL/6 mice (fasted) (Fig. 5A,B). There were no significant differences in the levels of the following mRNAs: Pdx1, Nkx2.2, and MafA (Fig. 5C–H). We also evaluated Ins1 and Ins2 mRNA levels in the embryonic pancreas of 1GG2GG mice. There were no significant differences between Ins1 and Ins2 mRNA levels in the embryonic pancreas of 1GG2GG mice and that of C57BL/6 mice (Supplementary Fig. 1).
Ins1/2, Pdx1, Nkx2.2, and MafA mRNA levels in pancreatic islets from 1GG2GG mice. (A, B) qRT-PCR analysis of Ins1 and Ins2 in pancreatic islets of 1GG2GG mice [(A): male, (B): female]. (C, D) qRT-PCR analysis of Pdx1 expression in pancreatic islets of 1GG2GG mice [(C): male, (D): female]. (E, F) qRT-PCR analysis of Nkx2.2 expression in pancreatic islets of 1GG2GG mice [(E): male, (F): female]. (G, H) qRT-PCR analysis of MafA expression in pancreatic islets of 1GG2GG mice [(G): male, (H): female]. Pancreatic islets (purity > 95%) of wild-type mice served as a control. The data are expressed as the gene-to-Gapdh ratio; that of the control cells was arbitrarily defined as 1 (n = 8). The error bars represent the standard error. *p < 0.05.
Glucose tolerance tests and body weight of 1GG2GG mice
Glucose tolerance tests showed significantly elevated glucose levels in male and female 1GG2GG mice (Fig. 6A,B). Both male and female 1GG2GG mice exhibited decreased body weights at 6 and 12 weeks (Fig. 6C,D).
Glucose tolerance test and body weights of 1GG2GG mice. (A, B) Intraperitoneal glucose tolerance testing of wild-type (blue: n = 6) and 1GG2GG mice (white: n = 6) at 12 weeks of age [(A): male, (B): female]. (C, D) Body weights of wild-type (n = 6) and 1GG2GG mice (n = 6) at 6 and 12 weeks of age [(C): male, (D): female]. The error bars represent the standard error. **p < 0.01.
1GG2GG mice with mutations in the highly conserved GG2–GG1/A2 elements of the Ins1 and Ins2 promoters developed diabetes, and the body weight of 1GG2GG mice was lower than that of wild type controls. However, 20–30% of insulin transcription in 1GG2GG mice remained, and they were fertile.
Discussion
Here, we used the CRISPR–Cas9 system to evaluate the functions of the promoter regions of Ins1 and Ins2 in vivo. The use of CRISPR–Cas9 systems to manipulate mammalian genomes presents enormous opportunities for curing human diseases28,29,30,31,32. The nonhomologous end joining (NHEJ) mechanism induces site-specific repair at the DNA break site, which causes different and unpredictable insertions or deletions of various sizes. Although the NHEJ features of the CRISPR–Cas9 system are disadvantageous for the generation of knockout mice via the deletion of a single protein-encoding gene, the system is advantageous for evaluating the functions of promoter regions in vivo because multiple mice with different alterations of promoter sequences can be generated concurrently.
We generated diabetic mice with deletions in the GG2–GG1/A2 elements of both the Ins1 and Ins2 promoters. Insulin promoter activity was significantly decreased for the 2 types of Ins1 and Ins2 promoters (Fig. 2C,D). Although the deletions in the GG2–GG1/A2 elements of the Ins1 promoters were complete, the deletions in the Ins2 promoter mainly involved the GG2 elements. In the human insulin gene, the GG2 element is a positive regulator of β-cell-specific transcription13,19. In contrast, it has been reported that the rodent GG2 element is negatively regulated by the Nkx2.2 transcription factor20,21. The sequence surrounding human GG2 is GGAAAT, and in mice, the GG2 is GCAAGT in Ins1 and GGAAGT in Ins2. In our study, the sequence of the deletions in the Ins2 promoter was CCAGGGAAGTGTTTG, and the deletions resulted in a decrease in insulin promoter activity. The sequences bordering the GG2 element (CCAG or GTTTG) may be important for insulin promoter activity.
Homozygous mice with deletions in only the Ins1 promoter or the Ins2 promoter were not diabetic (Fig. 2B). In the single mutant models, compensation of Ins1/Ins2 was demonstrated (Supplementary Fig. 2). Our previous study showed that compensatory transcription of a functional insulin gene in homozygous mice with deletions of the C1 element in either the Ins1 or Ins2 promoter did not lead to diabetes26. Moreover, it has been reported that mice with single homozygous null mutations in Ins1 or Ins2 were not diabetic because of compensatory responses, and there was a dramatic increase in Ins1 transcripts in the Ins2−/− mice33. Compensatory transcription results from nondiabetic mice with homozygous deletions in only the Ins1 promoter or the Ins2 promoter.
Although the overlapping sequence of the C1 element (AGCT) is deleted in 1GG of the insulin-1 promoter, the overlapping sequence of the C1 element is not deleted in 2GG of the insulin-2 promoter. 1GG2GG mice become diabetic, while 1GG mice do not become diabetic, suggesting that the deletion of the GG2–GG1/A2 element excluding the overlapping sequence of the C1 element in the insulin-2 promoter (CCAGGGAAGTGTTTG) is important for the positive regulation of the insulin-2 gene. Our data are the first to report that the CCAGGGAAGTGTTTG sequence in the insulin-2 promoter is important in vivo. On the other hand, 1GG2GG mice develop diabetes, while 2GG mice do not develop diabetes, suggesting that the deletion of the GG2–GG1/A2 element, including the overlapping sequence of the C1 element in the insulin-1 promoter (ACCAGGCAAGTGTTTGGAAACTGCAGCT), is important for the positive regulation of the insulin-1 gene in vivo. We cannot decide whether the CCAGGCAAGTGTTTG sequence or the overlapping AGCT sequence or both is important for the insulin-1 promoter.
The effect on fed and fasting glucose levels in 1GG2GG mice was very mild (Fig. 2B) compared to the inactivity of insulin-driven mutant luciferase activity (Fig. 2C,D), reduced insulin transcription (Fig. 5A,B), and the degree of glycemic excursion by glucose tolerance test (Fig. 6). This may be due to the remaining capacity of the pancreas. Living donor segmental pancreas transplants have been performed clinically, and most of the donors did not develop diabetes, at least not immediately after donation34. Half of the pancreas was donated at that time, but it did not induce diabetes, suggesting that pancreata have remaining capacity. Although insulin transcription is severely reduced in 1GG2GG mice, the degree of diabetes is mild, probably due to the remaining capacity of the pancreas.
In conclusion, the sequences of the GG2–GG1/A2 elements in the Ins1 and Ins2 promoters are required for Ins expression in vivo. To the best of our knowledge, our findings show for the first time that the GG2–GG1/A2 elements of the insulin promoter in mice are important for its activity in vivo. The CRISPR–Cas9 technique will provide a tool to generate knockout mice that can be used to evaluate promoter regions.
Methods
Generation of mice with insulin promoter mutations
We previously constructed two Cas9-single-guide RNA (sgRNA) expression vectors26. The 1st gRNA and 2nd gRNA were inserted into pX330 vectors35 (Addgene, Watertown, MA). Female C57BL/6J mice were injected with pregnant mare serum gonadotropin and human CG (hCG) at 48-h intervals and then were mated with male C57BL/6J mice. Fertilized one-cell embryos were collected from the oviducts. Subsequently, 5 ng/μL pX330-1st/2nd gRNA vectors were injected into the pronuclei of these one-cell embryos, which were transferred into pseudopregnant mice from the Institute of Cancer Research (ICR). F0 mice were genotyped to detect the presence of mutations in the Ins1/2 promoters. F0 mice were checked for the Cas9 transgene and for off-target effects36. F0 mice were mated with C57BL/6J mice to obtain F1 offspring.
The mutant mice and littermate wild-type mice were maintained on a C57BL/6 background. Mice were housed under a 12:12-h light/dark cycle in a room with controlled temperature and humidity. Food and water were provided without restrictions26. The Institutional Animal Care and Use Committee of the University of the Ryukyus and the University of Tsukuba approved the animal studies.
Construction of insulin promoter-luciferase plasmids
Wild-type or mutated Ins1/2 promoter cDNAs containing approximately 1000 bp of the 5′-flanking sequences of the Ins1/2 promoter regions were amplified using PCR with appropriate linker-containing primers, which were then used to replace the promoter region of the Rat Ins2 promoter–reporter (luciferase) plasmid37 using a ligation kit (TaKaRa, Tokyo, Japan) (Supplementary Fig. 3).
Reporter assay
βTC6 cells (β-cell line) (ATCC, Manassas, VA) were transfected using Lipofectamine (Thermo Fisher Scientific, Tokyo, Japan) according to the conditions recommended by the manufacturer. The cells were transfected with 1.0 μg of wild-type or mutated reporter (luciferase) plasmids containing the Ins1 or Ins 2 promoter in 35-mm culture dishes. Forty-eight hours after transfection, the cells were harvested and assayed (Promega, Madison, WI).
Immunofluorescence staining
A guinea pig anti-insulin antibody (Abcam, Tokyo, Japan) and a FITC-conjugated anti-guinea pig IgG (Abcam) were used for immunofluorescence staining of mouse pancreatic islets. Pancreatic tissues were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). Paraffin sections were mounted on slides. After blocking with 20% AquaBlock (EastCoast Bio, North Berwick, ME, USA) for 30 min at room temperature, the sections were incubated overnight at 4 °C with a guinea pig anti-insulin antibody (1:100); then, they were incubated for 1 h at room temperature with a FITC-conjugated anti-guinea pig IgG (1:250).
Islet isolation from mouse pancreas
Islets were isolated from the pancreata of normal and genome-edited mice38,39. For islet isolation, the common bile duct was cannulated and injected with cold Hank’s balanced salt solution (HBSS, Thermo Fisher Scientific) containing 1.5 mg/mL collagenase (Roche Boehringer Mannheim, Indianapolis, IN). The pancreas was digested at 37 °C. The islets were separated with a Histopaque 1077 (Merck) density gradient; then, they were hand-picked using a dissecting microscope to ensure pure islet preparation and were used immediately.
Quantitative RT-PCR analysis of isolated islets
Total RNA was extracted from isolated islets using an RNeasy Mini Kit (Qiagen, Tokyo, Japan). After quantifying the RNA using spectrophotometry, 2.5 µg of RNA was heated at 85 °C for 3 min and then reverse-transcribed in a 25-µL reaction mixture containing 200 units of Superscript II RNase H-RT (Thermo Fisher Scientific), 50 ng random hexamers (Thermo Fisher Scientific), 160 µmol/l dNTP and 10 nmol/l dithiothreitol. The reaction mixture was incubated for 10 min at 25 °C, 60 min at 42 °C and 10 min at 95 °C26.
Quantitative PCR amplification of cDNA from mouse islets was performed using a TaqMan universal PCR master mix core reagent kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). PCR was performed for 40 cycles, and the reactions were incubated for 2 min at 50 °C and 10 min at 95 °C for the initial steps. In each cycle, denaturation was performed for 15 s at 95 °C, and annealing/extension was performed for 1 min at 60 °C. PCR was performed in 20 µL of solution using cDNAs synthesized from 1.11 ng of total RNA. The amount of mRNA was normalized by dividing the amount of the mRNA of interest by that of Gapdh mRNA. Primers specific for mouse Ins1, Ins2, Pdx1, Nkx2.2, MafA and Gapdh were purchased as Assays-on-Demand Gene Expression Products (Applied Biosystems)26.
Glucose tolerance test
An intraperitoneal glucose tolerance test was performed on 6- and 12-week-old mice. The mice were fasted for 12 h and then were injected intraperitoneally (i.p.) with glucose (2.0 g/kg body weight). The blood glucose levels were measured before injection and at 5, 30, 60, 90 and 120 min after injection.
Statistics and reproducibility
Error bars indicate the standard error of at least three measurements. Student’s t tests were used to compare two samples from independent groups using Microsoft Excel. Repeated measures ANOVA was performed to compare data among groups. The differences between the groups were considered to be significant when p < 0.05.
All experiments were performed in accordance with relevant guidelines and regulations.
All methods were carried out in compliance with the ARRIVE guidelines.
Data availability
The data that support the findings of this study are available from the corresponding author, H.N., upon reasonable request.
References
Clark, A. R. & Docherty, K. How is the developmental timing and tissue-specificity of insulin gene expression controlled? J. Endocrinol. 136, 187–190 (1993).
Docherty, K. R.D. (1992) Lawrence lecture. The regulation of insulin gene expression. Diabet. Med. 9, 792–798 (1992).
Docherty, K. & Clark, A. R. Nutrient regulation of insulin gene expression. FASEB J. 8, 20–27 (1994).
Edlund, T., Walker, M. D., Barr, P. J. & Rutter, W. J. Cell-specific expression of the rat insulin gene: Evidence for role of two distinct 5′ flanking elements. Science 230, 912–916 (1985).
Walker, M. D., Edlund, T., Boulet, A. M. & Rutter, W. J. Cell-specific expression controlled by the 5′-flanking region of insulin and chymotrypsin genes. Nature 306, 557–561 (1983).
German, M. S., Moss, L. G., Wang, J. & Rutter, W. J. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol. Cell. Biol. 12, 1777–1788 (1992).
Peshavaria, M. et al. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol. Endocrinol. 8, 806–816 (1994).
Ohlsson, H., Thor, S. & Edlund, T. Novel insulin promoter- and enhancer-binding proteins that discriminate between pancreatic alpha- and beta-cells. Mol. Endocrinol. 5, 897–904 (1991).
Boam, D. S. & Docherty, K. A tissue-specific nuclear factor binds to multiple sites in the human insulin-gene enhancer. Biochem. J. 264, 233–239 (1989).
Shieh, S. Y. & Tsai, M. J. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J. Biol. Chem. 266, 16708–16714 (1991).
Karlsson, O., Edlund, T., Moss, J. B., Rutter, W. J. & Walker, M. D. A mutational analysis of the insulin gene transcription control region: Expression in beta cells is dependent on two related sequences within the enhancer. Proc. Natl. Acad. Sci. U. S. A. 84, 8819–8823 (1987).
Crowe, D. T. & Tsai, M. J. Mutagenesis of the rat insulin II 5′-flanking region defines sequences important for expression in HIT cells. Mol. Cell. Biol. 9, 1784–1789 (1989).
Boam, D. S., Clark, A. R. & Docherty, K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J. Biol. Chem. 265, 8285–8296 (1990).
German, M. et al. The insulin gene promoter. A simplified nomenclature. Diabetes 44, 1002–1004 (1995).
Hwung, Y. P., Gu, Y. Z. & Tsai, M. J. Cooperativity of sequence elements mediates tissue specificity of the rat insulin II gene. Mol. Cell. Biol. 10, 1784–1788 (1990).
Olbrot, M., Rud, J., Moss, L. G. & Sharma, A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. U. S. A. 99, 6737–6742 (2002).
Harrington, R. H. & Sharma, A. Transcription factors recognizing overlapping C1–A2 binding sites positively regulate insulin gene expression. J. Biol. Chem. 276, 104–113 (2001).
Nishimura, W., Salameh, T., Kondo, T. & Sharma, A. Regulation of insulin gene expression by overlapping DNA-binding elements. Biochem. J. 392, 181–189 (2005).
Tomonari, A., Yoshimoto, K., Mizusawa, N., Iwahana, H. & Itakura, M. Differential regulation of the human insulin gene transcription by GG1 and GG2 elements with GG- and C1-binding factors. Biochim. Biophys. Acta 1446, 233–242 (1999).
Le Lay, J., Matsuoka, T. A., Henderson, E. & Stein, R. Identification of a novel PDX-1 binding site in the human insulin gene enhancer. J. Biol. Chem. 279, 22228–22235 (2004).
Cissell, M. A., Zhao, L., Sussel, L., Henderson, E. & Stein, R. Transcription factor occupancy of the insulin gene in vivo. Evidence for direct regulation by Nkx2.2. J. Biol. Chem. 278, 751–756 (2003).
Zhang, C. et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 25, 4969–4976 (2005).
Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609 (1994).
Ahlgren, U., Jonsson, J., Jonsson, L., Simu, K. & Edlund, H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768 (1998).
Sussel, L. et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125, 2213–2221 (1998).
Noguchi, H. et al. Mutations in the C1 element of the insulin promoter lead to diabetic phenotypes in homozygous mice. Commun. Biol. 3, 309 (2020).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR–Cas9. Science 346, 1258096 (2014).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR–Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 21, 121–131 (2015).
Pickar-Oliver, A. & Gersbach, C. A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell. Biol. 20, 490–507 (2019).
Sander, J. D. & Joung, J. K. CRISPR–Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Leroux, L. et al. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes 50, S150-153 (2001).
Kirchner, V. A. et al. Long-term outcomes for living pancreas donors in the modern era. Transplantation 100, 1322–1328 (2016).
Mashiko, D. et al. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3, 3355 (2013).
Mizuno, S. et al. Simple generation of albino C57BL/6J mice with G291T mutation in the tyrosinase gene by the CRISPR/Cas9 system. Mamm. Genome 25, 327–334 (2014).
Noguchi, H., Kaneto, H., Weir, G. C. & Bonner-Weir, S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes 52, 1732–1737 (2003).
Noguchi, H. et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 10, 305–309 (2004).
Noguchi, H. et al. RCAN-11R peptide provides immunosuppression for fully mismatched islet allografts in mice. Sci. Rep. 7, 3043 (2017).
Acknowledgements
We thank Naomi Kakazu (University of the Ryukyus) for the office processing and Yuki Kawahira, Ikue Honda (University of the Ryukyus), Seiya Mizuno, Yoko Tanimoto, Satoru Takahashi, and Fumihiro Sugiyama (University of Tsukuba) for technical support. This work was supported in part by JSPS KAKENHI Grant Numbers JP20H03745, JP19K09051, and Okinawa Science and Technology Innovation System Construction Project.
Funding
This work was supported in part by JSPS KAKENHI Grant Numbers JP20H03745, JP19K09051, and Okinawa Science and Technology Innovation System Construction Project.
Author information
Authors and Affiliations
Contributions
H.N. designed the experiments and analyzed the data. H.N. carried out most of the experimental work with the help of C.M.-S. and T.K., I.S. and M.W. provided materials and participated in discussion. H.N. wrote the manuscript. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noguchi, H., Miyagi-Shiohira, C., Kinjo, T. et al. In vivo evaluation of GG2–GG1/A2 element activity in the insulin promoter region using the CRISPR–Cas9 system. Sci Rep 11, 20290 (2021). https://doi.org/10.1038/s41598-021-99808-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99808-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.