Abstract
Cognitive flexibility describes the ability of animals to alter cognitively mediated behaviour in response to changing situational demands, and can vary according to prevailing environemental conditions and individual caracteristics. In the present study, we investigated (1) how learning and reversal learning performance changes between seasons, and (2) how cognitive flexibility is related to sex in a free-living small mammal. We studied 107 African striped mice, Rhabdomys pumilio, in an arid semi-desert, 58 during the hot dry summer with low food availability, and 49 during the cold wet winter with higher food availability. We used an escape box task to test for learning and reversal learning performance. We found that learning and reversal learning efficiency varied seasonally by sex: females tested in summer were faster at solving both learning and reversal tasks than males tested in winter. Performance varied within sex: males tested in winter showed faster learning compared to males tested in summer. During reversal learning, females tested in summer were more efficient and solve the task faster compared to females tested in winter. We suggest that seasonal cognitive performance could be related to sex-specific behavioural characteristics of the species, resulting in adaptation for living in harsh environmental conditions.
Similar content being viewed by others
Introduction
Cognition enables organisms to process, use and store information gathered from their natural environment1 and contribute to reducing uncertainty about important aspects of the environment. There is growing evidence of relationships between cognitive abilities, such as learning, and the fitness benefits in free-living populations2. However, cognition is not cost-free, resulting in trade-offs in energy investment between neural or other physiological structures3. Therefore, individual variation in cognitive perrformance is expected to closely match the energy demands imposed by the prevailing environmental conditions4, an ability that might play an important role in adaptation to new environments, particularly under human-induced rapid environmental change5.
Cognitive flexibility is one way to mitigate the need for constant energy investment into cognition, yet there is currently little evidence of links between particular environmental demands and cognitive flexibility4. Cognitive flexibility can be defined as “the ability to adapt cognitively driven behaviour in response to changing situational demands”6. Because development and maintenance of cognitive processes are energy demanding7, energetic constraints in varying environments could induce reversible changes in the structure and functioning of the nervous system, both influencing cognitive performance8. However, the environmental conditions under which animals might directly reduce energy investment into cognitive processes (cognitive impairment) and the conditions under which they might keep energetic investment unchanged (cognitive resilience) or improved (cognitive enhancement) remain poorly understood8.
The “harsh environment hypothesis” emphasizes the role of variability in food availability in driving cognitive flexibility9. Several supporting examples are available in the literature. Comparisons between two distinct populations of black-capped chickadees, Poecile atricapillus, showed that birds living at higher altitude and thus experiencing longer and colder winters demonstrated heightened food-hoarding tendencies, more accurate spatial memory (i.e., collection, retention and use of information about the environment to evaluate the relationship between given locations1) and higher hippocampal neuron count10 but performed worse during a reversal learning task11. Woodpecker finches, Cactospiza pallida, from an arid, variable habitat performed a single reversal learning faster than birds from a humid, stable habitat12. Free living animals could also show adaptive cognitive performance in response to seasonal changes in available energy sources. Spatial learning abilities in the common shrew, Sorex araneus, were better in summer than winter, when individuals showed a reduction in brain size13. Thus, studying cognitive flexibility according to seasonal changes in food availaibility could help to disentangle different trade-off outcomes between energy saving and cognition8.
Reversal learning is a commonly used test of cognitive flexibility14, and consists of first training an animal to distinguish between two stimuli, usually through the use of a food incentive (reward). Once the animal is successful at discriminating between the stimuli, the outcome of the reward is reversed and the animal has to inhibit the previously learned response in order to obtain the reward1. Reversal learning is ecologically relevant, especially in unpredictable environments, when conditions change rapidly and individuals must be able to inhibit a previously learned behaviour to display a new, more appropriate response. The literature indicates that some species are better at reversal learning and hence displaying cognitive flexibility15. For example, pigeons, Columbia livia, were more flexible than goldfish, Carassius auratus, when given a color discrimination reversal learning task16. Several studies also indicate inter-individual variation in cognitive flexibility within species17 and suggest the influence of personality18 or sex19. For example, male and female guppies, Poecilia reticulata, learned equally rapidly in a foraging colour discrimination task but females were better at inhibiting the previous response in a reversal task and solved the task twice as fast as males20. The authors suggested that sex differences in cognitive flexibility could be related to different roles of males and females in mating competition, mate choice and reproductive behaviour or in social interactions even though it is not known whether differences in cognition that evolved in one context (e.g., reproductive strategies) may affect other behaviours (e.g., foraging)20. Although the evolutionary causes are still not clear, previous studies suggested that lower male flexibility and hence, greater male persistence in the same behaviour, may be selected for polygynous species as it helps males to overcome female resistance to mate20.
We investigated whether and how discriminating between two spatial stimuli (i.e., learning) and then inhibit the previously learned response (i.e., reversal learning) performance changes between seasons in a free-ranging population of the African striped mouse, Rhabdomys pumilio. In our study population, striped mice are diurnal, territorial, polygynous, facultatively group-living, with communal breeding, paternal care and helpers at the nest and mice forage alone during the day21,22. R. pumilio is an ideal model to study the influence of seasonal variation on cognitive flexibility because it inhabits semi desert areas in southern Africa where it faces marked seasonal changes in food availability23. Cognitive performance varied seasonally in striped-mice: mice tested in summer solved a new problem (a proxy of innovation14) faster compared to those tested in winter24. Furthermore, attention, spatial learning and memory and problem-solving varied seasonally by sex: in summer, males showed a faster attention toward a predator-stimulus, solved more novel problems, but made more errors and took longer in a spatial learning and memory task than males tested during winter25. In contrast, the performance of females did not differ seasonally, but their survival was correlated with faster attention to a predator stimulus whereas male survival was correlated with greater spatial memory26. Therefore, seasonal cognitive performance in striped mice could be related to sex-specific behavioural characteristics of this species, such as male biased dispersal27. Thus, the different sex roles, shaped by natural and/or sexual selection, may also result in sex-specific differences in attention, learning and/or memory, that is, in general cognitive abilities. Evaluating how cognitive flexibility changes according to environmental conditions and sex offer a unique framework to better understand how striped mice cope in challenging environments. Such an approach might also provide a better understanding of the evolutionary causes of different cognitive functions.
We studied sex differences in cognitive flexibility in response to seasonal changes in energy availability. We developed a new escape box protocol based on spatial cues without using food incentive to assess learning and reversal learning performance of free-living striped mice. Using a non-food incentive offers a better assessment of individual variation in cognition because it does not consider a feeding motivation, which is always a challenge in studies in the wild28. We tested striped mice during the summer dry and the winter wet seasons. We hypothesized that environemental energy availability (through food availability) changes between seasons will affect cognitive performance such as learning and reversal learning (“ecophysiology of cognition hypothesis”8). We predicted that: (1) learning and reversal learning performance will be heightened in summer and follows the harsh environment hypothesis described in birds; (2) seasonal variation in learning and reversal learning will be sex-specific; based on our previous research, we expected that males will show greater spatial learning performance in winter when they disperse whereas females will not differ between seasons but will show overall faster reversal learning performance as found in other species.
Results
Seasonal changes in weather, food availability and mice body condition
The weather was hot and dry during summer (temperature: 24.42 ± 0.36 °C; total rainfall: 0.60 mm) and temperatures were lower and rainfall was higher during the winter months (temperature: 13.47 ± 0.45 °C; total rainfall: 39.60 mm; LM: N = 138, F = 368.4, P < 0.001; F = 4.31, P = 0.039, respectively). Food availability for the striped mice increased within the study period from summer (2.35 ± 0.11 food plants/plot) to winter (3.27 ± 0.24 food plants/plot; LM: Nsummer plots = 48, Nwinter plots = 48, F = 12.12, P < 0.001).
Body mass and length were greater in winter than summer (body mass: summer: 41.33 ± 1.01 g; winter: 47.17 ± 1.29 g; LMM: N = 107, χ21 = 13.44, P < 0.001; Length: summer: 106.21 ± 0.95 mm; winter: 113.91 ± 1.21 mm; LMM: N = 107, χ21 = 30.41, P < 0.0001). Males were heavier than females in both seasons (LMM: N = 107, χ21 = 38.38, P < 0.0001; summer males: 44.46 ± 1.47 g; summer females: 38.09 ± 1.13 g, t-test: Nmales = 31, Nfemales = 27, t96 = − 3.33, P = 0.006; winter males: 52.20 ± 1.37 g; winter females: 40.29 ± 1.47 g; LMM: N = 44, χ21 = 31.54, t-test: Nmales = 30, Nfemales = 19, t96 = − 5.56, P < 0.001). Males were longer than females in both seasons (LMM: N = 107, χ21 = 34.55, P < 0.0001; summer: males: 109.21 ± 1.30 mm; females: 102.00 ± 0.99 mm, t-test: Nmales = 31, Nfemales = 27, t88 = − 3.62, P = 0.003; winter: males: 118.12 ± 1.42 mm; females: 108.37 ± 1.43 mm, t-test: Nmales = 30, Nfemales = 19, t96 = -− 4.71, P = 0.0001).
Seasonal changes in learning and reversal learning
Initial task acquisition
All mice (N = 107, Table 1) opened the door for 3 consecutive trials. Some mice did not succeed to open the door at their first attempt and hence needed 4 trials to open doors for 3 consecutive trials (summer: 0/58; winter: 4/49). There was no significant influence of season (GLMM, N = 107, χ21 = 0.01, P = 0.985) and sex (GLMM, N = 107, χ21 = 0.00, P = 0.999). There was no influence of the season and sex on latencies to open the doors during the 3 consecutive successful trials (LMM, N = 107, 1st trial: season: χ21 = 1.40, P = 0.235; sex: χ21 = 0.43, P = 0.509; 2nd trial: season: χ21 = 2.09, P = 0.148; sex: χ21 = 0.21, P = 0.649; 3rd trial: season: χ21 = 3.48, P = 0.062; sex: χ21 = 0.28, P = 0.597; Supplementary Information: Table 1). However, longer mice showed a greater latency to succeed during their first trial (LMM, N = 107, χ21 = 5.38, P = 0.020).
Learning task
Overall, we tested 87 of the 107 mice in the learning task since 20 mice were not re-trapped within 6 days after the initial task acquisition phase (Table 1). The learning task was achieved by all subjects within 13.48 ± 0.33 (range: 12–23) trials.
There was no significant influence of season and intrinsic (age, body mass, length) characteristics on the trials to criterion (TTC) and the overall accuracy during the learning task (Table 2). However, there was a significant interaction between season and sex for the mean latency to succeed (LMM, N = 87, χ21 = 6.67, P = 0.009). Males tested in winter showed longer latencies compared to females tested in summer (t-test : Nfemales summer = 24, Nmales winter = 22, t45 = − 3.32, P = 0.009; Nfemales summer = 24, Nfemales winter = 17, t40 = 0.63, P = 0.921; Nfemales summer = 24, Nmales summer = 24, t47 = − 1.23, P = 0.609; Nfemales winter = 17, Nmales summer = 24, t40 = − 1.10, P = 0.689; Nfemales winter = 17, Nmales winter = 22, t40 = − 1.84, P = 0.269; Nmales summer = 24, Nmales winter = 22, t45 = − 1.14, P = 0.667; Fig. 1a).
Mean latency ± SEM (in seconds) for all trials needed to reach the learning criterion by season and sex. Males tested in winter showed longer latencies both during the (a) the learning task (Nfemales = 41; Nmales = 46) and (b) the reversal learning task (Nfemales = 38; Nmales = 40). Post hoc t test with Tukey correction, *P ≤ 0.05.
Reversal learning
We tested 78 of the 87 mice in the reversal learning task since 9 mice were not re-trapped within 6 days after the learning task (Table 1). Reversal was attained by all tested subjects within 14.37 ± 0.28 (range: 12–24) trials. There was no significant influence of the season and intrinsic characteristics on the accuracy during the reversal learning task (Table 3). However, there was a significant interaction between season and sex on the trials to criterion (LMM, N = 78, χ21 = 4.02, P = 0.045). Females tested in summer tended to need fewer trials to reach the learning criterion compared to females tested in winter (t-test: Nfemales summer = 22, Nfemales winter = 16, t37 = − 2.57, P = 0.058; Nfemales summer = 22, Nmales summer = 23, t44 = − 1.15, P = 0.660; Nfemales summer = 22, Nmales winter = 17, t38 = − 0.44, P = 0.971; Nfemales winter = 16, Nmales summer = 23, t38 = 1.26, P = 0.589; Nfemales winter = 16, Nmales winter = 17, t32 = 1.54, P = 0.419; Nmales summer = 23, Nmales winter = 17, t39 = 0.49, P = 0.959). There was an influence of season on the mean latency to succeed (LMM, N = 78, χ21 = 6.67, P = 0.009). Mice tested in winter showed longer latencies compared to those tested in summer (t-test: Nsummer = 45, Nwinter = 33, t39 = − 3.27, P = 0.011; Fig. 1b).
Learning curve
Learning curves representing accuracy computed for the 12 first successive trials showed a significant effect of trial number (GLMM, N = 87, χ21 = 44.59, P < 0.001), which is indicative of learning since the accuracy increased with trial number from the 3rd trial (t-test: Trial 1 versus Trial 3 to 12, P < 0.05 for all, Fig. 2a). In addition, there was a significant interaction between season and sex on the response accuracy (GLMM, N = 87, χ21 = 4.44, P = 0.035; Fig. 2a). Males tested in summer showed slower improvement in accuracy in learning compared to males tested in winter (t-test: Nmales summer = 24, Nmales winter = 22, t45 = − 2.97, P = 0.015; Nmales summer = 24, Nfemales summer = 24, t47 = 1.89, P = 0.231; Nmales summer = 24, Nfemales winter = 17, t40 = 0.17, P = 0.998; Nfemales summer = 24, Nfemales winter = 17, t40 = − 0.12, P = 0.999; Nfemales summer = 24, Nmales winter = 22, t45 = − 1.13, P = 0.670; Nfemales winter = 17, Nmales winter = 22, t40 = 0.08, P = 0.999; Fig. 2a). For the reversal learning task, there was a significant effect of trial number (GLMM, N = 78, χ21 = 150.47, P < 0.001): accuracy increased with trials number from the 3rd trial onwards (t-test: Trial 1 versus Trial 3 to 12, P < 0.05 for all, Fig. 2b). There was a significant interaction between season and sex on the response accuracy (GLMM, N = 78, χ21 = 6.97, P = 0.008; Fig. 2b). Females tested in summer showed faster improvement in accuracy in reversal learning compared to females tested in winter (t-test: Nfemales summer = 22, N females winter = 16, t36 = 2.81, P = 0.025; Nfemales summer = 22, Nmales summer = 23, t44 = 1.86, P = 0.246; Nfemales summer = 22, Nmales winter = 17, t38 = 0.49, P = 0.961; Nfemales winter = 16, N males summer = 23, t38 = − 1.11, P = 0.685; Nfemales winter = 16, Nmales winter = 17, t32 = − 2.15, P = 0.138; Nmales summer = 23, Nmales winter = 17, t39 = − 1.16, P = 0.648; Fig. 2b).
Learning curves in (a) the learning (Nfemales = 41; Nmales = 46) and (b) the reversal learning tasks (Nfemales = 38; Nmales = 40), for the 12 first successive trials. The left panels show males and right panel females. Mice tested in summer are represented with black dots and full black lines and mice tested in winter are represented with grey triangles and gray dashed lines. Post hoc t test with Tukey correction, *P ≤ 0.05.
Speed-accuracy trade-off
For the learning task, there was a significant influence of the latency (LMM: N = 87, χ21 = 8.57, P = 0.004) and the interaction between latency and season (LMM: N = 87, χ21 = 7.16, P = 0.009), but no influence of sex (LMM: N = 87, χ21 = 1.08, P = 0.301) on accuracy: in winter, mice of both sexes which required more time to solve the task were less accurate (Spearman rank correlation, summer: rs = − 0.23, P = 0.111; winter: rs = − 0.61, P < 0.001; Fig. 3a). For the reversal learning task, there was no significant influence of the latency (LMM: N = 78, χ21 = 0.11, P = 0.741), the interaction between latency and season (LMM: N = 78, χ21 = 0.99, P = 0.321) and sex (LMM: N = 78, χ21 = 0.17, P = 0.683) on accuracy (Fig. 3b).
Influence of the season on speed accuracy trade-off measured by the relationship between mean latency (seconds) calculated from all trials during (a) the learning (N = 87) task and (b) the reversal learning tasks (N = 78); and accuracy measured as the number of correct responses divided by the total number of responses (Spearman rank correlation, ***P < 0.001).
Discussion
We showed that learning and reversal learning performance vary seasonally in a sex-dependent way in a free-living rodent population experiencing seasonal changes in food availability. Learning efficiency, measured with the trial to criterion, as well as accuracy did not differ between seasons and sex. However, males tested in winter showed longer latencies to solve the task compared to females tested in summer. Furthermore, we showed within sex differences in performance according to the season: males tested in winter showed earlier learning curve increase compared to males tested in summer. During the reversal learning task, females tested in summer showed faster reversal learning, needing fewer trials to reach the criterion and showed shorter latencies to solve the task compared to females tested in winter. Thus, in striped mice, there is an inter-relationship between intrinsic characteristics and environemental/ecological influence on cognitive abilities such as learning and reversal learning.
In contrast to previous studies on free-living animals showing increased cognitive performance under harsh conditions, striped mice learning and reversal learning efficiency did not change seasonally, even though summer in our study site was characterized by hot and dry weather with low food availability. This supports the cognitive resilience hypothesis which maintains that cognition is so essential for fitness that animals might keep this energetic investment unchanged, and energy is channelled into cognition even under harsh conditions29. Cognitive resilience could rely on specific physiological mechanisms (e.g., brain-centered gluco-regulatory system30) protecting the brain against seasonal variation in energy availability and associated changes in hormone secretion8. An alternative explanation could be the lower than expected food availability in winter during our study. In other words, cognitive resilience occurred or food availability might not have reached a sufficeint level to induce significant differences in cognitive processes at the population level but could individually influence mice with particular intrinsic (e.g., sex) characteristics.
We showed that learning and reversal learning performance vary seasonally in a sex-dependent way, both between and within the sexes according to the season. Males tested in winter required more time to solve the learning and reversal learning tasks compare to females. This sex effect is unlikely to reflect sex differences in locomotor or motor abilities since the proportion of individuals opening the doors during the initial task acquisition did not differ between males and females. Therefore, this sex difference is likely to reflect the time required for a mouse to “make a decision”. Interestingly, regardless of sex, higher speed (measured as the mean latency) was positively related to the accuracy. Although there is some debate as to whether speed of solving a task is a suitable method to test cognitive proficiency31,32, our findings indicate that the mean latency to solve the task is an important metric of cognitive abilities. It appears that striped mice do not suffer of a “speed-accuracy trade-off” where slower individuals might have greater accuracy33. Faster striped mice showed greater accuracy, an important adaptation in a prey species which needs to make quick decisions to avoid predators by choosing their route to reach the nest. Males required more time to solve the tasks in winter, indicating that they devoted more time to collect information or were more persistent i.e., time that an animal spent interacting with the task/device34 depending on the season. Males disperse in winter and information gathering might be an important adaptation during an important phase of their lives27. Alternatively, higher persistence male in winter before the breeding season could be related to the different sex-specific reproductive tactics in this polygynous species35. In sum, between sex differences in learning latency according to the season could be related to functional specific behavioural characteristics of this species, such as male biased dispersal27 and mating system35.
We did not find significant sex differences in the proportion of successful animals or in learning and reversal learning efficiency (TTC and accuracy). Generally, males of several taxa possess better spatial navigation abilities than females36,37. Sex differences in spatial abilities may be coupled with instrinsic sex-specific developmental trajectories and subsequent sex differences in reproductive behaviour38. For instance, males in some polygynous species have better spatial learning ability compared to females, possibly as a result of greater spatial demands to search for females and consequently have larger home range sizes38. In our present study, males did not outperform females in efficiency (TTC and accuracy) during the learning task based on spatial stimuli in each season. These findings could be due to seasonal similarities in activities, such as territorial defense, solitary foraging, facultatively group-living22. Furthermore, both sexes were under similar environmental pressure e.g., food availability. Thus, behavioural characteristics do not differ between sexes outside the breeding season and mice were under similar environmental challenges regardless of sex which might explain the absence of spatial learning efficiency difference between males and females.
Interestingly, we found within sex differences according to the season. The faster learning (learning curve) of males in winter compared to summer, concurs with the results of a previous study which showed improved performance within males from summer to winter in spatial learning and memory tests25. Improved spatial performance in winter may be adaptive for males that disperse and then breed in spring39. For example, spatial memory improved in dispersing male meadow voles, Microtus pennsylvaniscus, and deer mice, Peromyscus maniculatus39,40, during the breeding season relative to the non-breeding season; differences were related to structural modifications in the hippocampus41. In striped mice, male dispersal starts in winter, several weeks before reproduction in spring and males will travel distances of up to several kilometres to find new territories27. Dispersal will increase demands on spatial learning and memory processing, and thus might explain faster spatial learning improvement in winter as in our study.
During reversal learning, females tested in summer showed faster learning improvement (learning curve) compared to females tested in winter and hence required fewer trials to reach the learning criterion (TTC). This suggests that females potentially respond quicker to environemental changes under harsh conditions9. Our results also support previous results on mammals, birds and fish showing that females are more flexible than males20,42,43. Several hypothesis have been proposed to explain why females showed greater cognitive flexibility20. Males appear to pay a cost for their reduced flexibility, being less ready to modify their behaviour in response to environmental change35. Males could show a greater degree of behavioural persistence in the reversal learning task i.e., persistently executing a previously learned behaviour which will result in more errors being made when the contingencies are changed14. Such male persistence is supported by our results since males tested in winter had longer latencies to solve the reversal learning task. Thus, males might face trade-offs between the ability to learn new information and the ability to retain old memories1. In mammals and birds, sex differences in cognitive flexibility appears to be related to the influence of testosterone level on cholinergic activity in specific brain regions implicated in memory42,43. The causes for sex differences in persistence are still not clear, but an adaptive hypothesis suggests that greater male persistence is selected in polygynous species in which males search for reproductively available females44. Striped mice in our population are polygynous21 which might explain the sex differences in reversal learning in our study in parallel to higher cognitive flexibility for females under harsh conditions.
To our knowledge, this is the first study of reversal learning performance in rodents under natural conditions. However, testing two distinct groups between seasons is an important consideration to test the extent of cognitive flexibility, involving repeat sampling of the same individuals in summer and winter. However, we were mindful of testing the same individual twice since some mice could have remembered the task25. Furthermore, it was not possible to test the same individual in both seasons because free-ranging striped mice disperse in early winter45 or disappear due to predation. Other studies also reach conclusions about cognitive flexibility between distinct populations e.g., high versus low elevation10, urban versus rural area4, humid versus arid area12 and between seasons (winter versus summer25,46; breeding versus non-breeding39,40), and the same individuals were not re-sampled. Finally, it would have been interesting to perform a serial reversal task and paid attention to the improvement in performance over successive reversals47 according to the season. However, to perform a serial reversal task with free-ranging animals is challenging, it requires tracking the same individuals in space and time which is difficult with prey species showing dispersal and high predation pressure such as striped mice45,48.
In conclusion, our study showed that learning and reversal learning varies according to sex and season in a free-ranging striped mice population. Seasonal differences in cognitive performance could be related to sex-specific behavioural characteristics of the species, such as male biased dispersal27 leading to faster spatial learning in winter, and females potentially responding quicker to environemental changes in summer. Such variability in responses indicates cognitive flexibility for males in winter and for females under harsh conditions in summer. This also shows an inter-relationship between intrinsic characteristics and ecological conditions on the influence of cognitive abilities. Thus, the present study creates new areas of investigation on particular environmental demands under which animals might show cognitive impairment, resilience or enhancement and on the relationship with a potential by-product of a sexual selection on other traits. Further studies taking into account the trade-off between cognitive process and energy-demanding physiological processes and their fitness outcomes under harsh seasonal condition are required.
Materials and methods
Ethical note
Animal ethical clearance was provided by the University of the Witwatersrand, Johannesburg, South Africa (No. 2018/09/46B). All procedures were in accordance with the ethical standards of the institution or practice at which the studies were conducted. We took care in ensuring the animals welfare throughout the experimental procedures and thereafter. The study was carried out in compliance with the ARRIVE guidelines.
Study location, period and animals
We conducted our study in Goegap Nature Reserve, South Africa (S 29 41.56, E 18 1.60) during the 2019 dry summer and wet winter seasons. We measured food availability49 twice a month and temperature and rainfall daily. Our free-living striped mice population was monitored continuously using behavioural observations, trapping and radiotracking22. Mice were trapped using baited metal live traps (26 × 9 × 9 cm) placed under bushes where striped mice were nesting. Trapping sessions were conducted each morning (5 days a week) within the first hour after sunrise. Traps were checked 30 and 60 min after they were set. Evening trapping sessions were conducted 5 days a week within 30 min after sunset22. During trapping, mice were sexed and body mass (± 1 g) and length (± 1 mm, from tip of the nose to the anus) were measured. Mice were permanently marked with numbered metal ear tags (National Band and Tag Co., Newport, USA) and with commercial hair dye (Rapido, Pinetown, South Africa) for visual identification.
Striped mice feed on all parts (leaves, flowers and seeds) of annual and periannual plants. We assessed food availability through plant surveys using the Braun-Blanquet method50 twice a month on the 1st and the 15th of each month of the year. We recorded the number of food plant species for striped mice as a measure of food availability (palatability is known from behavioural observations51), in eight monitoring plots of 4 m2 each that were located within the home ranges of the different social groups52.
We assessed learning and reversal learning performance using a novel escape box with a door opening task, in which a test individual could reach its nest as the incentive. We tested 58 mice (27 females and 31 males, originating from 13 different social groups) in summer and 49 other mice (19 females and 30 males, originating from 16 groups including the same 13 groups as in summer) in winter. In order to avoid any carry over effects because of learning, none of the individuals were tested in both seasons. Furthermore, it was not possible to test the same individual in both seasons because striped mice disperse in early winter45 or disappear due to predation (e.g., 22 of 55 mice (40%) tested in summer either dispersed or were dead at the beginning of winter).
Learning and reversal learning tasks
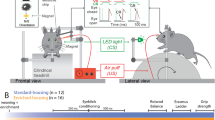
Mice were tested directly at their nest in the field in a sequential learning—reversal learning task in which they had to choose between opening a left or right door to reach their nests (Fig. 4). Using a non-food incentive does not test a feeding motivation28. After being trapped directly at their nests in both the morning and afternoon (usual trapping sessions), mice were placed by an experimenter (CR) in a black testing box (40 × 30 × 35 cm) equipped with 2 black doors, situated 17 cm apart on the same side of the box and equipped with a transparent perpex lid to prevent mice jumping out of the box (Electronic Supplementary Material). The box was bottomless in order to let mice experience the natural surface. The door system was designed for the mouse to escape and return to its nest (i.e., the incentive) by pushing the door with its head and/or fore paws. The doors were hinged, so that even a light mouse e.g., 25 g, could easily open it. Doors were shorter than the bottom of the box wall, allowing for a strip of external light entering the box and indicating their position (Fig. 4). The escape box was located at 50 cm in front of the nest, with the doors facing the nest. The subject was video recorded using a go pro camera (AEE LYFE, S91B) mounted on the top of the box. Subjects were given a maximum of 5 min to complete the task whereafter those that did not complete the task were released53.
Graphical representation of part of the experimental protocol and device. The mouse has to open one door to escape from the box and return back to its nest. In this example, the mouse chooses the left side (at least 2 left trials out of 3) in the initial task acquisition phase and is then allowed to access the left side (preferred side) during the learning phase whereas the right side door is locked from outside (non-preferred side). After reaching the learning criterion of a total of 10 consecutive correct trials out of 12 during the learning phase, the mouse starts the reversal learning in which it can exit on the right side (non-preferred side) and prevented from exiting the left side (closed door from outside). The mouse had to reach the learning criterion (10 correct responses out of 12 consecutives trials).
Each time a mouse was trapped, its identity was checked by the experimenter and it was placed into the escape box. Each mouse was first tested for 3 times with both doors open and could swing freely (i.e., initial task acquisition47) and the door (right or left) it selected 2/3 times was assigned as its preferred one47. Once a mouse ended the initial task acquisition (100% of tested mice), it was subjected to the learning task at the next trapping session, where the preferred side was kept open (‘door open’ to reach the nest) and the non-preferred side was locked shut from the outside (‘door closed’ from outside to prevent visual cues of the lock, Fig. 4). An individual was considered to have succeeded at the learning task when it reached the learning criterion of selecting its preferred door at least 10 out of 12 consecutive trials (10/12 correct represents a significant deviation from random binomial probability). A mouse could have as much trials as possible to reach the learning criterion. A trial was defined as each time a mouse was trapped and placed into the apparatus. A mouse was tested only once per trapping session because the incentive was to return to the nest.
During the trapping session after the completion of the learning task, the reversal learning test began where the incentive switched spatially. The initial preferred side was locked shut (‘door closed’) and the previous non-preferred side was rewarded (‘door opened’). Mice had to reach the same learning criterion of at least 10 out of 12 consecutive correct choice trials. Again, a mouse could have as much trials as possible until it reached the 10 correct consecutive trials criterion. If a mouse was not re-trapped within 6 days, it was excluded from the experiment.
Data collection
We assessed initial task acquisition performance by recording the number of trials needed to open one door for 3 consecutive trials and the latency of each trial as the time required to open the door from the time the mouse was in the escape box. For each experimental task (learning and reversal learning tasks), we assessed the accuracy of each trial, by measuring if the response was correct, scored as 1, or incorrect, scored as 0. Response accuracy was computed in sequence for the 12 first trials (regardless of the total number of trials required to attain the 10 consecutive criterion) to assess whether accuracy improved in time47 and was used to obtain learning curves. Next, we calculated the trials to criterion (TTC) as the total number of trials (sum of correct and incorrect responses) needed to reach the learning criterion47. We computed the accuracy for all trials as the number of correct responses divided by the total number of responses. We also measured the latency to open the door for each trial and calculated the mean latency in all trials. The TTC and accuracy are measures of performance efficiency and mean latency is a measure of speed efficiency i.e., how fast the mouse took to solve the tasks.
Statistical analyses
The video-recordings of the tests were scored using the software Kinovea (Kinovea 0.8.15). Scoring was done by the same observer (C.R.) and also by one field assistant who was naïve to the experimental treatment (10% of test sessions, Spearman correlation rank test, Nmice = 12, rs = 0.85). All statistics were performed with R v. 3.6.154. The significance level was set at 0.05. Descriptive statistics are reported as means and standard error of mean (SEM). Independence and homogeneity of variances of the models were assessed by inspection of the residuals of the fitted values using the plotresid function in RVAideMemoire package55 and by reporting conditional R2 using r.squaredGLMM function of the MuMln package56. Mixed models were constructed using the lm/lmer or glm/glmer function in lme4 package57 and statistical tests were performed using the Anova function in car package58. Statistical tests (Likelihood Ratio Test) were performed with loglink function for Gaussian and Poisson distribution and logitlink function for Binomial distribution58. Post hoc tests were performed using the emmeans function in the emmeans package, along with the contrast pairwise comparision function (emmeans, “pairwise”) using t-test with a Tukey correction59. We log transformed latencies prior to analysis to achieve improved normality of the model residuals. We checked for outliers and found that the data from some individuals were outliers in one variable and for others in another variable.We maintain that analyses of such experiment should include individual data points as a measure of individual ability and variation, and should not exclude outliers because these account for the population-level variation60.
The seasonal changes in weather and food availability were analysed using several linear models (LM) with daily temperature, daily rainfall and bi-monthly food availability measurements as the dependent variables and season as the fixed effect. The seasonal changes in body condition were analysed by using different linear mixed models (LMM) with body mass and length as the dependent variables and season, sex and the interaction between season and sex as fixed effects. We specified group identity as a random factor to account for potential confounding effects of group origin (litter and/or ecology).
When analysing the predictors of initial task training, season, sex, the interaction between season and sex to test sex-specific seasonal variation, age, group size and body condition (body mass and length) were included as fixed effects. We specified group identity as a random factor. We first performed a generalized linear mixed model (GLMM) for Poisson family distribution with the total number of trials needed to successfully open one door for 3 consecutive trials as the dependent variables We performed linear mixed models for the 1st, 2nd and 3rd latencies of successful door opening as the dependent variables.
The influence of season on the learning and reversal learning tasks was analysed using a separate linear mixed model for each task with (i) the trial to criterion (TTC), (ii) the accuracy, or (iii) the mean latency to open the doors as the dependent variables and season, sex, the interaction between season and sex, age, group size and body condition as fixed effect and group ID as a random factor. We analysed predictors of learning curve by fitting GLMMs for Binomial family distribution either for learning accuracy or reversal learning accuracy as dependent variables and trial number, season, sex, the interaction between trial number, season and sex, and age, group size and body condition as fixed effect and mice ID as a random factor.
We analysed the seasonal influence on the speed-accuracy trade-off by testing whether mean latency to open the doors during the learning and reversal learning tasks predicted individual accuracy. We fitted LMMs for each task with the accuracy as a dependent variable and the mean latency, season, sex and the interaction between latency, season and sex as fixed effect and mice ID as a random factor. Post-hoc test were performed with Spearman rank correlation test.
For all previous models, nonsignificant predictors were excluded based on stepwise backward model selection using log-likelihood ratio tests and the Akaike’s Information Criterion (AIC) comparison (‘lrtest’, ‘lmtest’ package61).
References
Shettleworth, S. J. Cognition, Evolution and Behavior 2nd edn. (Springer, 2010).
Morand-Ferron, J. Why learn? The adaptive value of associative learning in wild populations. Curr. Opin. Behav. Sci. 16, 73–79 (2017).
Mery, F. & Kawecki, T. J. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 270, 2465–2469 (2003).
Morand-Ferron, J., Hermer, E., Jones, T. B. & Thompson, M. J. Environmental variability, the value of information, and learning in winter residents. Anim. Behav. 147, 137–145 (2019).
Chevin, L. M. & Hoffmann, A. A. Evolution of phenotypic plasticity in extreme environments. Philos. Trans. R. Soc. B. 372, 20160138 (2017).
Klanker, M., Feenstra, M. & Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 7, 201 (2013).
Laughlin, S. B. Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol. 11, 475–480 (2001).
Maille, A. & Schradin, C. Ecophysiology of cognition: How do environmentally induced changes in physiology affect cognitive performance?. Biol. Rev. 92, 1101–1112 (2016).
Pravosudov, V. V. & Clayton, N. S. A test of the adaptive specialization hypothesis: Population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522 (2002).
Pravosudov, V. V., Roth, T. C. I., LaDage, L. D. & Freas, C. A. Environmental influences on spatial memory and the hippocampus in food-caching chickadees. Comp. Cogn. Behav. Rev. 10, 25–43 (2015).
Croston, R. et al. Predictably harsh environment is associated with reduced cognitive flexibility in wild food-caching mountain chickadees. Anim. Behav. 123, 139–149 (2017).
Tebbich, S. & Teschke, I. Coping with uncertainty: Woodpecker finches (Cactospiza pallida) from an unpredictable habitat are more flexible than birds from a stable habitat. PLoS ONE 9, e91718 (2014).
Lázaro, J. et al. Cognitive skills of common shrews (Sorex araneus) vary with seasonal changes in skull size and brain mass. J. Exp. Biol. https://doi.org/10.1242/jeb.166595 (2017).
Audet, J.-N. & Lefebvre, L. What’s flexible in behavioral flexibility?. Behav. Ecol. 28, 943–947 (2017).
Tello-Ramos, M. C. et al. Memory in wild mountain chickadees from different elevations: Comparing first-year birds with older survivors. Anim. Behav. 137, 149–160 (2018).
Gonzalez, R. C., Behrend, E. R. E. & Bitterman, M. E. Reversal learning and forgetting in bird and fish. Science 158, 519–521 (1967).
Tello-Ramos, M. C., Branch, C. L., Kozlovsky, D. Y., Pitera, A. M. & Pravosudov, V. V. Spatial memory and cognitive flexibility trade-offs: To be or not to be flexible, that is the question. Anim. Behav. 147, 129–136 (2019).
Mazza, V., Eccard, J. A., Zaccaroni, M., Jacob, J. & Dammhahn, M. The fast and the flexible: Cognitive style drives individual variation in cognition in a small mammal. Anim. Behav. 137, 119–132 (2018).
Branch, C. L. et al. Testing the greater male variability phenomenon: Male mountain chickadees exhibit larger variation in reversal learning performance compared with females: Sex differences in reversal learning. Proc. R. Soc. B Biol. Sci. 287, 13–16 (2020).
Lucon-Xiccato, T. & Bisazza, A. Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol. Lett. 10, 20140604 (2014).
Schubert, M., Pillay, N. & Schradin, C. Parental and alloparental care in a polygynous mammal. J. Mammal. 90, 724–731 (2009).
Schradin, C. & Pillay, N. The striped mouse (Rhabdomys pumilio) from the Succulent Karoo, South Africa: A territorial group-living solitary forager with communal breeding and helpers at the nest. J. Comp. Psychol. 118, 37–47 (2004).
Schradin, C. et al. Social flexibility and social evolution in mammals: A case study of the African striped mouse (Rhabdomys pumilio). Mol. Ecol. 21, 541–553 (2012).
Rochais, C., Schradin, C. & Pillay, N. Seasonal changes in problem-solving in wild African striped mice. Review.
Maille, A., Pillay, N. & Schradin, C. Seasonal variation in attention and spatial performance in a wild population of the African striped mouse (Rhabdomys pumilio). Anim. Cogn. 18, 1231–1242 (2015).
Maille, A. & Schradin, C. Survival is linked with reaction time and spatial memory in African striped mice. Biol. Lett. 12, 20160346 (2016).
Solmsen, N., Johannesen, J. & Schradin, C. Highly asymmetric fine-scale genetic structure between sexes of African striped mice and indication for condition dependent alternative male dispersal tactics. Mol. Ecol. 20, 1624–1634 (2011).
Cauchard, L., Boogert, N. J., Lefebvre, L., Dubois, F. & Doligez, B. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim. Behav. 85, 19–26 (2013).
Buchanan, K. L., Grindstaff, J. L. & Pravosudov, V. V. Condition dependence, developmental plasticity, and cognition: Implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296 (2013).
Schwartz, M. W. et al. Cooperation between brain and islet in glucose homeostasisand diabetes. Nature 503, 59–66 (2013).
Griffin, A. S., Guillette, L. M. & Healy, S. D. Cognition and personality: An analysis of an emerging field. Trends Ecol. Evol. 30, 207–2014. https://doi.org/10.1016/j.tree.2015.01.012 (2015).
Rowe, C. & Healy, S. D. Measuring variation in cognition. Behav. Ecol 25, 1287–1292 (2014).
Sih, A. & Del Giudice, M. Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philos. Trans. R. Soc. B 367, 2762–2772 (2012).
Griffin, A. S. & Guez, D. Innovation and problem solving: A review of common mechanisms. Behav. Process. 109, 121–134 (2014).
Lucon-Xiccato, T. & Bisazza, A. Male and female guppies differ in speed but not in accuracy in visual discrimination learning. Anim. Cogn. 19, 733–744 (2016).
Healy, S. D., Bacon, I. E., Haggis, O., Harris, A. P. & Kelley, L. A. Explanations for variation in cognitive ability: Behavioural ecology meets comparative cognition. Behav. Process. 80, 288–294 (2009).
Jones, C. M. & Healy, S. D. Differences in cue use and spatial memory in men and women. Proc. R. Soc. B 273, 2241–2247 (2006).
Jones, C. M., Braithwaite, V. A. & Healy, S. D. The evolution of sex differences in spatial ability. Behav. Neurosci. 117, 403–411 (2003).
Galea, L. A. M., Kavaliers, M. & Ossenkopp, K. P. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J. Exp. Biol. 199, 195–200 (1996).
Galea, L. A., Kavaliers, M., Ossenkopp, K. P., Innes, D. & Hargreaves, E. L. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 635, 18–26 (1994).
Galea, L. A. M. & McEwen, B. S. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89, 955–964 (1999).
Rogers, L. J. Persistence and search influenced by natural levels of androgens in young and adult chickens. Physiol. Behav. 12, 197–204 (1974).
Guillamón, A., Valencia, A., Calés, J. & Segovia, S. Effects of early postnatal gonadal steroids on the successive conditional discrimination reversal learning in the rat. Physiol. Behav. 38, 845–849 (1986).
Rowe, L., Cameron, E. & Day, T. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 165, S5-18 (2005).
Vuarin, P., Pillay, N. & Schradin, C. Elevated basal corticosterone levels increase disappearance risk of light but not heavy individuals in a long-term monitored rodent population. Horm. Behav. 113, 95–102 (2019).
Rochais, C., Maille, A., Jäger, J., Pillay, N. & Schradin, C. How does cognitive performance change in relation to seasonal and experimental changes in blood glucose levels?. Anim. Behav. 158, 149–159 (2019).
Cauchoix, M., Hermer, E., Chaine, A. S. & Morand-Ferron, J. Cognition in the field: Comparison of reversal learning performance in captive and wild passerines. Sci. Rep. 7, 1–10 (2017).
Lonnstedt, O. M., Mccormick, M. I., Meekan, M. G., Ferrari, M. C. O. & Chivers, D. P. Learn and live : Predator experience and feeding history determines prey behaviour and survival. Proc. R. Soc. B 279, 2091–2098 (2012).
Schradin, C. & Pillay, N. Demography of the striped mouse (Rhabdomys pumilio) in the succulent karoo. Mamm. Biol. 70, 84–92 (2005).
Wikum, D. A. & Shanholtzer, G. F. Application of the Braun–Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manage. 2, 323–329 (1978).
Schradin, C. Whole-day follows of striped mice [Rhabdomys pumilio], a diurnal murid rodent. J. Ethol. 24, 37–43 (2006).
Schradin, C. & Pillay, N. Intraspecific variation in the spatial and social organization of the African striped mouse. J. Mammal. 86, 99–107 (2005).
Rochais, C., Pillay, N. & Schradin, C. Do alternative reproductive tactics predict problem-solving performance in African striped mice?. Anim. Cogn. https://doi.org/10.1007/s10071-020-01459-z (2021).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020) https://www.R-project.org/.
Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9–73. (2019).
Barton, K. Mu-MIn: Multi-model inference. R Package Version 0.12.2/r18. http://R-Forge.R-project.org/projects/mumin/ (2009).
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–10 (2015).
Fox, J. & Weisberg, S. An R Complanion to Applied Regression 3rd edn. (Sage, 2019).
Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.2. https://cran.r-project.org/package=emmeans (2019).
Rowell, M. K., Pillay, N. & Rymer, T. L. Problem solving in animals: Proposal for an ontogenetic perspective. Animals 11, 1–21 (2021).
Hothorn, T. et al. Package ‘lmtest’ (Version 0.9-37). https://CRAN.R-project.org/Package. (2019).
Acknowledgements
This study was made possible by the administrative and technical support of the Succulent Karoo Research Station (registered South African NPO 122-134). We thank C. Schradin for his help on designing the study. We thank J. Anver, N. Den hartog, L. Kotze, G. Lemonnier, J. Sommer, R. Weil and L. Wolhuter for assistance in the field. We are grateful L. Kotze for the blind video analyses. We also thank two anonymous referees for comments that greatly improved the manuscript. This research was supported by a fellowship (to C.R.) by the University of the Witwatersrand and the National Research Fundation (South Africa).
Author information
Authors and Affiliations
Contributions
C.R. collected and analysed the data. C.R., and N.P. designed the study and drafted the article. H.H. designed and built the device. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rochais, C., Hotte, H. & Pillay, N. Seasonal variation in reversal learning reveals greater female cognitive flexibility in African striped mice. Sci Rep 11, 20061 (2021). https://doi.org/10.1038/s41598-021-99619-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99619-9
This article is cited by
-
Cognitive flexibility in urban yellow mongooses, Cynictis penicillata
Animal Cognition (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.