Abstract
Biomarkers of exposure to harmful tobacco constituents are key tools for identifying individuals at risk and developing interventions and tobacco control measures. However, tobacco biomarker studies are scarce in many parts of the world with high prevalence of tobacco use. Our goal was to establish a robust method for simultaneous analysis of urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), N′-nitrosonornicotine (NNN), and cotinine at the Advanced Centre for Treatment, Research and Education in Cancer (ACTREC) in Mumbai, India. These biomarkers are validated measures of exposure to the carcinogenic tobacco nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and NNN and the addictive alkaloid nicotine, respectively. The established method is characterized by excellent accuracy, linearity, and precision, and was successfully applied to the analysis of 15 smokeless tobacco (SLT) users and 15 non-users of tobacco recruited in Mumbai. This is the first report of establishment of such procedure in a laboratory in India, which offers the first in-country capacity for research on tobacco carcinogenesis in Indian SLT users.
Similar content being viewed by others
Introduction
Tobacco use remains the leading preventable cause of morbidity and mortality globally, accounting for 8 million annual deaths worldwide1. While steady declines in smoking prevalence have been registered over the last decades in some high-income countries, the prevalence of tobacco use in many parts of the world remains unchanged or even increasing. For example, use of smokeless tobacco (SLT) products remains high in India and other Southeast Asian countries2,3,4, and smoking is on the rise in several countries with low-income economies that became targets for cigarette advertising by transnational tobacco companies5.
Research employing biomarkers of toxic and carcinogenic tobacco constituents can provide important insights for identifying individuals at risk and developing interventions and tobacco control measures. Biomarkers of nicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and N′-nitrosonornicotine (NNN) are of particular importance because these constituents are specific to tobacco and play important roles in tobacco-associated health outcomes. Nicotine is the major known addictive constituent in tobacco, and its biomarker cotinine has been historically used as a measure of tobacco product consumption6,7. NNK and NNN are potent organ-specific carcinogens in laboratory animals: NNK causes lung cancer independent of the route of administration, and NNN causes cancers of oral cavity and esophagus2,8,9. In tobacco users, exposure to NNK can be measured via its biomarker urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and exposure to NNN is measured via urinary total NNN10,11,12. Epidemiological studies in smokers showed that levels of urinary total NNAL and NNN are prospectively associated with the risk for developing lung cancer13,14 and esophageal cancer15,16, respectively. In the United States, studies assessing cotinine, total NNAL, and total NNN in tobacco users generated important evidence to support tobacco control measures such as clean air laws and the proposal by the Food and Drug Administration to regulate levels of NNN in SLT products to protect public health17. However, because of the lack of expertise and resources, tobacco biomarker studies are scarce in parts of the world that could benefit the most from such research.
The overarching goal of our research efforts is to develop capacity for tobacco biomarker research in India. Here we report a method for simultaneous analysis of cotinine, NNAL and NNN in human urine, which was optimized at the Advanced Centre for Treatment, Research and Education in Cancer (ACTREC) in Mumbai, India. Previously reported methods measured either a single biomarker or a combination of two biomarkers18,19,20,21,22. In our ongoing and future research efforts in India, we aim to analyze all three biomarkers in large numbers of urine samples in order to characterize the links between the chemical composition of SLT and other tobacco products and the exposures and health outcomes in users. The ability to analyze all three biomarkers in one assay is essential for accelerating such research. Furthermore, simultaneous analysis of these three important tobacco biomarkers offers a cost-effective solution for conducting tobacco control research in low-resource settings.
Results
Development and optimization of the assay
The liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for the analysis of NNAL, NNN, and cotinine in the purified urine samples was essentially based on the previously reported methods21,22,23,24,25, except that a non-capillary system and a QTRAP instrument were used in this study. Given the higher flow rate used in this system, we optimized the HPLC mobile phase gradient to allow for chromatographic resolution of analytes from the potential peaks of residual interfering metabolites that may be present in the complex urine matrix. Representative chromatograms obtained upon LC–MS/MS analysis of a standard mix containing NNAL, NNN, and cotinine and corresponding labeled internal standards [13C6]NNAL, 13C6NNN, and [D3]cotinine are shown in Fig. 1A.
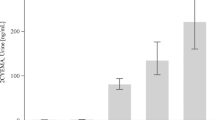
The method reported here also includes a newly developed sample purification procedure to allow for simultaneous extraction and enrichment of all three biomarkers, which distinguishes it from the previously published assays for urinary total NNAL, NNN, and cotinine19,20,21,22. The characteristics of the method are summarized in Table 1. Accuracy of the assay (added vs. measured) in pooled non-tobacco user urine averaged 110.9% for total NNAL, 89.2% for total NNN, and 107.2% for cotinine. Precision (coefficient of variation, CV) of measurements at the lowest levels of addition were 4.2% for NNAL, 13.8% for NNN, and 2.6% for cotinine (Supplemental Table S1). Linearity of the method in the assessed range of concentrations is illustrated in Fig. 2.
The performance of the method was assessed by using the positive control urine data (pooled smokers’ urine) that is being routinely used as a quality control for tobacco biomarker analyses in Dr. Stepanov’s laboratory at University of Minnesota. The levels of NNAL, NNN, and cotinine measured in the positive control urine by the developed method were 1.3 (± 0.01) pmol/mL, 0.055 (± 0.1) pmol/mL, and 20 (± 1.8) nmol/mL, respectively. These levels were comparable with the values measured in the same positive control stock in Dr. Stepanov’s laboratory: 1.4 (± 0.20) pmol/mL, 0.052 (± 0.005) pmol/mL, and 22.5 (± 2.1) nmol/mL, respectively. Intra- and inter-day variations for biomarker measurement in these samples, as analyzed by the developed method, are shown in Table 1.
Analysis of urine samples from SLT users and non-users of tobacco products
The method was applied to the analysis of 30 urine samples: 15 SLT users and 15 non-users of tobacco. Water blanks, which were used as negative controls, did not contain detectable levels of any of the biomarkers. Typical chromatograms obtained upon analysis of urine samples are illustrated in Fig. 1B,C. Levels of urinary total NNAL, NNN and cotinine in all subjects are summarized in Table 2. The limit of quantitation (LOQ, determined at signal-to noise ratio of 5) for total NNAL was 0.05 pmol/mL, for total NNN 0.01 pmol/mL, and for cotinine 0.05 nmol/mL. All SLT users had quantifiable total NNAL, NNN and cotinine in their urine. Levels of urinary total NNAL, NNN, and cotinine in these subjects averaged 3.67 (± 5.8) pmol/mL, 0.18 (± 0.3) pmol/mL, and 14.33 (± 13.9) nmol/mL, respectively. In non-users of tobacco, total cotinine was present in urine of 12 out of 15 subjects, ranging from LOQ to 0.07 nmol/mL, while total NNAL and total NNN were non-detectable.
Discussion
Analysis of tobacco biomarkers is an important tool in studies of risks associated with tobacco use. We present here the development of the procedure for the analysis of urinary total NNAL, NNN, and cotinine, to achieve simultaneous measurement of these important biomarkers in a resource-efficient way. This is the first report of establishment of such procedure in a laboratory in Mumbai, India, which offers the first in-country capacity for research on tobacco carcinogenesis in Indian SLT users. While the majority of published studies on the analysis of urinary total NNAL and NNN used low-flow capillary HPLC coupled with triple-quadrupole mass-spectrometry detectors21,22,23,24,25, the assay reported here utilizes a non-capillary, low-flow HPLC and AB Sciex QTRAP system. Such analytical equipment is widely used for robust analyses of drug metabolites in pharmacokinetics studies worldwide26,27 and therefore is more likely to be available in countries where the procurement of dedicated mass-spectrometry equipment for tobacco biomarker research may not be feasible due to limited resources.
Our reported method leverages the previously developed approach that takes advantage of the presence of 13C-cotinine in urine and monitors the mass-spectrometry signal for its [M + H]+ ion with m/z 178.1, which accounts for only 11% of the 12C-cotinine [M + H]+ signal21. Because levels of cotinine in urine of tobacco users are several thousand-fold higher than those of total NNAL and total NNN, such reduction in the cotinine signal is key to avoiding the ion source or detector saturation and the resulting challenges in simultaneous accurate quantitation of all three biomarkers28. In addition, to achieve simultaneous purification of NNAL and cotinine with NNN, it was necessary to increase the strength of the elution solvent during the normal-phase extraction, which is an important step in sample purification for urinary total NNN analysis20,23,25. Addition of 10% MeOH allowed for removal of more polar NNAL and cotinine from the Silica Bond Elut cartridges together with NNN, which normally can be removed with 100% EtOAc. We note that a combined assay of urinary NNAL and NNN with the use of normal-phase solid phase extraction has been previously proposed22. However, this is the first report of the method for simultaneous analysis of all three biomarkers.
We used ascorbic acid to prevent artefactual formation of NNN via nitrosation of nornicotine which is present in urine of tobacco users23,29,30. Urine also contains nitrates and nitrites, and sample processing exposes samples to nitrogen oxides in the air, all of which can produce nitrosating species capable of reacting with nornicotine to form NNN30,31. Ascorbic acid acts as a scavenger of such nitrosating species, and we previously used its addition to prevent nornicotine nitrosation and artefactual NNN formation during sample processing23,32,33. Similar approach with ammonium sulfamate as the inhibitor of nitrosation was also used in the published method for combined NNAL and NNN measurement22. Our method is different in that we add ascorbic acid prior to purification of urine samples by MCX, instead of after such purification. This assures that NNN formation does not take place when sample is acidified prior to loading on MCX cartidges31, and preserves the purity of samples after the desalting that occurs during MCX purification. We previously employed the addition of [pyridine-D4]nornicotine to biological samples and subsequent monitoring of [pyridine-D4]NNN to demonstrate that ascorbic acid, as used here, prevents artefactual NNN formation23.
The optimized method is characterized by excellent accuracy, linearity, and precision (Table 1, Fig. 2). We note that method accuracy for NNN was somewhat lower than for other biomarkers (Fig. 1C). This is potentially due to lower levels of this biomarker compared to other two, and higher background noise resulting from interfering polar compounds present in the residual sample matrix. The original method for separate analysis of total NNN does not add 10% MeOH to the elution solvent at the last step of normal-phase extraction, which facilitates the removal of interfering polar metabolites, reduces the background noise, and improves the accuracy of NNN assay. However, the accuracy for NNN in the combined procedure reported here is still in the acceptable range for analytical assays (80–120% per U.S. FDA guidelines for bioanalytical method validation).
We applied the developed method to the analysis of a subset of urine samples that are being collected as part of our on-going research on tobacco carcinogenesis in Mumbai (Table 2). In the urine of SLT users, levels of NNAL and NNN were comparable to, and cotinine levels were lower than, those reported for smokers and SLT users in the US20,21,24,34. It is important to note that 10 out of 15 SLT users reported using gutkha and betel quid—SLT products in India that contain only small amounts of tobacco mixed with other ingredients35,36. Many other popular SLT products in India contain extremely high levels of NNN, NNK, and nicotine37,38, and exposures in users of such products are expected to be significantly higher than in smokers. Efforts are underway to analyze urine samples collected from users of various SLT products. In non-users of tobacco, only cotinine was present at trace levels, while total NNAL and total NNN were below LOD (Table 2). Exposures to secondhand cigarette smoke or to environmental residues of smokeless tobacco could be responsible for the presence of cotinine in the urine of non-users of tobacco products. The trace levels of cotinine in some non-users are likely due to exposures to secondhand cigarette smoke (SHS) or to environmental residues of SLT. It is important to note that SHS exposure is also a source of exposure to NNK and NNN; however, levels of these constituents in tobacco products are much lower than those of nicotine. Indeed, previous studies of SHS exposure in adults report a range of 0.33 × 103–1.04 × 103 for the ratio of urinary total NNAL to cotinine, with some individuals having no detectable NNAL even if cotinine is present39. Lastly, due to low prevalence of smoking, the frequency of exposure to SHS is expected to be low in India. Future studies aimed at characterizing SHS or other environmental exposures to NNK and NNN in non-tobacco users in India should use larger starting volumes of urine to assure accurate quantitation of respective urinary biomarkers.
In summary, we report here a robust and resource-effective method for simultaneous analysis of three important tobacco-specific biomarkers in human urine. The method has been established in the analytical laboratory at ACTREC in Mumbai, India, creating a vital resource for conducting tobacco carcinogenesis research in this country. Future efforts are warranted to adapt this method to 96-well format to facilitate tobacco biomarker measurements in large epidemiological studies. Such research will serve to support tobacco control efforts in India and potentially in other countries in Southeast Asia.
Methods
Caution
NNAL and NNN are carcinogenic and mutagenic and should be handled with extreme care, using appropriate protective clothing and ventilation at all times.
Materials and reagents
Analytical standards for NNAL, NNN, cotinine and their respective isotope-labeled analogues [13C6]NNAL, 13C6NNN, and [D3]cotinine were obtained from Toronto Research Chemicals, Inc. (Canada). β-Glucuronidase (Type IX from E. coli) was purchased from Sigma Aldrich Chemicals (Bangalore, India). Diatomaceous Earth Chem Elut (5 mL unbuffered) cartridges and Bond Elut 1 cc (100 mg, LRC-SI) cartridges were purchased from Agilent Technologies (Bangalore, India). Oasis MCX 1 cc (30 mg) extraction cartridges were obtained from Waters (Bangalore, India). CH2Cl2, EtOAc, MeOH, and other sample preparation reagents were purchased either from Sigma Aldrich, Fisher scientific or Sisco Research Laboratories (Mumbai, India).
Urine sample collection
Single-void urine samples used in this study were collected as part of an ongoing research that aims to characterize tobacco exposures and cancer risk in SLT users in Mumbai, India. Non-users of any tobacco products were recruited among staff workers at the ACTREC. Users of SLT were recruited among healthy individuals (friends and relatives) who accompanied patients visiting Head and Neck Cancer Clinic at Tata Memorial Hospital in Mumbai. For analyses reported here, we selected urine samples from those SLT users who reported regular use of any SLT product for more than 5 years, were current users (within the last 24 h), reported not smoking a combustible product such as cigarettes or bidis, and reported being in generally good health. Urine was collected by a standard “clean-catch” technique into 30-mL collection cups, placed in a portable cooler with ice-packs until same-day delivery to the laboratory at ACTREC, and stored in the laboratory at − 20 °C until analyses.
Subject recruitment and sample collection were approved by the Institutional Ethics Committee of ACTREC, Tata Memorial Centre (study number 900095). The study was carried out in accordance with the Declaration of Helsinki and International Conference on Harmonization-Good Clinical Practice guidelines. All participants were adults over 18 years of age, and provided informed consent.
Sample preparation
Sample preparation procedure included 4 key steps: (1) treatment with β-glucuronidase to cleave free NNAL, NNN, and cotinine from their glucuronides; (2) supported liquid extraction on Chem Elut cartridges; (3) solid-phase extraction by mixed phase cation exchange on Oasis MCX cartridges; and (4) solid-phase extraction by normal-phase Bond Elut Silica cartridges. Steps (1)–(3) were performed essentially as previously described, with minor modifications21,22,23,25. Briefly, 2 mL of urine sample (thawed at room temperature) was mixed with 1.5 mL 30 mM KH2PO4 buffer (pH 7), a mixture of isotope-labeled internal standards was added (100 pg of 13C6-NNN, 200 pg of 13C6-NNAL and 200 ng of [D3]cotinine per sample), and samples were treated with 15,000 units β-glucuronidase overnight at 37 °C. Next day, samples were purified on Chem Elut cartridges, with the eluants (2 × 8 mL of CH2Cl2) being collected into tubes containing 20 µL of ascorbic acid (1.2 µmol/µL) to prevent artefactual formation of NNN22,23,30. The eluants were dried (SpeedVac vacuum concentrator, Thermo Scientific) reconstituted in 500 µL water, and acidified with 50 µL of 1 N HCl. Ascorbic acid was added again, and samples were loaded on 30 mg Oasis MCX SPE cartridges and purified as described21,25. Additional detail can be found in Supplemental Figure S1. In step (4), the normal-phase extraction procedure that is used in sample purification for NNN analysis23,25 was modified so that NNAL and cotinine can be eluted together with NNN. Samples dried after the MCX purification were re-dissolved in 500 µL of CH2Cl2, loaded on 100 mg Bond Elut cartridges pre-equilibrated with 1 mL CH2Cl2, and the cartridges were then washed with 1 mL CH2Cl2 and 1 mL CH2Cl2:EtOAc (50:50) sequentially, with the eluants going to waste. The analytes were then eluted with 3 mL EtOAc:MeOH (90:10). The collected eluants were dried and transferred to glass LC microinsert vials with two 100-µL volumes of ACN. The transferred samples were dried and stored at − 20 °C until analysis. At the time of analysis samples were re-dissolved 25 µL deionized H2O and 10 µL were injected into the LC–MS/MS system.
Biomarker analysis by LC–MS/MS
LC–MS/MS analysis was performed on a Shimadzu Nexera X2 ultra performance liquid chromatography system (Japan) coupled with an AB Sciex QTRAP-4500 (USA) system comprising of Turbo V™ source with ESI probe. Chromatographic separation was achieved on a Zorbax SB C18, 1.8 µ, 3.0 × 150 mm (Agilent Technologies) column eluted in gradient mode with a flow rate of 0.4 mL/min. Column temperature was maintained at 40 °C. 15 mM ammonium acetate (A) and 100% MeOH (B) were used as mobile phase for elution. Gradient was maintained at 5% B for initial three minutes, which was gradually increased to 70% B from 3 to 10 min. Composition of B was then decreased to 40% from 10 to 12 min and maintained at 40% for next three minutes. Column was re-equilibrated by returning to 5% B for 5 min before next run.
MS/MS detection was conducted by electrospray ionization in the positive ion mode using multiple reaction monitoring (MRM). Ion source parameters were optimized and applied as follows: mass spectrometer ion spray voltage of 5500 V; source temperature 500 °C; nebulizer gas and heater gas were set at 50 psi and curtain gas at 40 psi. Collision energy of 32 V was applied for cotinine analysis and 14.5 V for NNAL and NNN. Resolution of the quadrupoles (Q1 and Q3) was set at unit mass. MRM transitions monitored for quantitative analysis are listed in Table 3. To overcome the wide differences in typical urinary concentration ranges between cotinine and the other two biomarkers, we monitored 13C cotinine, which naturally occurs in urine, as previously described21.
Method characterization
Accuracy of the assay was determined by adding different amounts of NNAL, NNN, and cotinine to pooled urine from several non-tobacco users and performing the assay as described above. The levels of added biomarkers were in the range and at ratios typically reported for tobacco users:21,22,24 NNAL was added at 0.1, 0.5, 2.4, 4.8 and 23.9 pmol/mL urine, NNN was added at 0.06, 0.3, 1.4, 2.8 and 5.6 pmol/mL urine, and cotinine at 0.6, 5.6, 28.2, 56.5 and 84.7 nmol/mL urine. At each level, five replicates were analyzed and precision was assessed as % CV. Intra-day and inter-day variation were determined by analyzing positive control urine samples (pooled smokers’ urine with no biomarkers added to it) on two separate days. Calculations of the S/N ratios for the determination of LOQ and in the analyses of urine samples from SLT users were performed in analyst software version 1.7.1.
Analyses of urine samples from SLT users
Urine samples from SLT users were analyzed by following the purification and LC–MS/MS analysis procedures described above. Quantitation of biomarkers was based on the analyte-to-internal standard ratio, using calibration curves constructed with variable levels of each biomarker (in the range of values reported in Table 1) and constant levels of the corresponding internal standards: 10 pg/µL for 13C6-NNAL and 13C6-NNN and 10 ng/µL for [D3]cotinine. Because of the complexity and inter-individual variation of urine matrix, S/N ratios were monitored to ensure that the values are above 5 (the LOQ parameter) for the reported values.
References
World Health Organization. Tobacco Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/tobacco (2020).
International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco-Specific Nitrosamines IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol 89 (International Agency for Research on Cancer, 2007).
National Cancer Institute and Centers for Disease Control and Prevention. Smokeless Tobacco and Public Health: A Global Perspective (U.S. Department of Health and Human Services, National Cancer Institute and Centers for Disease Control and Prevention, 2014).
The Ministry of Health and Family Welfare (MoHFW) and Tata Institute of Social Sciences (TISS). Global Adult Tobacco Survey 2 India Report 2016–2017.
Gilmore, A. B., Fooks, G., Drope, J., Bialous, S. A. & Jackson, R. R. Exposing and addressing tobacco industry conduct in low-income and middle-income countries. Lancet 385, 1029–1043. https://doi.org/10.1016/S0140-6736(15)60312-9 (2015).
Benowitz, N. L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev 18, 188–204 (1996).
Benowitz, N. L., Hukkanen, J. & Jacob, P. III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_2 (2009).
Hecht, S. S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 11, 559–603 (1998).
Hecht, S. S. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis 23, 907–922 (2002).
Hecht, S. S., Stepanov, I. & Carmella, S. G. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 49, 106–114. https://doi.org/10.1021/acs.accounts.5b00472 (2016).
Stepanov, I. et al. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol. Biomark. Prev. 17, 1764–1773 (2008).
Stepanov, I. et al. Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 69, 8236–8240 (2009).
Yuan, J. M. et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 69, 2990–2995 (2009).
Yuan, J. M., Butler, L. M., Stepanov, I. & Hecht, S. S. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 74, 401–411 (2014).
Yuan, J. M. et al. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32, 1366–1371 (2011).
Stepanov, I. et al. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: Remarkable coherence with rat tumor sites. Int. J. Cancer 134, 2278–2283. https://doi.org/10.1002/ijc.28575 (2014).
Food and Drug Administration, HHS. Federal Register 82 FR 8004, January 23, 2017. Tobacco Product Standard for N-Nitrosonornicotine Level in Finished Smokeless Tobacco Products. https://www.federalregister.gov/d/2017-01030 (2017).
Dunlop, A. J., Clunie, I., Stephen, D. W. & Allison, J. J. Determination of cotinine by LC-MS-MS with automated solid-phase extraction. J. Chromatogr. Sci. 52, 351–356. https://doi.org/10.1093/chromsci/bmt038 (2014).
Carmella, S. G., Han, S., Fristad, A., Yang, Y. & Hecht, S. S. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol. Biomark. Prev. 12, 1257–1261 (2003).
Stepanov, I. & Hecht, S. S. Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol. Biomark.s Prev. 14, 885–891 (2005).
Kotandeniya, D., Carmella, S. G., Ming, X., Murphy, S. E. & Hecht, S. S. Combined analysis of the tobacco metabolites cotinine and 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine. Anal. Chem. 87, 1514–1517 (2015).
Kotandeniya, D., Carmella, S. G., Pillsbury, M. E. & Hecht, S. S. Combined analysis of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the urine of cigarette smokers and e-cigarette users. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1007, 121–126. https://doi.org/10.1016/j.jchromb.2015.10.012 (2015).
Knezevich, A., Muzic, J., Hatsukami, D. K., Hecht, S. S. & Stepanov, I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine Tob. Res. 15, 591–595 (2012).
Hatsukami, D. K. et al. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev. Res. (Phila) 8, 20–26. https://doi.org/10.1158/1940-6207.CAPR-14-0250 (2015).
Bustamante, G. et al. Presence of the carcinogen N’-nitrosonornicotine in saliva of e-cigarette users. Chem. Res. Toxicol. 31, 731–738. https://doi.org/10.1021/acs.chemrestox.8b00089 (2018).
Heffernan, A. L. et al. Rapid, automated online SPE-LC-QTRAP-MS/MS method for the simultaneous analysis of 14 phthalate metabolites and 5 bisphenol analogues in human urine. Talanta 151, 224–233. https://doi.org/10.1016/j.talanta.2016.01.037 (2016).
Dai, T. et al. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed. Chromatogr. 33, e4573. https://doi.org/10.1002/bmc.4573 (2019).
Staeheli, S. N., Poetzsch, M., Kraemer, T. & Steuer, A. E. Development and validation of a dynamic range-extended LC-MS/MS multi-analyte method for 11 different postmortem matrices for redistribution studies applying solvent calibration and additional 13C isotope monitoring. Anal. Bioanal. Chem. 407, 8681–8712. https://doi.org/10.1007/s00216-015-9023-5 (2015).
Hukkanen, J., Jacob, P. III. & Benowitz, N. L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 57, 79–115 (2005).
Mirvish, S. S., Sams, J. & Hecht, S. S. Kinetics of nornicotine and anabasine nitrosation in relation to N’-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. JNCI 59, 1211–1213 (1977).
Mirvish, S. S. Formation of N-nitroso compounds: Chemistry, kinetics, and in vivo occurrence. Toxicol. Appl. Pharmacol. 31, 325–351 (1975).
Mirvish, S. S., Wallcave, L., Eagen, M. & Shubik, P. Ascorbate-nitrite reaction: Possible means of blocking the formation of carcinogenic N-nitroso compounds. Science 177, 65–67 (1972).
Mirvish, S. S. Effects of vitamins C and E on N-nitroso compound formation, carcinogenesis, and cancer. Cancer 58, 1842–1850 (1986).
Hecht, S. S. et al. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol. Biomark. Prev. 16, 1567–1572 (2007).
Bhisey, R. A. Chemistry and toxicology of smokeless tobacco. Indian J. Cancer 49, 364–372. https://doi.org/10.4103/0019-509X.107735 (2012).
Gupta, P. C. & Ray, C. S. Smokeless tobacco and health in India and South Asia. Respirology 8, 419–431 (2003).
Stepanov, I. et al. Constituent variations in smokeless tobacco purchased in Mumbai, India. Tob. Regul. Sci. 3, 305–314 (2017).
Stepanov, I., Hecht, S. S., Ramakrishnan, S. & Gupta, P. C. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Intl. J. Cancer 116, 16–19 (2005).
Vogel, R. I., Carmella, S. G., Stepanov, I., Hatsukami, D. K. & Hecht, S. S. The ratio of a urinary tobacco-specific lung carcinogen metabolite to cotinine is significantly higher in passive than in active smokers. Biomarkers 16(6), 491–497 (2011).
Acknowledgements
We would like to thank Dr. Vipin Jain and Anshu Jain (members of Dr. Stepanov laboratory at the University of Minnesota at the time of the study) for technical support. We also thank Robert Carlson for editorial assistance. The work reported here was supported by funds from the University of Minnesota’s Center for Global Health and Social Responsibility (Khariwala), FIC/NIH Grant R01-TW010651 (Khariwala/Stepanov/Chaturvedi, MPIs), and in part by R01-CA180880 (Stepanov). LC-MS/MS analysis was supported in part by XII Plan project funding from the Department of Atomic Energy, Government of India. The content is solely the responsibility of the authors and does not represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
P.C., S.S.K., and I.S. collaborated on the study design and project development. I.S. and V.G. collaborated on method development and overseeing laboratory procedures. S.S.N., M.G., and A.P. performed laboratory work on method development and optimization. S.S.N. completed all sample processing and analyses. H.S. and A.S. performed sample collection. S.N.N., V.G., and I.S. wrote the main manuscript text. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nikam, S.S., Gurjar, M., Singhavi, H. et al. Simultaneous analysis of urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, N′-nitrosonornicotine, and cotinine by liquid chromatography-tandem mass-spectrometry. Sci Rep 11, 20007 (2021). https://doi.org/10.1038/s41598-021-99259-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99259-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.