Abstract
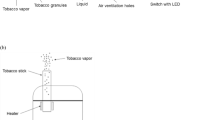
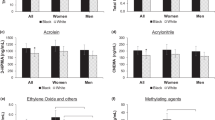
Tobacco products present a deadly combination of nicotine addiction and carcinogen exposure resulting in millions of cancer deaths per year worldwide. A plethora of smokeless tobacco products lead to unacceptable exposure to multiple carcinogens, including the tobacco-specific nitrosamine N′-nitrosonornicotine, a likely cause of the commonly occurring oral cavity cancers observed particularly in South-East Asian countries. Cigarettes continue to deliver a large number of carcinogens, including tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons and volatile organic compounds. The multiple carcinogens in cigarette smoke are responsible for the complex mutations observed in critical cancer genes. The exposure of smokeless tobacco users and smokers to carcinogens and toxicants can now be monitored by urinary and DNA adduct biomarkers that may be able to identify those individuals at highest risk of cancer so that effective cancer prevention interventions can be initiated. Regulation of the levels of carcinogens, toxicants and nicotine in tobacco products and evidence-based tobacco control efforts are now recognized as established pathways to preventing tobacco related cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hecht, S. S. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nat. Rev. Cancer 3, 733–744 (2003). This is the forerunner to the present Review; although there are similarities, the field has advanced considerably.
US Department of Health and Human Services. The Health Consequences of Smoking — 50 Years of Progress. A Report of the Surgeon General (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014). This report summarizes the health effects of smoking on the basis of 50 years of research.
Sinha, D., Agarwal, N. & Gupta, P. Prevalence of smokeless tobacco use and number of users in 121 countries. Br. J. Med. Med. Res. 9, 1–20 (2015).
National Cancer Institute & Centers for Disease Control and Prevention. Smokeless Tobacco and Public Health: A Global Perspective. NIH publication no. 14-7983 (US Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health, National Cancer Institute, 2014). This report summarizes information on worldwide smokeless tobacco products and their health effects.
International Agency for Research on Cancer. Personal habits and indoor combustions. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 100E. (IARC, 2012).
National Cancer Institute. Smokeless tobacco and cancer https://www.cancer.gov/about-cancer/causes-prevention/risk/tobacco/smokeless-fact-sheet (2010).
American Cancer Society. Health risks of smokeless tobacco https://www.cancer.org/healthy/stay-away-from-tobacco/health-risks-of-tobacco/smokeless-tobacco.html (2020).
Khan, Z., Tonnies, J. & Muller, S. Smokeless tobacco and oral cancer in South Asia: a systematic review with meta-analysis. J. Cancer Epidemiol. 2014, 394696 (2014).
Warnakulasuriya, S. & Straif, K. Carcinogenicity of smokeless tobacco: evidence from studies in humans & experimental animals. Indian J. Med. Res. 148, 681–686 (2018).
Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral. Oncol. 45, 309–316 (2009).
Sinha, D. N. et al. Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: systematic review and meta-analysis. Tob. Control. 27, 35–42 (2018). This is a systematic review and meta-analysis of studies investigating the association between smokeless tobacco use and all-cause mortality.
Siddiqi, K. et al. Global burden of disease due to smokeless tobacco consumption in adults: an updated analysis of data from 127 countries. BMC Med. 18, 222 (2020).
International Agency for Research on Cancer. Smokeless tobacco and some tobacco-specific N-nitrosamines, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 89 41–583 (IARC, 2007).
GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389, 1885–1906 (2017). This article provides critical data on smoking and disease burden worldwide.
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21660 (2021).
US Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General (Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010).
Duong, M. et al. Effects of bidi smoking on all-cause mortality and cardiorespiratory outcomes in men from south Asia: an observational community-based substudy of the Prospective Urban Rural Epidemiology Study (PURE). Lancet Glob. Health 5, e168–e176 (2017).
Bhatnagar, A. et al. Water pipe (hookah) smoking and cardiovascular disease risk: a scientific statement from the American Heart Association. Circulation 139, e917–e936 (2019).
Lawler, T. S., Stanfill, S. B., Zhang, L., Ashley, D. L. & Watson, C. H. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem. Toxicol. 57, 380–386 (2013).
Edwards, S. H. et al. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of U.S. commercial cigarettes. Chem. Res. Toxicol. 30, 540–551 (2017).
Stanfill, S. B. et al. Chemical characterization of smokeless tobacco products from South Asia: nicotine, unprotonated nicotine, tobacco-specific N′-nitrosamines, and flavor compounds. Food Chem. Toxicol. 118, 626–634 (2018).
Edwards, S. H. et al. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of commercial little cigars. Chem. Res. Toxicol. 34, 1034–1045 (2021).
Hecht, S. S. & Hoffmann, D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 9, 875–884 (1988).
Hecht, S. S., Stepanov, I. & Carmella, S. G. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 49, 106–114 (2016).
Stepanov, I. et al. Evidence for endogenous formation of N′-nitrosonornicotine in some long term nicotine patch users. Nicotine Tob. Res. 11, 99–105 (2009).
Knezevich, A., Muzic, J., Hatsukami, D. K., Hecht, S. S. & Stepanov, I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N′-nitrosonornicotine in humans. Nicotine Tob. Res. 15, 591–595 (2013).
Ding, Y. S. et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol. Biomarkers Prev. 17, 3366–3371 (2008).
Benowitz, N. L. et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res. 22, 1086–1097 (2020).
Gupta, A. K., Tulsyan, S., Bharadwaj, M. & Mehrotra, R. Grass roots approach to control levels of carcinogenic nitrosamines, NNN and NNK in smokeless tobacco products. Food Chem. Toxicol. 124, 359–366 (2019).
Kumar, A. et al. Regulation of toxic contents of smokeless tobacco products. Indian. J. Med. Res. 148, 14–24 (2018).
Stepanov, I. et al. High levels of tobacco-specific N-nitrosamines and nicotine in Chaini Khaini, a product marketed as snus. Tob. Control. 24, e271–e274 (2015).
Nasrin, S., Chen, G., Watson, C. J. W. & Lazarus, P. Comparison of tobacco-specific nitrosamine levels in smokeless tobacco products: High levels in products from Bangladesh. PLoS ONE 15, e0233111 (2020).
Lawler, T. S. et al. Chemical analysis of snus products from the United States and northern Europe. PLoS ONE 15, e0227837 (2020).
Hatsukami, D. K. et al. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev. Res. 8, 20–26 (2015).
Oldham, M. J. et al. Variability of TSNA in U.S. tobacco and moist smokeless tobacco products. Toxicol. Rep. 7, 752–758 (2020).
US Surgeon General. The Health Consequences of Smoking: Nicotine Addiction. DHHS Publication (CDC) 88-8406 (US Department of Health and Human Services, US Government Printing Office, 1988).
Hatsukami, D., Stead, L. F. & Gupta, P. C. Tobacco addiction. Lancet 371, 2027–2038 (2008).
Hoffmann, D., Raineri, R., Hecht, S. S., Maronpot, R. & Wynder, E. L. Effects of N′-nitrosonornicotine and N′-nitrosoanabasine in rats. J. Natl Cancer Inst. 55, 977–981 (1975).
Balbo, S. et al. (S)-N′-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis 34, 2178–2183 (2013). This study demonstrates the oral carcinogenesis of (S)-NNN in rats.
Schuller, H. M. Nitrosamines as nicotinic receptor ligands. Life Sci. 80, 2274–2280 (2007).
Hukkanen, J., Jacob, P. III & Benowitz, N. L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 57, 79–115 (2005).
Murphy, S. E. Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem. 296, 100722 (2021). This is a current review of nicotine metabolism.
Castagnoli, N., Rimoldi, J., Bloomquist, J. & Castagnoli, K. Potential metabolic bioactivation pathways involving cyclic tertiary amines and azaarenes. Chem. Res. Toxicol. 10, 924–940 (1997).
Wong, H. L., Murphy, S. E. & Hecht, S. S. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N′-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol. 18, 61–69 (2004).
Zarth, A. T., Upadhyaya, P., Yang, J. & Hecht, S. S. DNA adduct formation from metabolic 5′-hydroxylation of the tobacco-specific carcinogen N′-nitrosonornicotine in human enzyme systems and in rats. Chem. Res. Toxicol. 29, 380–389 (2016).
Zhao, L. et al. Quantitation of pyridyloxobutyl-DNA adducts in tissues of rats treated chronically with (R)- or (S)-N′-nitrosonornicotine (NNN) in a carcinogenicity study. Chem. Res. Toxicol. 26, 1526–1535 (2013).
Carmella, S. G., McIntee, E. J., Chen, M. & Hecht, S. S. Enantiomeric composition of N′-nitrosonornicotine and N′-nitrosoanatabine in tobacco. Carcinogenesis 21, 839–843 (2000).
Stepanov, I., Yershova, K., Carmella, S., Upadhyaya, P. & Hecht, S. S. Levels of (S)-N′-nitrosonornicotine in U.S. tobacco products. Nicotine Tob. Res. 15, 1305–1310 (2013).
Haussmann, H. J. & Fariss, M. W. Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Crit. Rev. Toxicol. 46, 701–734 (2016).
Song, M. A. et al. Chemical and toxicological characteristics of conventional and low-TSNA moist snuff tobacco products. Toxicol. Lett. 245, 68–77 (2016).
Arain, S. S. et al. Scalp hair and blood cadmium levels in association with chewing gutkha, mainpuri, and snuff, among patients with oral cancer in Pakistan. J. Oral. Pathol. Med. 44, 707–713 (2015).
Hecht, S. S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 11, 559–603 (1998).
Ge, G. Z., Xu, T. R. & Chen, C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 47, 477–487 (2015).
Peterson, L. A. Context matters: contribution of specific DNA adducts to the genotoxic properties of the tobacco-specific nitrosamine NNK. Chem. Res. Toxicol. 30, 420–433 (2017). This article provides an overview of the consequences of DNA damage by NNK.
Ma, B., Stepanov, I. & Hecht, S. S. Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics 7, 16 (2019).
Belinsky, S. A., Foley, J. F., White, C. M., Anderson, M. W. & Maronpot, R. R. Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 50, 3772–3780 (1990).
Balbo, S. et al. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis 35, 2798–2806 (2014).
Du, H., Leng, J., Wang, P., Li, L. & Wang, Y. Impact of tobacco-specific nitrosamine-derived DNA adducts on the efficiency and fidelity of DNA replication in human cells. J. Biol. Chem. 293, 11100–11108 (2018).
Hoffmann, D., Hecht, S. S., Ornaf, R. M. & Wynder, E. L. N′-Nitrosonornicotine in tobacco. Science 186, 265–267 (1974). This study first identifies NNN in tobacco.
Hecht, S. S. et al. Reaction of nicotine and sodium nitrite: formation of nitrosamines and fragmentation of the pyrrolidine ring. J. Org. Chem. 43, 72–76 (1978). This study first demonstrates the formation of NNK from nicotine.
Hecht, S. S. et al. Tobacco-specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J. Natl Cancer Inst. 60, 819–824 (1978).
Bhutani, P., Murray, M. T., Sommer, C. W., Wilson, K. A. & Wetmore, S. D. Structural rationalization for the nonmutagenic and mutagenic bypass of the tobacco-derived O4-4-(3-pyridyl)-4-oxobut-1-yl-thymine lesion by human polymerase eta: a multiscale computational study. Chem. Res. Toxicol. 34, 1619–1629 (2021).
Hu, S. C. et al. Toxicokinetic and genotoxicity study of NNK in male Sprague-Dawley rats following nose-only inhalation exposure, intraperitoneal injection, and oral gavage. Toxicol. Sci. 182, 10–28 (2021).
Peterson, L. A. et al. Coexposure to inhaled aldehydes or carbon dioxide enhances the carcinogenic properties of the tobacco-specific nitrosamine 4-methylnitrosamino-1-(3-pyridyl)-1-butanone in the A/J mouse lung. Chem. Res. Toxicol. 34, 723–732 (2021).
Snook, M. E., Severson, R. F., Arrendale, R. F., Higman, H. C. & Chortyk, O. T. Multi-alkyated polynuclear aromatic hydrocarbons of tobacco smoke: separation and identification. Beiträge Tabakforsch 9, 222–247 (1978).
Rodgman, A. & Perfetti, T. The Chemical Components of Tobacco and Tobacco Smoke. 1483–1784 (CRC Press, 2009).
International Agency for Research on Cancer. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 92 (IARC, 2010).
Snook, M. E., Severson, R. F., Arrendale, R. F., Higman, H. C. & Chortyk, O. T. The identification of high molecular weight polynuclear aromatic hydrocarbons in a biologically active fraction of cigarette smoke condensate. Beitr. Tab. Int. 9, 79–101 (1977).
International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 83 (IARC, 2004).
Basu, A. K. DNA damage, mutagenesis and cancer. Int. J. Mol. Sci. 19, 970 (2018).
Delaney, J. C. & Essigmann, J. M. Biological properties of single chemical-DNA adducts: a twenty year perspective. Chem. Res. Toxicol. 21, 232–252 (2008).
Geacintov, N. E. & Broyde, S. Repair-resistant DNA lesions. Chem. Res. Toxicol. 30, 1517–1548 (2017). This is a current review of repair-resistant DNA lesions.
Leemans, C. R., Snijders, P. J. F. & Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282 (2018).
Phillips, D. H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 23, 1979–2004 (2002).
Phillips, D. H. & Venitt, S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int. J. Cancer 131, 2733–2753 (2012).
Boysen, G. & Hecht, S. S. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat. Res. 543, 17–30 (2003).
Hecht, S. S. Oral cell DNA adducts as potential biomarkers for lung cancer susceptibility in cigarette smokers. Chem. Res. Toxicol. 30, 367–375 (2017).
Khariwala, S. S. et al. High level of tobacco carcinogen-derived DNA damage in oral cells is an independent predictor of oral/head and neck cancer risk in smokers. Cancer Prev. Res. 10, 507–513 (2017).
Villalta, P. W., Hochalter, J. B. & Hecht, S. S. Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal. Chem. 89, 12735–12742 (2017).
Jokipii Krueger, C. C. et al. Urinary N7-(1-hydroxy-3-buten-2-yl) guanine adducts in humans: temporal stability and association with smoking. Mutagenesis 35, 19–26 (2020).
Chung, F. L., Young, R. & Hecht, S. S. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 44, 990–995 (1984).
Minko, I. G. et al. Chemistry and biology of DNA containing 1,N2-deoxyguanosine adducts of the α,ß-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem. Res. Toxicol. 22, 759–778 (2009).
Paiano, V. et al. Quantitative liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of acrolein-DNA adducts and etheno-DNA adducts in oral cells from cigarette smokers and nonsmokers. Chem. Res. Toxicol. 33, 2197–2207 (2020). This study demonstrates high levels of DNA adducts in oral cell DNA of smokers.
Yang, J., Balbo, S., Villalta, P. W. & Hecht, S. S. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung DNA from smokers and nonsmokers. Chem. Res. Toxicol. 32, 318–325 (2019).
Zhang, S., Balbo, S., Wang, M. & Hecht, S. S. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem. Res. Toxicol. 24, 119–124 (2011).
Alexandrov, L. B. et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622 (2016). This study identifies mutational signatures associated with smoking in human cancer.
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020).
Campbell, J. D. et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 48, 607–616 (2016).
The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582 (2015).
India Project Team of the International Cancer Genome Consortium. Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat. Commun. 4, 2873 (2013).
Upadhyay, P. et al. Genomic characterization of tobacco/nut chewing HPV-negative early stage tongue tumors identify MMP10 asa candidate to predict metastases. Oral Oncol. 73, 56–64 (2017).
Al-Hebshi, N. N. et al. Exome sequencing of oral squamous cell carcinoma in users of Arabian snuff reveals novel candidates for driver genes. Int. J. Cancer 139, 363–372 (2016).
Li, Y. & Hecht, S. S. Identification of an N′-nitrosonornicotine-specific deoxyadenosine adduct in rat liver and lung DNA. Chem. Res. Toxicol. 34, 992–1003 (2021).
Li, Y., Carlson, E. S., Zarth, A. T., Upadhyaya, P. & Hecht, S. S. Investigation of 2′-deoxyadenosine-derived adducts specifically formed in rat liver and lung DNA by N′-nitrosonornicotine metabolism. Chem. Res. Toxicol. 34, 1004–1015 (2021).
Benowitz, N. L., St Helen, G., Nardone, N., Cox, L. S. & Jacob, P. Urine metabolites for estimating daily intake of nicotine from cigarette smoking. Nicotine Tob. Res. 22, 288–292 (2020).
Murphy, S. E. et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 35, 2526–2533 (2014).
Murphy, S. E. et al. Tobacco biomarkers and genetic/epigenetic analysis to investigate ethnic/racial differences in lung cancer risk among smokers. NPJ Precis. Oncol. 2, 17 (2018). This is a review of tobacco biomarkers and genetic and epigenetic analyses of ethnic differences in lung cancer in smokers.
Yuan, J. M. et al. CYP2A6 genetic polymorphisms and biomarkers of tobacco smoke constituents in relation to risk of lung cancer in the Singapore Chinese Health Study. Carcinogenesis 38, 411–418 (2017).
Park, S.-L. et al. Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risk of lung cancer and effect on their smoking behavior. Carcinogenesis 37, 269–279 (2016).
Xia, B. et al. Tobacco-specific nitrosamines (NNAL, NNN, NAT, and NAB) exposures in the US Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Nicotine Tob. Res. 23, 573–583 (2020). This study reports current data on tobacco-specific nitrosamine biomarkers in US tobacco users.
Rostron, B. L., Chang, C. M., van Bemmel, D. M., Xia, Y. & Blount, B. C. Nicotine and toxicant exposure among U.S. smokeless tobacco users: results from 1999 to 2012 National Health and Nutrition Examination Survey data. Cancer Epidemiol. Biomarkers Prev. 24, 1829–1837 (2015).
Park, S. L. et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol. Biomarkers Prev. 24, 561–569 (2015).
Jain, R. B. Contributions of dietary, demographic, disease, lifestyle and other factors in explaining variabilities in concentrations of selected monohydroxylated polycyclic aromatic hydrocarbons in urine: data for US children, adolescents, and adults. Environ. Pollut. 266, 115178 (2020).
Conney, A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 42, 4875–4917 (1982).
Zhong, Y., Carmella, S. G., Hochalter, J. B., Balbo, S. & Hecht, S. S. Analysis of r-, t-8,9, c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem. Res. Toxicol. 24, 73–80 (2011).
Hochalter, J. B., Zhong, Y., Han, S., Carmella, S. G. & Hecht, S. S. Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem. Res. Toxicol. 24, 262–268 (2011).
Welch, R. M., Harrison, Y. E., Conney, A. H., Poppers, P. J. & Finster, M. Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science 160, 541–542 (1968).
Luo, K. et al. Cigarette smoking enhances the metabolic activation of the polycyclic aromatic hydrocarbon phenanthrene in humans. Carcinogenesis 42, 570–577 (2020).
Yuan, J. M. et al. Genetic determinants of cytochrome P450 2A6 and biomarkers of tobacco smoke exposure in relation to risk of lung cancer development in the Shanghai Cohort Study. Int. J. Cancer 138, 2161–2171 (2015).
Yuan, J. M., Butler, L. M., Stepanov, I. & Hecht, S. S. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 74, 401–411 (2014).
Yuan, J. M. et al. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis 33, 804–809 (2012).
Yuan, J. M. et al. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2α to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis 39, 948–954 (2018).
Hecht, S. S. Tobacco smoke carcinogens and lung cancer. in Chemical Carcinogenesis (ed. Penning, T. M.) 53–74 (Springer, 2011).
Hecht, S. S. Tobacco smoke carcinogens and lung cancer. J. Natl Cancer Inst. 91, 1194–1210 (1999).
Abati, S., Bramati, C., Bondi, S., Lissoni, A. & Trimarchi, M. Oral cancer and precancer: a narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph17249160 (2020).
Hoffman, R. M., Atallah, R. P., Struble, R. D. & Badgett, R. G. Lung cancer screening with low-dose CT: a meta-analysis. J. Gen. Intern. Med. 35, 3015–3025 (2020).
Selph, S. et al. Primary care-relevant interventions for tobacco and nicotine use prevention and cessation in children and adolescents: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 323, 1599–1608 (2020).
World Health Organization. Guidelines for Implementation of Article 5.3 of the WHO Framework Convention on Tobacco Control https://www.who.int/fctc/guidelines/article_5_3.pdf (2008).
Berman, M. L. & Hatsukami, D. K. Reducing tobacco-related harm: FDA’s proposed product standard for smokeless tobacco. Tob. Control. 27, 352–354 (2018). This article discusses the FDA’s regulatory approaches to smokeless tobacco harm reduction.
Swedish Match. GOTHIATEK limits for undesired components https://www.swedishmatch.com/Snus-and-health/GOTHIATEK/GOTHIATEK-standard/ (2016).
Stepanov, I. & Hatsukami, D. Call to establish constituent standards for smokeless tobacco products. Tob. Reg. Sci. 2, 9–30 (2016).
Wyss, A. B. et al. Smokeless tobacco use and the risk of head and neck cancer: pooled analysis of US studies in the INHANCE consortium. Am. J. Epidemiol. 184, 703–716 (2016).
World Health Organization. WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Third Report of a WHO Study Group. WHO Technical Report Series (World Health Organization, 2009).
US Food and Drug Administration. Tobacco product standard for N-nitrosonornicotine level in finished smokeless tobacco products. Fed. Regist. 82, 8004–8053 (2017).
World Health Organization. Smokeless tobacco products: research needs and regulatory recommendations. in Report on the Scientific Basis of Tobacco Product Regulation: Fifth Report of the WHO Study Group. WHO Technical Report Series 989 Ch. 2 17–30 (World Health Organization, 2015).
World Health Organization. WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a WHO Study Group. WHO Technical Report Series Vol. 1015 (World Health Organization, 2019).
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. N. Engl. J. Med. 331, 123–125 (1994).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377, 1111–1114 (2017).
Hatsukami, D. K. et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA 320, 880–891 (2018). This randomized clinical trial demonstrates the effects of immediate reduction in nicotine content of cigarettes on biomarkers of smoke exposure.
Hammond, D. & O’Connor, R. J. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol. Biomarkers Prev. 23, 2032–2040 (2014).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373, 1340–1349 (2015).
Cassidy, R. N. et al. Age moderates smokers’ subjective response to very-low nicotine content cigarettes: evidence from a randomized controlled trial. Nicotine Tob. Res. 21, 962–969 (2019).
Higgins, S. T. et al. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 randomized clinical trials. JAMA Netw. Open 3, e2019311 (2020).
Tidey, J. W. et al. Effects of 6-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob. Res. 21, S38–S45 (2019).
Pacek, L. R. et al. Evaluation of a reduced nicotine product standard: moderating effects of and impact on cannabis use. Drug Alcohol. Depend. 167, 228–232 (2016).
Dermody, S. S. et al. The impact of smoking very low nicotine content cigarettes on alcohol use. Alcohol. Clin. Exp. Res. 40, 606–615 (2016).
Shiffman, S., Kurland, B. F., Scholl, S. M. & Mao, J. M. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: a randomized double-blind clinical trial. JAMA Psychiatry 75, 995–1002 (2018).
Shiffman, S., Scholl, S. M. & Mao, J. M. Very-low-nicotine-content cigarettes and dependence among non-daily smokers. Drug Alcohol. Depend. 197, 1–7 (2019).
World Health Organization. WHO study group on tobacco product regulation: global nicotine reduction strategy. in WHO Technical Report Series 1015 (World Health Organization, 2015). This article explains the WHO’s approach to global nicotine reduction in tobacco products.
Wayne, G. F., Donny, E. & Ribisl, K. M. A global nicotine reduction strategy: state of the science in WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a Who Study Group WHO Technical Report Series Ch. 4 75–110 (World Health Organization, 2019).
Apelberg, B. J. et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N. Engl. J. Med. 378, 1725–1733 (2018).
US Food and Drug Administration. Tobacco product standard for nicotine level of combusted cigarettes. Fed. Regist. 83, 11818–11843 (2018).
Glynn, T. J., Hays, J. T. & Kemper, K. E-cigarettes, harm reduction, and tobacco control: a path forward? Mayo Clin. Proc. https://doi.org/10.1016/j.mayocp.2020.11.022 (2021).
Ministry of Health. Proposals for a Smokefree Aotearoa 2025 Action Plan: Discussion Document (Ministry of Health, 2021).
Hatsukami, D. K., Xu, D. & Ferris Wayne, G. Regulatory approaches and implementation of minimally addictive combusted products. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab138 (2021).
Hecht, S. S. & Hatsukami, D. K. A regulatory strategy for reducing exposure to toxicants in cigarette smoke. in WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a WHO Study Group WHO Technical Report Series Ch. 5, 111–124 (World Health Organization, 2019).
Song, M. A. et al. Cigarette filter ventilation and its relationship to increasing rates of lung adenocarcinoma. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djx075 (2017).
Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations (Center for Tobacco Products, Food and Drug Administration, 2011).
Food and Drug Administration. Preliminary scientific evaluation of the possible public health effects of menthol versus nonmenthol cigarettes (Food and Drug Administration, 2013).
World Health Organization. Advisory note: banning menthol in tobacco products. in WHO Study Group on Tobacco Product Regulation (TobReg). (World Health Organization, 2016).
Acknowledgements
The authors’ research is supported by US National Cancer Institute grants P01 CA-138338 (S.S.H.), R01 CA-081301 (S.S.H.) and P01 CA-217806 (D.K.H.) and by US National Institute on Drug Abuse grant U54 DA-031659 (D.K.H.). The studies reported here were accomplished by an outstanding team of researchers. We thank all team members for their contributions.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article. S.S.H. wrote the sections on carcinogens and their effects, and D.K.H. wrote the sections on regulating tobacco products.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks David Ashley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Catalogue of Somatic Mutations In Cancer (COSMIC) database: https://cancer.sanger.ac.uk/cosmic
Glossary
- DNA adducts
-
Compounds formed by the reaction of DNA bases with certain electrophilic intermediates generated during metabolism, or with inherently reactive substances.
- Odds ratio
-
A statistic that explains the association between two events.
- Areca nut
-
The seed of the areca palm, which grows in South-East Asia; it is a common constituent of a quid known as paan, which is chewed by millions of people in the region.
- Agronomics
-
The branch of economics dealing with the distribution, management and productivity of land.
- Apurinic sites
-
Sites in DNA lacking the usual guanine or adenine bases. The sites can be formed when certain relatively unstable DNA adducts, such as 7-methyldeoxyguanosine, lose their purine base (in this case 7-methylguanine) due to spontaneous decomposition or hydrolysis.
- Creatinine
-
A waste product from normal metabolism that is commonly used as a denominator in biomarker studies.
- Advance notice of proposed rulemaking
-
(ANPRM). A document that an agency such as the US Food and Drug Administration may choose to issue before it is ready to issue a notice of proposed rulemaking. It is the first public step in the notice and comment rulemaking process, and the comments received could affect the final rule making process.
- Cigarette filter ventilation
-
The practice of incorporating holes near the cigarette filter to allow air to mix with the smoke stream, leading to overall lower constituent concentrations. It is a defective design because the holes may be blocked during smoking but not during machine measurement of smoke constituents.
Rights and permissions
About this article
Cite this article
Hecht, S.S., Hatsukami, D.K. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer 22, 143–155 (2022). https://doi.org/10.1038/s41568-021-00423-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00423-4
This article is cited by
-
The global, regional, and national disease burden of breast cancer attributable to tobacco from 1990 to 2019: a global burden of disease study
BMC Public Health (2024)
-
Cigarette smoking and progression of kidney dysfunction: a longitudinal cohort study
Clinical and Experimental Nephrology (2024)
-
Mortality burden and future projections of major risk factors for esophageal cancer in China from 1990 to 2019
General Thoracic and Cardiovascular Surgery (2024)
-
Short sleep duration and smoking initiation in university students: a retrospective cohort study
Sleep and Breathing (2024)
-
When smoke meets gut: deciphering the interactions between tobacco smoking and gut microbiota in disease development
Science China Life Sciences (2024)