Abstract
We aimed to compare refractive outcomes between total keratometry using a swept-source optical biometer and conventional keratometry in cataract surgery with refractive multifocal intraocular lens (IOL) implantation. We included patients who underwent cataract surgery with refractive multifocal IOL implantation. The IOL power was calculated using conventional formulas (Haigis, SRK/T, Holladay 2, and Barrett Universal II) as well as a new formula (Barrett TK Universal II). The refractive mean error, mean absolute error, and median absolute error were compared, as were the proportions of eyes within ± 0.25 diopters (D), ± 0.50 D, and ± 1.00 D of prediction error. In total 543 eyes of 543 patients, the absolute prediction error of total keratometry was significantly higher than that of conventional keratometry using the SRK/T (P = 0.034) and Barrett Universal II (P = 0.003). The proportion of eyes within ± 0.50 D of the prediction error using the SRK/T and Barrett Universal II was also significantly higher when using conventional keratometry than total keratometry (P = 0.010 for SRK/T and P = 0.005 for Barrett Universal II). Prediction accuracy of conventional keratometry was higher than that of total keratometry in cataract surgery with refractive multifocal IOL implantation.
Similar content being viewed by others
Introduction
When estimating total keratometry (TK), clinicians must consider posterior as well as anterior keratometry to ensure they obtain accurate measurements. Traditionally, anterior corneal measurements have been used to estimate posterior corneal power based on a fixed posterior:anterior curvature ratio. Both TK and astigmatism can then be estimated based on anterior corneal measurements combined with an estimated posterior corneal power. In one published study, the measured TK showed a strong correlation with the TK estimated using a Goggin nomogram, which is based on the anterior keratometric value1.
The IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany), which is based on swept-source optical coherence tomography, was developed to allow more precise optical biometry. It can measure the correct axial length (AXL) by imaging a cross section of the eye. The IOLMaster 700 also can measure the TK and calculate the exact intraocular lens (IOL) power using exclusive formulas, such as the Barrett TK Universal II and the Barrett TK toric2. Several articles have applied the currently used IOL calculation formulas to both the measured TK and conventional keratometry (K) to calculate the refractive outcomes of cataract surgery using monofocal or toric IOLs, while others have described cataract surgery performed after refractive surgery2,3,4,5,6.
Cataract surgery technology is constantly evolving, with increasing predictive accuracy and more sophisticated refractive results. In cataract surgery with multifocal IOL implantation, which has recently become more common, accurate IOL power is important for postoperative distant and near visual quality and is determined using optical biometric measurements.
Our group recently reported differences between prediction errors in applying K and TK data according to the type of diffractive multifocal IOL7. According to another recently published paper, the application of TK to formulas used after refractive surgery was not beneficial in cataract surgery after refractive surgery8. As such, it remains unclear whether TK in cataract surgery with refractive multifocal IOL implantation is useful, and long-term data are needed. In the present study, we compared the prediction accuracy of TK with that of conventional K in cataract surgery with refractive multifocal IOL implantation.
Results
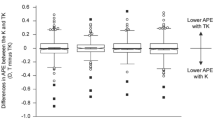
A total 543 eyes of 543 patients were included in the present study. The preoperative demographics of the patients and number of patients in each AXL subgroup are shown in Table 1. Table 2 shows the refractive mean error (ME), mean absolute error (MAE), and median absolute error (MedAE) according to each formula using either K or TK. When comparing the absolute predictive error (APE) using the K and TK calculated by each formula, the SRK/T and Barrett Universal II formulas showed significantly lower APE with K compared to TK (P = 0.127 for Haigis, P = 0.034 for SRK/T, P = 0.097 for Holladay 2, and P = 0.003 for Barrett Universal II). Figure 1 shows the Bland–Altman plot of the APE differences between K and TK calculated using each formula. The proportions of eyes within ± 0.25 diopters (D), ± 0.50 D, and ± 1.00 D of the prediction error using K and TK according to each formula are shown in Table 3 and Fig. 2. When comparing the proportion of eyes within ± 0.50 D between K and TK, SRK/T (P = 0.010) and Barrett Universal II (P = 0.005) formulas showed a significantly higher proportion of K compared to TK.
Bland–Altman plots comparing absolute predictive error between conventional keratometry and total keratometry using each formula (A) Haigis; (B) SRK/T; (C) Holladay 2; (D) Barrett Universal II. Ranges within 95% of values are indicated by dot lines. APE absolute predictive error, K conventional keratometry, TK total keratometry.
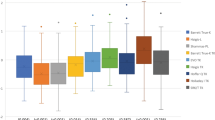
There was a significant difference in APE among all the formulas (P < 0.001). Post hoc analysis was conducted using the Wilcoxon signed-rank test, with a Bonferroni correction applied, resulting in a significance level of P < 0.0018. The overall comparison and post hoc analysis showed that the APE was lowest in the Barrett Universal II using K, followed in order by the Barrett TK Universal II, the Holladay 2 using K, the SRK/T using K, the Holladay 2 using TK, the SRK/T using TK, the Haigis using TK, and the Haigis using K (Fig. 3). When each formula was compared with all others, the APE of the Haigis using K was significantly higher from that of all other formulas, and the APE of the Haigis using TK was significantly higher from that of all formulas except for SRK/T using TK. Among the other formulas, there were significant differences between SRK/T using K and Barrett Universal II using K (adjusted P < 0.001), between SRK/T using TK and Barrett Universal II using K (adjusted P < 0.001), between SRK/T using TK and Barrett TK Universal II (adjusted P = 0.009), and between Holladay 2 using TK and Barrett Universal II using K (adjusted P = 0.007).
Table 4 shows the AXL subgroup analysis. In short eyes, the Barrett TK Universal II had the lowest MAE, while the Haigis using K had the highest MAE. In long eyes, the Barrett Universal II using K had the lowest MAE, while the Haigis using TK had the highest MAE.
Discussion
In the present study, we demonstrated that the prediction accuracy of conventional K was higher than that of TK in cataract surgery with refractive multifocal IOL implantation. When using the SRK/T and Barrett Universal II formulas, K had a significantly higher prediction accuracy than TK. Previous studies on the clinical usefulness of TK have used several kinds of monofocal or toric IOLs. In the present study, we focused on the use of refractive multifocal IOLs to investigate the accuracy of TK in IOL power prediction. In addition, one strength of the study was its large sample size.
With all formulas except the Haigis formula, MAE and MedAE were higher when using TK than when using K, and the difference was significant in the SRK/T and Barrett Universal II formulas. When comparing the proportion of eyes within ± 0.50 D of the prediction error, K showed a significantly higher proportion than TK in the SRK/T and Barrett Universal II formulas. Previous studies have reported that, when the Barrett Universal II and SRK/T formulas were applied to conventional K, the MAE and MedAE were higher than when those formulas were applied to TK2,3. The present study contradicts this finding. It may be that the SRK/T formula showed more accurate results when using conventional K because it was developed using that measurement. However, the Barrett TK Universal II was developed based on TK, so by the same reasoning one would expect more accurate results when TK was applied to Barrett TK Universal II than when K was applied to Barrett Universal II.
These unexpected results may have occurred because the two previous studies used the 601P/PY IOL (Carl Zeiss Meditec, Jena, Germany) and Asphina 409M/MP IOL (Carl Zeiss Meditec, Jena, Germany), respectively, whereas the present study used multifocal IOLs from different manufacturer. In addition, when comparing the proportion of eyes within ± 0.50 D of the prediction error, Fabian et al. reported 77% and 84%, respectively, when applying K to the Haigis and Barrett Universal II formulas, and 79% and 86% when applying TK to the same formulas2. Sirvannaboon et al. reported 62% and 60%, respectively, when applying K to the Haigis and Barrett Universal II formulas, and 63% and 67% when applying TK to them3. In the present study, we found values of 82.5% and 89.9%, respectively when applying K to the Haigis and Barrett Universal II formulas, and 82.1% and 86.6% when the TK was applied. Compared to the two studies mentioned, when using TK, the prediction accuracy was similar or higher, while when using K, the accuracy was much higher. Therefore, the unexpected results of the present study likely resulted from higher accuracy when using K rather than from low accuracy when using TK. The patients included in the present study underwent multifocal IOL implantation to correct presbyopia. As such, they were younger and better preoperative best corrected visual acuity (BCVA) than the patients in other IOL power calculation studies. Therefore, it may be that we could make more accurate biometric measurements and that the IOL power calculation had greater accuracy.
The Haigis formula was the only one that showed lower MAE when using TK than when using K. In a previous study, Haigis using TK exhibited higher accuracy than Haigis using K2. When estimating the cumulative percentage of eyes, Haigis K showed 77% within 0.50 D and 95% within 1.00 D, while Haigis TK showed 79% within 0.50 D and 96% within 1.00 D. All of these proportions were higher in the present study than in the previous study2.
When comparing the APE between formulas, not all comparisons were significant, but the overall comparison was significant (Fig. 3). In a recently published study on TK, when using both K and TK, MAE and MedAE was lowest when applying Barrett Universal II, followed by SRK/T, Holladay 2, and Haigis3. Our study found similar results, although the order between SRK/T and Holladay 2 was reversed, and the difference was not significant using either K or TK. As mentioned above, the Barrett TK Universal II formula showed significantly inferior results than the Barrett Universal II using K, but it did show superior results compared to other formulas using K, perhaps because the Barrett TK Universal II was developed based on TK. Conversely, when the previously used formulas SRK/T, Holladay 2, and Haigis used TK, the result was inferior to all formulas using K except Haigis.
A review article on IOL power calculation mentioned that the Haigis and Holladay 2 formulas have higher accuracy than the Barrett Universal II formula in short eyes, and that the Barrett Universal II, Haigis, Olsen, and SRK/T formulas have greater accuracy in long eyes9. In the AXL subgroup analyses of the present study, Barrett formulas resulted in the lowest MAE, regardless of the AXL—Barrett TK Universal II in short eyes, Barrett Universal II in medium and long eyes, while Haigis formulas conferred the highest MAE—Haigis K in short and medium eyes, Haigis TK in long eyes. Both Haigis and Holladay 2, which were mentioned as high accuracy in the review article, showed high MAE in short eyes, and Haigis even showed high MAE in long eyes, whereas Barrett and SRK/T showed lower MAE in long eyes than in short eyes, showing high accuracy in long eyes. Although these results were somewhat contrary to those previously published, our results included only 11.0% of all subjects for short eyes and 3.87% for long eyes, so a larger number of patients should be analysed in a future study.
The present study was limited by its retrospective nature and because the average ME was not set to zero using the A constant as a User Group of the Laser Interference Biometry (ULIB). To allow more accurate IOL power prediction, an optimally personalized lens constant that can set the average ME to 0 should be applied to eliminate bias caused by the lens factor. Excluding this limitation, the present study followed all protocols suggested for studies into IOL formula accuracy proposed by Hoffer et al.10. The cataract surgery in the present study was performed by one expert surgeon using only one IOL model, one eye from each patient was randomly included, and postoperative refraction was measured 2 months after surgery.
In conclusion, when applying the formulas currently in use to TK during cataract surgery with refractive multifocal IOL implantation, the refractive results were not superior to those of K. The APE was higher when using TK than when using K, so more TK data must be gathered for each formula to allow more accurate IOL power calculation using TK.
Methods
Subject
This study was conducted with the approval of the Institutional Review Board of the Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea (Approval number: 2020-1290). The study adhered to the tenets of the Declaration of Helsinki and followed good clinical practice guidelines. This study was conducted retrospectively and the informed consent from the patients was waived.
In this retrospective study, all patients underwent cataract surgery with phacoemulsification and implantation of refractive multifocal IOL (Lentis M plus; Oculentis GmbH., Berlin, Germany). All procedures were performed by a single experienced surgeon. In previous studies, this refractive multifocal IOL has shown stable distant and near vision, as well as fewer photopic phenomena and better intermediate vision than diffractive multifocal IOLs11,12. The study included patients who underwent cataract surgery between January 2019 and December 2019, who underwent a complete preoperative examination, uncomplicated cataract surgery, and a follow-up of at least 2 months. Patients who had undergone previous intraocular surgery were excluded, as were those with a BCVA of less than 0.1 logMAR at 2 months after surgery. One eye was randomly selected to participate in the study. All subjects underwent a complete ophthalmologic examination before surgery, including BCVA and automated keratometry using the RK-F2 Full Auto Ref-Keratometer (Canon, Tokyo, Japan). The AXL, anterior chamber depth, K, and TK were measured using the IOLMaster 700. At least 2 months after surgery, patients underwent ophthalmologic examination, including BCVA, automated keratometry, and manifest refraction. The parameters were measured twice by an experienced technician to ensure accuracy. Patients were categorised into three groups according to the preoperative axial length: short (< 22.5 mm), medium (22.5–25.5 mm), and long (> 25.5 mm).
Calculating IOL prediction error
IOL power calculations were carried out on an IOLMaster 700 unit (Barrett Suite, software version 1.80.6.60340). We applied the TK and K of all patients to the currently widely used formulas (Haigis, SRK/T, Holladay 2). In the Barrett formula, K is applied to Barrett Universal II, and TK is applied to Barrett TK Universal II, a new formula based on TK. We used the lens constants of the User Group of the Laser Interference Biometry (ULIB) website.
ME was defined as the mean value obtained by subtracting the preoperative predicted spherical equivalent from the actual spherical equivalent. APE was defined as the absolute value obtained by subtracting the actual spherical equivalent from the preoperative predicted spherical equivalent. MAE was defined as the mean APE value, while MedAE was defined as the median APE. The proportion of eyes within ± 0.25 D, ± 0.50 D, and ± 1.00 D of the prediction error were measured.
Statistical analyses
A Wilk–Shapiro test was used to assess the distribution of numerical data. The Wilcoxon signed-rank test was used to compare the APE between K and TK. The APE was compared between the formulas using the Friedman test, with Bonferroni post-hoc correction for multiple comparisons. The proportions of eyes within ± 0.25 D, ± 0.50 D, and ± 1.00 D were compared using the McNemar’s test. For each formula, APE differences between conventional K and TK were obtained, the differences were analysed using the Bland–Altman plot. All data were statistically analysed using SPSS software version 25.0 (IBM, Armonk, NY, USA), and all P-values less than 0.05 were considered statistically significant.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
LaHood, B. R., Goggin, M., Beheregaray, S., Andrew, N. H. & Esterman, A. Comparing total keratometry measurement on the IOLMaster 700 with Goggin nomogram adjusted anterior keratometry. J. Refract. Surg. 34, 521–526. https://doi.org/10.3928/1081597X-20180706-01 (2018).
Fabian, E. & Wehner, W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J. Refract. Surg. 35, 362–368. https://doi.org/10.3928/1081597X-20190422-02 (2019).
Srivannaboon, S. & Chirapapaisan, C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol. 257, 2677–2682. https://doi.org/10.1007/s00417-019-04443-7 (2019).
Wang, L., Spektor, T., de Souza, R. G. & Koch, D. D. Evaluation of total keratometry and its accuracy for intraocular lens power calculation in eyes after corneal refractive surgery. J. Cataract. Refract. Surg. 45, 1416–1421. https://doi.org/10.1016/j.jcrs.2019.05.020 (2019).
Lawless, M. et al. Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin. Exp. Ophthalmol. 48, 749–756. https://doi.org/10.1111/ceo.13760 (2020).
Yeo, T. K., Heng, W. J., Pek, D., Wong, J. & Fam, H. B. Accuracy of intraocular lens formulas using total keratometry in eyes with previous myopic laser refractive surgery. Eye (Lond.) https://doi.org/10.1038/s41433-020-01159-5 (2020).
Lee, H., Chung, J. L., Kim, Y. J., Kim, J. Y. & Tchah, H. Prediction accuracy of standard and total keratometry by swept-source optical biometer for multifocal intraocular lens power calculation. Sci. Rep. 11, 4794. https://doi.org/10.1038/s41598-021-84238-1 (2021).
Sandoval, H. P., Serels, C., Potvin, R. & Solomon, K. D. Cataract surgery after myopic LASIK: Objective analysis to determine best formula and keratometry to use. J. Cataract. Refract. Surg. https://doi.org/10.1097/j.jcrs.0000000000000472 (2020).
Hoffer, K. J. & Savini, G. IOL power calculation in short and long eyes. Asia Pac. J. Ophthalmol. (Phila.) 6, 330–331. https://doi.org/10.22608/APO.2017338 (2017).
Hoffer, K. J. et al. Protocols for studies of intraocular lens formula accuracy. Am. J. Ophthalmol. 160, 403–405. https://doi.org/10.1016/j.ajo.2015.05.029 (2015).
Alio, J. L., Plaza-Puche, A. B. & Pinero, D. P. Rotationally asymmetric multifocal IOL implantation with and without capsular tension ring: Refractive and visual outcomes and intraocular optical performance. J. Refract. Surg. 28, 253–258. https://doi.org/10.3928/1081597X-20120314-01 (2012).
Moore, J. E., McNeely, R. N., Pazo, E. E. & Moore, T. C. Rotationally asymmetric multifocal intraocular lenses: Preoperative considerations and postoperative outcomes. Curr. Opin. Ophthalmol. 28, 9–15. https://doi.org/10.1097/ICU.0000000000000339 (2017).
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing. This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 9991006821, KMDF_PR_20200901_0148), by a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (2021IP0059-1, 2021IP0061-1), and by Research and Business Development Program through the Korea Institute for Advancement of Technology(KIAT) funded by the Ministry of Trade, Industry and Energy(MOTIE) (Grant number : P0014063). The funding agency had no role in the design or conduct of this study, in the collection, management, analysis, or interpretation of the data, in the preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.S.C., J.L.C., Y.J.K., H.L., J.Y.K., and H.T. designed and conducted the study, analysed the data, wrote the original draft, and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, H.S., Chung, J.L., Kim, Y.J. et al. Comparing prediction accuracy between total keratometry and conventional keratometry in cataract surgery with refractive multifocal intraocular lens implantation. Sci Rep 11, 19234 (2021). https://doi.org/10.1038/s41598-021-98491-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98491-x
This article is cited by
-
Calculation of the total corneal astigmatism using the virtual cross cylinder method on the secondary principal plane of the cornea
Scientific Reports (2024)
-
Comparison of the accuracy of 9 intraocular lens power calculation formulas after SMILE in Chinese myopic eyes
Scientific Reports (2023)
-
Lower refractive prediction accuracy of total keratometry using intraocular lens formulas loaded onto a swept-source optical biometer
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.